Open Access Article
Open Access ArticleBinding of enterolactone and enterodiol to human serum albumin: increase of cysteine-34 thiol group reactivity
Marija M.
Takić
a,
Vesna B.
Jovanović
b,
Ivan D.
Pavićević
b,
Tamara N.
Uzelac
b,
Jelena M.
Aćimović
b,
Danijela K.
Ristić-Medić
a and
Ljuba M.
Mandić
*b
aInstitute for Medical Research, Center of research excellence in nutrition and metabolism, University of Belgrade, Belgrade, Serbia
bDepartment of Biochemistry, Faculty of Chemistry, University of Belgrade, Studentski trg 12-16, Belgrade 11158, Serbia. E-mail: ljmandic@chem.bg.ac.rs; Tel: +381 11 333 66 76
First published on 22nd January 2016
Abstract
The interaction of polyphenolic molecules with human serum albumin (HSA) could lead to changes in the reactivity of the HSA Cys34 thiol group (HSA-SH). The influences of enterolactone (EL) and enterodiol (ED) binding on HSA-SH reactivity in fatty acid (FA)-free HSA, and in HSA with bound stearic acid (S) in S/HSA molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, were investigated by the determination of the pseudo first order rate constants (k′) for the thiol reaction with 5,5′-dithiobis-(2-nitrobenzoic acid). The binding affinities and binding sites of EL and ED were also determined, using fluorescence measurements of the intrinsic fluorescence of Trp214 and diazepam (binding site marker). EL and ED binding to HSA increased the reactivity of HSA-SH in all assayed HSA-enterolignan complexes by 9.1–33.1%. The strongest effects were obtained for FA-free HSA-enterolignan complexes. S modulated/reduced the effect of EL on HSA-SH reactivity, while its influence on the effect of ED was negligible. The binding of enterolignans to HSA was investigated: the binding constants were the highest for FA-free HSA (EL: 11.64 × 104 M−1 and ED: 5.59 × 104 M−1 at 37 °C) and the lowest for S/HSA 4
1, were investigated by the determination of the pseudo first order rate constants (k′) for the thiol reaction with 5,5′-dithiobis-(2-nitrobenzoic acid). The binding affinities and binding sites of EL and ED were also determined, using fluorescence measurements of the intrinsic fluorescence of Trp214 and diazepam (binding site marker). EL and ED binding to HSA increased the reactivity of HSA-SH in all assayed HSA-enterolignan complexes by 9.1–33.1%. The strongest effects were obtained for FA-free HSA-enterolignan complexes. S modulated/reduced the effect of EL on HSA-SH reactivity, while its influence on the effect of ED was negligible. The binding of enterolignans to HSA was investigated: the binding constants were the highest for FA-free HSA (EL: 11.64 × 104 M−1 and ED: 5.59 × 104 M−1 at 37 °C) and the lowest for S/HSA 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-enterolignan complexes (EL: 2.43 × 104 M−1 and ED: 1.92 × 104 M−1). When the S/HSA ratio was increased, the binding affinities and number of binding sites for EL and ED were decreased. At the same time, a high correlation between binding constants and increased Cys34 reactivity was found (r = 0.974). Competitive experiments using diazepam indicated that the binding of ED and of EL was located in the hydrophobic pocket of site II in HSA. Overall, it is evident that stearic acid could modulate the enterolignan effects on HSA-SH reactivity as well as their binding to HSA. This finding could be important for pharmacokinetics and the expression of enterolignan antioxidant effects in vivo after an intake of lignan rich food.
1-enterolignan complexes (EL: 2.43 × 104 M−1 and ED: 1.92 × 104 M−1). When the S/HSA ratio was increased, the binding affinities and number of binding sites for EL and ED were decreased. At the same time, a high correlation between binding constants and increased Cys34 reactivity was found (r = 0.974). Competitive experiments using diazepam indicated that the binding of ED and of EL was located in the hydrophobic pocket of site II in HSA. Overall, it is evident that stearic acid could modulate the enterolignan effects on HSA-SH reactivity as well as their binding to HSA. This finding could be important for pharmacokinetics and the expression of enterolignan antioxidant effects in vivo after an intake of lignan rich food.
1. Introduction
Human serum albumin (HSA) is the most abundant protein in plasma, being present at a high concentration of about 0.6 mM,1 and having a half-life of 19 days. As the α-helix and random structure contents in HSA are 70% and 30%, respectively (with almost no β-sheets), the HSA molecule has a high degree of conformational flexibility.2 Crystallographic data show that HSA contains three homologous domains (I, II and III) of 10 helices that can be divided into two subdomains (A and B), also displaying partial inter-subdomain homology.3 HSA is a transporter of many endogenous substances (non-esterified fatty acids, bilirubin, bile salts, steroid hormones, hematin, tryptophan, thyroxin and some vitamins), several metal ions and different exogenous molecules (e.g. polyphenols and drugs), that greatly augments the transport capacity of blood plasma.4 HSA has seven binding sites (of varying affinities) for medium and long-chain fatty acids (FA) distributed throughout the molecule, which involve all six subdomains.5 There are also four thyroxin binding sites, several metal binding sites including albumin's N-terminus, and a site centered around residue Cys34.6 HSA's exogenous ligands are accommodated primarily at one of two major sites with binding association constants in the range from 104 to 106 M.2 Drug binding site I is located in subdomain IIA (and overlaps with FA binding site 7), and drug site II is in subdomain IIIA (and overlaps with FA binding sites 3 and 4).7Dietary phenolic substances have received much attention as numerous studies have revealed their various protective effects in vitro and in vivo.8,9 Enterolactone (EL) and enterodiol (ED) (Fig. 1) are produced from several dietary plant lignans (with polyphenolic structure) through extensive metabolism by the gut microflora.10 They are especially abundant in seeds, whole grains and berries.11 Results of our previous study12 indicated that when dietary milled sesame/pumpkin/flax seed mixtures, rich in polyunsaturated FA and lignans, were added to the habitual diet, triglyceride and inflammatory marker levels were lowered, glycemic control was affected, and the FA profile and pruritus symptoms in hemodialysis patients were improved. Because of their antioxidant and weak estrogenic effect, enterolignans may have many beneficial effects on human liver function,13 and decrease the risk of breast14 and prostate cancers15 and of cardiovascular diseases.16 Variations in enterolignan levels in serum and urine are high and most of the differences within the population can be attributed to different dietary habits. Besides dietary intake, metabolism by intestinal bacteria, endogenous hormones, and antibiotic use also influence lignan levels. EL and ED are mainly present as glucuronide and sulfate conjugates in body fluids and are eliminated slowly via the urine.17 The binding of absorbed phytoestrogens to albumin is an important factor in determining their pharmacokinetics, pharmacodynamics and biological activities. Numerous studies reported to date have dealt with native polyphenol binding to albumin and noticeable differences in their binding behavior, due to subtle differences in structure, have been revealed.18 To our knowledge, interactions between enterolignans and albumin have not yet been studied.
HSA has 17 disulfide bridges and one free Cys34 thiol group (HSA-SH).8 HSA-SH accounts for approximately 80% of the total free thiols in plasma.19 About 70% of circulating HSA contains Cys34 in its reduced thiol state. The rest of the Cys34 residues consist mostly of mixed disulfides with cysteine and other low molecular weight thiols.20 A minor fraction is oxidized to higher oxidation states such as sulfinic (HSA-SO2H) and sulfonic acids (HSA-SO3H).21 Therefore, HSA is recognized as a very important antioxidant in plasma. Under normal physiological conditions, between 0.1 and 2 mol of free FA are bound to HSA, but the molar ratio of FA/HSA can rise above 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in fasting, intensive exercise or under pathological conditions such as diabetes, liver and cardiovascular diseases.22 Binding of FA is associated with significant structural changes in the HSA molecule,23 which can cause the Cys34 residue to be more or less exposed to the surrounding environment, leading to differential reactivity and susceptibility to oxidative stress.19–21,24–27 These findings lead to the question: Does the binding of EL and ED to HSA (for which the antioxidant effect is proven) influence HSA-SH reactivity and therefore its antioxidant potential?
1 in fasting, intensive exercise or under pathological conditions such as diabetes, liver and cardiovascular diseases.22 Binding of FA is associated with significant structural changes in the HSA molecule,23 which can cause the Cys34 residue to be more or less exposed to the surrounding environment, leading to differential reactivity and susceptibility to oxidative stress.19–21,24–27 These findings lead to the question: Does the binding of EL and ED to HSA (for which the antioxidant effect is proven) influence HSA-SH reactivity and therefore its antioxidant potential?
Although numerous studies on the interactions between polyphenols and HSA have been performed, the changes in HSA-SH reactivity that can occur upon polyphenol binding (which may be relevant to antioxidant properties) have not been considered yet. Therefore, in this study the pseudo first order rate constants for the reaction between HSA-SH and 5, 5′-dithiobis-(2-nitrobenzoic acid) (DTNB) in the presence of EL and ED were determined. As the HSA binding sites for FA and polyphenols are overlapping, cooperative and competitive interactions between FA and enterolignans on HSA-SH reactivity were also investigated. Two stearic acid/HSA (S/HSA) molar ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) that correspond to normal and pathological conditions were used. In order to better understand the results obtained, the interactions of EL and ED with HSA were also investigated. The binding affinities and binding sites of EL and ED were determined, using fluorescence measurements of the intrinsic fluorescence of Trp214 and diazepam (binding site marker).
1) that correspond to normal and pathological conditions were used. In order to better understand the results obtained, the interactions of EL and ED with HSA were also investigated. The binding affinities and binding sites of EL and ED were determined, using fluorescence measurements of the intrinsic fluorescence of Trp214 and diazepam (binding site marker).
2. Materials and methods
2.1. Chemicals
All chemicals were purchased from Sigma (Steinheim, Germany) unless otherwise noted. The 20% solution of HSA was purchased from Baxter (Vienna, Austria). All chemicals used were of analytical grade.2.2. Preparation of FA-free HSA and reduced HSA
Commercial HSA contains bound FA and contains approximately 40% of HSA-SH in its reduced form. For experimental purposes, HSA was defatted according to Chen's charcoal treatment method,27 and then reduced with dithiothreitol (DTT) as described by Penezic et al.28 In all experiments, HSA with a free thiol group content of about 60–70% (FA-free HSA) was prepared by mixing appropriate volumes of defatted HSA and reduced defatted HSA.2.3. Preparation of FA-bound HSA samples, and HSA-EL and HSA-ED complexes
A solution of S (50 mM) in 99% ethanol was mixed with the solution of FA-free HSA (0.25 mM in 0.1 M sodium phosphate buffer, pH 7.4) at molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (S/HSA 1
1 (S/HSA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 4
1) and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (S/HSA 4
1 (S/HSA 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The mixtures were incubated at room temperature overnight and then centrifuged (12
1). The mixtures were incubated at room temperature overnight and then centrifuged (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 5 min). The highest increase of thiol group reactivity has been observed when polyunsaturated FA (PUFA) are bound to HSA25 (and it is known that phytoestrogens influence the oxidative metabolism of PUFA29), but they were avoided because of their oxidizability.
000 rpm, 5 min). The highest increase of thiol group reactivity has been observed when polyunsaturated FA (PUFA) are bound to HSA25 (and it is known that phytoestrogens influence the oxidative metabolism of PUFA29), but they were avoided because of their oxidizability.
HSA enterolignan complexes (HSA-EL and HSA-ED) were prepared by mixing FA-free HSA, S/HSA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or S/HSA 4
1 or S/HSA 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with appropriate volumes of EL or ED solutions (25 mM in DMSO) to get a final HSA/enterolignan molar ratio of 1
1 with appropriate volumes of EL or ED solutions (25 mM in DMSO) to get a final HSA/enterolignan molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and incubating at 37 °C for one hour.
1, and incubating at 37 °C for one hour.
2.4. Quantification of HSA and HSA-SH group content
A biuret assay was used for the quantification of total protein content.30 Free HSA-SH content was determined spectrophotometrically according to a modified Ellman's method.31 All reagents were kept at room temperature for 30 min before determination. DTNB reagent (100 μL of 2 mM solution) was mixed with equal volumes of sample and 1 M Tris buffer (pH 8.0) and brought up to 1000 μL with water. The absorbance was measured after 30 min at room temperature at 412 nm against the sample and reagent blanks. The concentration of thiols was calculated by using the molar extinction coefficient (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 M−1 cm−1).32
150 M−1 cm−1).32
2.5. Determination of the pseudo first order constant for the reaction of HSA-SH with DTNB
Reaction kinetics were monitored spectrophotometrically using the method described in detail elsewhere.25 Briefly, a sample (100 μL) of FA-free HSA or S/HSA complex (with EL or ED) (0.25 mM HSA, with HSA-SH content of about 60–70%) was mixed with 1 M Tris buffer pH 8.0 (100 μL), water (700 μL) and 3.5 mM DTNB reagent (100 μL). The concentration of the DTNB reagent represents a twenty-fold pseudo first order excess compared to the HSA-SH concentration. After mixing, the absorbance at 412 nm was recorded every 5 s for the first 90 s, then every 10 s for 270 s, and finally every 30 s for the remaining 30 min of total reaction time. Values of k′ were determined by fitting the natural logarithm of unreacted thiol group concentration versus time using the linear least squares model.2.6. Fluorescence measurements
The binding of EL and ED to HSA was studied using a fluorescence quenching titration method using the intrinsic fluorescence of HSA as the probe. The fluorescence measurements were performed on a FluoroMax-4 Jobin Yvon (Horiba Scientific, Japan) spectrofluorometer equipped with a 1.0 cm quartz cell and thermostat bath. The excitation and emission slit widths were set at 5.0 nm.Solutions of FA-free HSA, S/HSA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and S/HSA 4
1 and S/HSA 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were prepared daily by diluting the stock solutions of HSA (0.25 mM in 100 mM sodium phosphate buffer pH 7.4) and enterolignans (25 mM in DMSO) with 100 mM sodium phosphate buffer pH 7.4 to the final concentrations of HSA (0.5 μM) and enterolignans (100 μM) in all experiments. Small aliquots of 100 μM ligand solutions were added to 2.5 ml of 0.5 μM HSA solution. Thus the final concentrations of ligands were 0.125, 0.25, 0.5, 1.0, 1.5, 2.0 and 2.5 μM. Emission spectra were recorded in the range 300 to 500 nm at 37 °C with an excitation wavelength of 280 nm.
1 were prepared daily by diluting the stock solutions of HSA (0.25 mM in 100 mM sodium phosphate buffer pH 7.4) and enterolignans (25 mM in DMSO) with 100 mM sodium phosphate buffer pH 7.4 to the final concentrations of HSA (0.5 μM) and enterolignans (100 μM) in all experiments. Small aliquots of 100 μM ligand solutions were added to 2.5 ml of 0.5 μM HSA solution. Thus the final concentrations of ligands were 0.125, 0.25, 0.5, 1.0, 1.5, 2.0 and 2.5 μM. Emission spectra were recorded in the range 300 to 500 nm at 37 °C with an excitation wavelength of 280 nm.
Quencher ligands can absorb energy at both the HSA excitation and emission wavelengths. In order to overcome the inner-filter effect, the absorbance values of the ligands used were recorded on a Shimadzu UV 1800 (Japan) and corresponding corrections were made during the calculation of binding parameters according to eqn (1):33
| Fc = Fu × 10(Aex×dex + Aem×dem)/2 | (1) |
The quenching constants of HSA/enterolignan complexes were determined using the Stern–Volmer eqn (2):
| F0/F−1 = 1 + kqτ0[Q] = 1 + Ksv[Q] | (2) |
The estimations of association (binding) constants (Ka) and number of binding sites (n) of HSA and enterolignans (EL and ED) were done using eqn (3):35
log(F0 − F)/F = −nlog(1/([Q] − [P] × (F0 − F)/F0) + nlog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka Ka | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) respectively.
1) respectively.
In the binding site marker experiments, diazepam was used as the marker for site II. A volume of 2.5 ml of 0.5 μM HSA-EL or HSA-ED was titrated with 100 μM diazepam (prepared daily by diluting a stock solution of 50 mM diazepam in DMSO with 100 mM sodium phosphate buffer, pH 7.4); the final concentration of diazepam varied from 0–1.5 μM at increments of 0.125 μM.
2.7. Statistical analysis and graph generation
All statistical analysis and graphical representations of data were performed using the Origin 9.0 statistical program.3. Results and discussion
The cooperative and competitive interactions between FA and polyphenolic molecules (e.g. enterolignans) could be important factors affecting HSA-SH reactivity. Therefore, the reactivities of FA-free HSA-SH, FA/HSA-SH and their complexes with enterolignans were first investigated. Appropriate physiological models of FA/HSA were used: S/HSA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (normal physiological condition) and S/HSA 4
1 (normal physiological condition) and S/HSA 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (in fasting, intensive exercise or under pathological conditions).
1 (in fasting, intensive exercise or under pathological conditions).
3.1. Influence of stearic acid on the reactivity of HSA-SH
The reactivity of HSA-SH was studied with the low molecular weight disulfide DTNB reagent, at a concentration twenty-fold higher than that of HSA-SH. This represented a pseudo first order excess. The reaction between the HSA Cys34 thiol group and DTNB can be written as follows:| HSA–S− + DTNB → HSA–S-TNB + TNB− | (4) |
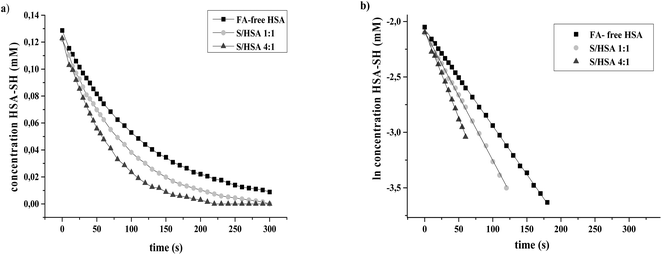
The reactions of HSA-SH were monitored spectrophotometrically over 30 minute time courses. Graphics obtained after the linearization of kinetics data show that reactions followed pseudo first order reaction kinetics (Fig. 2). The values of k′ obtained for FA-free HSA and S/HSA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were 8.9 ± 0.1 × 10−3 s−1, 11.6 ± 0.3 × 10−3 s−1 and 15.3 ± 0.1 × 10−3 s−1, respectively. They are in accordance with previous results obtained under similar conditions.25 The reactivity of the HSA-SH in the S/HSA 4
1 were 8.9 ± 0.1 × 10−3 s−1, 11.6 ± 0.3 × 10−3 s−1 and 15.3 ± 0.1 × 10−3 s−1, respectively. They are in accordance with previous results obtained under similar conditions.25 The reactivity of the HSA-SH in the S/HSA 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex was almost two times higher than that of FA-free HSA. The free thiol group of albumin, present at a concentration of 0.6 mM in plasma, constitutes the largest pool of reactive thiols in plasma and acts as a key oxidant scavenger.19–21 Therefore, some physiological conditions (e.g. exercise, fasting) and diseases (hemodialysis, preeclampsia, diabetes) which are associated with oxidative stress, tend to increase the amount of FA bound to HSA,19 which could be a protective adaptation. The reactivity of HSA-SH is increased upon FA binding and is dependent on the type of FA bound to HSA.25 It is reported that upon FA binding, the environment of the HSA-SH could become more polar, and induced conformational changes may cause increased accessibility of the Cys34 group.20
1 complex was almost two times higher than that of FA-free HSA. The free thiol group of albumin, present at a concentration of 0.6 mM in plasma, constitutes the largest pool of reactive thiols in plasma and acts as a key oxidant scavenger.19–21 Therefore, some physiological conditions (e.g. exercise, fasting) and diseases (hemodialysis, preeclampsia, diabetes) which are associated with oxidative stress, tend to increase the amount of FA bound to HSA,19 which could be a protective adaptation. The reactivity of HSA-SH is increased upon FA binding and is dependent on the type of FA bound to HSA.25 It is reported that upon FA binding, the environment of the HSA-SH could become more polar, and induced conformational changes may cause increased accessibility of the Cys34 group.20
3.2. Binding of enterolignans to HSA influences Cys34 thiol group reactivity
The influence of enterolignan (ED and EL) binding on the reactivity of HSA-SH was investigated after incubation of FA-free HSA or S/HSA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 4
1 or 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (0.25 mM) at 37 °C for one hour with EL and ED in the molar ratio 1
1 (0.25 mM) at 37 °C for one hour with EL and ED in the molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
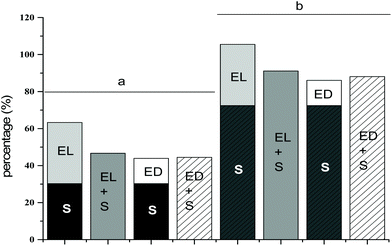
Significant increases in the reactivity of HSA-SH were found after the binding of enterolignans to all HSA complexes without and with FA (Fig. 3 and Table 1). The highest effect on HSA-SH reactivity was obtained for FA-free HSA-EL followed by FA-free HSA-ED (33.1 and 13.6%, respectively), even though the thiol group of the formed complex FA-free HSA-EL had a reactivity similar to S/HSA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (k′ values 11.8 ± 0.4 × 10−3 s−1vs. 11.6 ± 0.3 × 10−3 s−1, Table 1). Thus, the values obtained for k′ constants lead to the conclusion that enterolignan binding to HSA leads to an increase in HSA-SH reactivity and that this effect is more pronounced at lower molar ratios of FA/HSA.
1 (k′ values 11.8 ± 0.4 × 10−3 s−1vs. 11.6 ± 0.3 × 10−3 s−1, Table 1). Thus, the values obtained for k′ constants lead to the conclusion that enterolignan binding to HSA leads to an increase in HSA-SH reactivity and that this effect is more pronounced at lower molar ratios of FA/HSA.
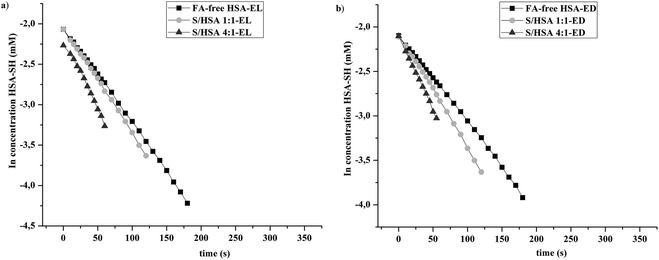 | ||
Fig. 3 Linear models of pseudo first order reaction kinetics of the thiol group of FA-free HSA, and S/HSA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4 1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with DTNB, obtained after binding of (a) enterolactone and (b) enterodiol. 1 with DTNB, obtained after binding of (a) enterolactone and (b) enterodiol. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and their complexes with enterolignans. Each experiment was done in triplicate
1), and their complexes with enterolignans. Each experiment was done in triplicate
| Complex | k′ × 10−3 s−1 | Increase (%) of HSA-SH reactivity after binding of | |
|---|---|---|---|
| Enterolignansa | S + enterolignansb | ||
a Compared to FA-free HSA, S/HSA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and S/HSA (4 1) and S/HSA (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), respectively.
b Compared to FA-free HSA. 1), respectively.
b Compared to FA-free HSA.
|
|||
| FA-free HSA | 8.9 ± 0.1 | ||
| FA-free HSA-EL | 11.8 ± 0.4 | 33.1 | |
| FA-free HSA-ED | 10.1 ± 0.1 | 13.6 | |
S/HSA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
11.6 ± 0.3 | ||
S/HSA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-EL 1)-EL |
13.0 ± 0.6 | 12.6 | 46.7 |
S/HSA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-ED 1)-ED |
12.8 ± 0.3 | 10.9 | 44.4 |
S/HSA (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
15.3 ± 0.1 | ||
S/HSA (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-EL 1)-EL |
17.0 ± 0.3 | 10.8 | 91.1 |
S/HSA (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-ED 1)-ED |
16.7 ± 0.9 | 9.1 | 88.1 |
In S/HSA complexes (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the reactivity of Cys34 was increased by 30.3% and 72.4% respectively, compared with FA-free HSA. Overall, it was evident that both enterolignan (EL and ED) and S interactions with HSA increased the reactivity of HSA-SH in all investigated HSA complexes (Table 1). Singular contributions of S and enterolignans (ED, EL) to the increase of HSA-SH reactivity were summed and compared with cumulative increases of HSA-SH reactivity (Fig. 4). As can be seen from Table 1, EL led to an increase of FA-free HSA-SH reactivity by 33.1%. If this value were summed with the 30.3% increase seen in HSA-SH reactivity contributed by S interactions with FA-free HSA, the total would be 63.4% (Fig. 4), but the obtained increase of HSA-SH reactivity in the S/HSA (1
1), the reactivity of Cys34 was increased by 30.3% and 72.4% respectively, compared with FA-free HSA. Overall, it was evident that both enterolignan (EL and ED) and S interactions with HSA increased the reactivity of HSA-SH in all investigated HSA complexes (Table 1). Singular contributions of S and enterolignans (ED, EL) to the increase of HSA-SH reactivity were summed and compared with cumulative increases of HSA-SH reactivity (Fig. 4). As can be seen from Table 1, EL led to an increase of FA-free HSA-SH reactivity by 33.1%. If this value were summed with the 30.3% increase seen in HSA-SH reactivity contributed by S interactions with FA-free HSA, the total would be 63.4% (Fig. 4), but the obtained increase of HSA-SH reactivity in the S/HSA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-EL complex was 46.7% (Table 1 and Fig. 4). Thus, it is evident that S modulated/reduced the effect of EL on the reactivity of the HSA-SH group and that this influence was, to a small extent, more pronounced at lower molar ratios of FA/HSA (decrease of 16.7% for the 1
1)-EL complex was 46.7% (Table 1 and Fig. 4). Thus, it is evident that S modulated/reduced the effect of EL on the reactivity of the HSA-SH group and that this influence was, to a small extent, more pronounced at lower molar ratios of FA/HSA (decrease of 16.7% for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in comparison to 14.4% for the 4
1 ratio in comparison to 14.4% for the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio). At the same time, the negative effect of S on the ED increase in HSA-SH reactivity was not found (Fig. 4). The finding that the reactivity of HSA-SH was increased in HSA-enterolignan complexes could be important for the possible modulation of HSA-SH reactivity by the dietary intake of lignan rich food. In some physiological conditions and diseases with elevated plasma levels of non-esterified FA, the effects of a dietary intake of lignan rich food could be modulated by FA.
1 ratio). At the same time, the negative effect of S on the ED increase in HSA-SH reactivity was not found (Fig. 4). The finding that the reactivity of HSA-SH was increased in HSA-enterolignan complexes could be important for the possible modulation of HSA-SH reactivity by the dietary intake of lignan rich food. In some physiological conditions and diseases with elevated plasma levels of non-esterified FA, the effects of a dietary intake of lignan rich food could be modulated by FA.
3.3. Effect on intrinsic fluorescence of enterolactone and enterodiol binding to HSA
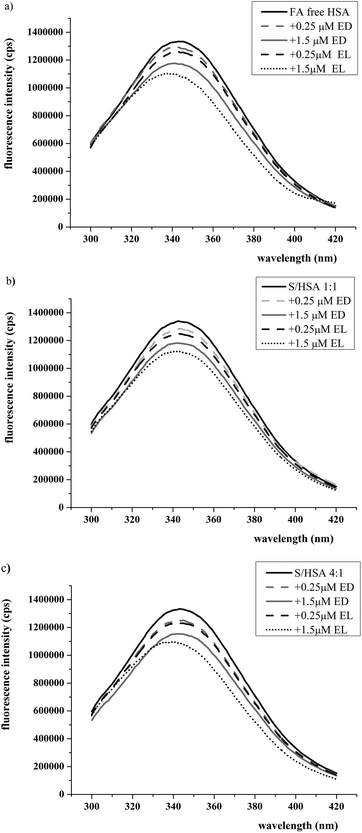
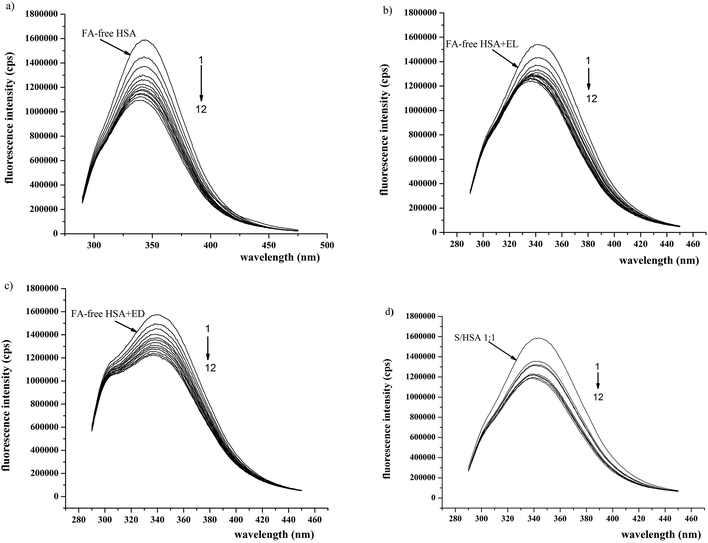
In order to better understand the influences of the enterolignans on HSA-SH reactivity, the interactions of EL and ED with HSA and FA/HSA were investigated using fluorescence spectroscopy. The excitation wavelength was 280 nm, at which both Trp214 and Tyr residues emit fluorescence. In spite of the presence of 18 Tyr residues in the HSA molecule,36 the HSA intrinsic fluorescence is dominated by the single Trp214 in subdomain IIA.34The binding of EL or ED to FA-free HSA and S/HSA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes resulted in the quenching of fluorescence intensity at λem (340 nm) compared to the FA-free HSA and S/HSA 1
1 complexes resulted in the quenching of fluorescence intensity at λem (340 nm) compared to the FA-free HSA and S/HSA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (results for enterolignan/HSA molar ratios of 0.5
4 (results for enterolignan/HSA molar ratios of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 are shown in Fig. 5). In the presence of EL and ED, the intrinsic fluorescence decreased in a concentration-dependent manner, suggesting that EL and ED interact with HSA. A blue shift in the emission maximum wavelength occurred between FA-free HSA-EL and S/HSA 4
1 are shown in Fig. 5). In the presence of EL and ED, the intrinsic fluorescence decreased in a concentration-dependent manner, suggesting that EL and ED interact with HSA. A blue shift in the emission maximum wavelength occurred between FA-free HSA-EL and S/HSA 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-EL, suggesting that the intrinsic fluorophore could be forced into a more hydrophobic protein environment37 in the presence of high concentrations of EL. The more pronounced HSA intrinsic fluorescence changes observed (i.e. conformational changes) induced by EL binding in comparison to ED binding are in accordance with their effects on HSA-SH reactivity.
1-EL, suggesting that the intrinsic fluorophore could be forced into a more hydrophobic protein environment37 in the presence of high concentrations of EL. The more pronounced HSA intrinsic fluorescence changes observed (i.e. conformational changes) induced by EL binding in comparison to ED binding are in accordance with their effects on HSA-SH reactivity.
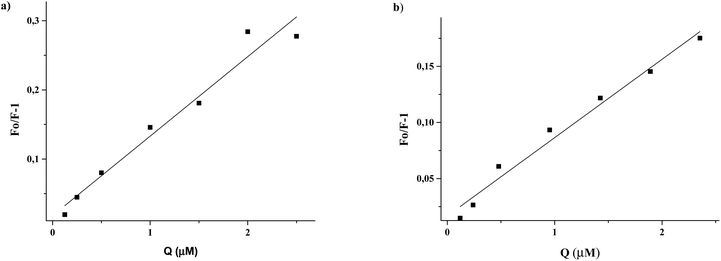
After correction of the fluorescence intensities of EL and ED at 340 nm for the inner-filter effect (Materials and methods, eqn (1)), the Stern–Volmer constants could be calculated (Materials and methods, eqn (2)) from the slope of the regression curves Fo/F−1versus [Q] (Fig. 6). The obtained Stern–Volmer plots were linear at all applied conditions, and the Stern–Volmer quenching constants (Ksv) are shown in Table 2.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with increasing concentrations of quencher (EL and ED) from 0.125 to 2.5 μM, at 37 °C
1 with increasing concentrations of quencher (EL and ED) from 0.125 to 2.5 μM, at 37 °C
| Complex | K sv × 104 (M−1) | r |
|---|---|---|
| Enterolactone | ||
| +FA-free HSA | 11.49 | 0.985 |
+S/HSA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9.55 | 0.989 |
+S/HSA 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5.87 | 0.979 |
| Enterodiol | ||
| +FA-free HSA | 6.98 | 0.989 |
+S/HSA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6.95 | 0.974 |
+S/HSA 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7.02 | 0.984 |
Given an average lifetime of a biomolecule of 10−8 s, the quenching rate constants (kq) were calculated. The obtained kq values (5.87–11.49 × 1012 M−1 s−1) are two orders of magnitude higher than the limiting diffusion rate constant of the biomolecule (∼1010 M−1 s−1),30 indicating a static type mechanism of fluorophore quenching.38
3.4. Binding parameters of HSA-enterolignan complexes
Polyphenols are a large and heterogeneous group of phytochemicals present in foods. Lignans are polyphenolic structured phytochemicals with antiestrogenic, antimitotic and anticancer effects.39 The transport of absorbed phytoestrogens, and in particular their binding to specific estrogen carrier proteins (steroid hormone binding protein (SBP)40 and α-fetoprotein (AFP)30) or to the nonspecific serum carrier protein, albumin, are important factors in determining pharmacodynamics, pharmacokinetics and their biological activities. The inhibitory effects of phytoestrogens on the binding of sex hormones to SBP and AFP30,40 and unsaturated FA to AFP have been reported.30 The dissociation constants obtained for EL-AFP and ED-AFP complexes were 1.7 × 10−5 and 2.2 × 10−5 M, respectively.29 The interactions between flavonoids, phenolic acids, anthocyanins, catechins, resveratol and albumins have been reported.18,41–43 In most studies, the interactions of polyphenols (but not of EL and ED) with HSA were studied using FA-free HSA.42–44The values obtained for the binding constants of EL and ED to HSA (Materials and methods, eqn (3)) were from 1.92 to 11.64 × 104 M−1 (Table 3), suggesting that the binding between enterolignans and HSA is moderate. These results are also in accordance with the finding that binding constants of polyphenols typically range from 104 to 106 M−1.44 Soybean isoflavone genistein is a naturally occurring estrogen-like molecule with a binding constant to HSA of 1.5 × 105 M−1.45 In addition, the obtained values show that EL and ED can be stored and transported by HSA as it is the most abundant protein in plasma with a concentration of about 0.6 mM versus the concentration of the specific estrogen carrier protein SBP which is in the nM range.46 However, EL and ED are mainly present as glucuronide and sulfate conjugates in body fluids.18 As the conjugation with glucuronate and sulfate enable an increase of the solubility and consequently the elimination of xenobiotics, we considered that EL and ED are in the most part bound to HSA in unconjugated form. Most studies to date have dealt with native polyphenols, but it has also been shown that the polyphenol conjugates (glucuronide and sulfate) circulate bound to albumin.47,48 For hydroxycinnamic acid conjugates, the affinities to albumin were found to be of the same order as those of aglycons.47 The changes in the binding activities of quercetin sulfate conjugates depended on the conjugation site.48 At the same time, a recent study demonstrated that β-glucuronidase from human neutrophils is able to deconjugate and thus activate glucuronide conjugates during inflammation (in vitro).49
| Complex | K a × 104 (M−1) 37 °C | n |
|---|---|---|
| Enterolactone | ||
| +FA-free HSA | 11.64 | 0.896 |
+S/HSA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4.35 | 0.679 |
+S/HSA 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.43 | 0.578 |
| Enterodiol | ||
| +FA-free HSA | 5.59 | 0.818 |
+S/HSA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.50 | 0.610 |
+S/HSA 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.92 | 0.507 |
The highest values of Ka were obtained for FA-free HSA-enterolignan complexes at 37 °C (EL: 11.64 × 104 M−1 and ED: 5.59 × 104 M−1) and the lowest for S/HSA 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-enterolignan complexes (EL: 2.43 × 104 M−1 and ED: 1.92 × 104 M−1). Thus, with an increased ratio of S/HSA, the binding affinity of HSA decreased from 2.7 to 4.8 times for EL and from 2.2 to 2.9 times for ED (Table 3). The number of binding sites also reduced, from 0.896 to 0.578 for EL and from 0.818 to 0.507 for ED.
1-enterolignan complexes (EL: 2.43 × 104 M−1 and ED: 1.92 × 104 M−1). Thus, with an increased ratio of S/HSA, the binding affinity of HSA decreased from 2.7 to 4.8 times for EL and from 2.2 to 2.9 times for ED (Table 3). The number of binding sites also reduced, from 0.896 to 0.578 for EL and from 0.818 to 0.507 for ED.
Simultaneous binding of various ligands to HSA can result in changes in their affinity. This can occur when conformational changes of the albumin appear or when ligands occupy the same binding site in serum albumin. Cooperative and competitive interactions between FA and different classes of ligands have been observed in numerous studies.50–52 There is little literature data for the influence of FA on polyphenols and phytoestrogens binding to HSA. Pantusa et al.53 found that resveratol binds to HSA and that its interaction is modulated by S. The binding constant for the resveratol-HSA interaction does not change with up to 3–4 molecules of S per HSA molecule, but it markedly decreases at S/HSA molar ratios of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 6
1 and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. From our results regarding the Ka values for HSA-enterolignan complexes, it is evident that S modulates the binding affinity of the HSA for ED and EL, decreasing the Ka value by approximately 2.5 times even at the S/HSA molar ratio of 1
1. From our results regarding the Ka values for HSA-enterolignan complexes, it is evident that S modulates the binding affinity of the HSA for ED and EL, decreasing the Ka value by approximately 2.5 times even at the S/HSA molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The increase of the S/HSA molar ratio to 4
1. The increase of the S/HSA molar ratio to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 3) leads to a further significant decrease of binding affinity of the HSA to the enterolignans, especially to EL. These findings are very important as 0.1–2 mole of FA is bound to HSA under physiological conditions, and the FA/HSA molar ratio can rise above 6
1 (Table 3) leads to a further significant decrease of binding affinity of the HSA to the enterolignans, especially to EL. These findings are very important as 0.1–2 mole of FA is bound to HSA under physiological conditions, and the FA/HSA molar ratio can rise above 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the peripheral vasculature during fasting or extreme exercise,54 or under pathological conditions such as diabetes, liver disease and cardiovascular disease.55
1 in the peripheral vasculature during fasting or extreme exercise,54 or under pathological conditions such as diabetes, liver disease and cardiovascular disease.55
Modulation of HSA binding affinity to ED and EL could be a consequence of conformational changes in the HSA molecules by S that influence the binding of ligands to HSA.50–52 On the other hand, the ED and EL binding affinities could decrease in the presence of S due to the overlap of two principal drug binding sites with FA binding sites: drug site I in subdomain IIA overlaps with FA site 7; and drug site II located in subdomain IIIA overlaps with FA sites 3 and 4.7
The values for the number of binding site for enterolignans on FA-free HSA are close to one (Table 3). When the S/HSA ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the decreases in these values were pronounced. Further decreases caused by a FA/HSA ratio of 4
1, the decreases in these values were pronounced. Further decreases caused by a FA/HSA ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 are much smaller. We found that EL and ED bind to the diazepam binding site (given below). As shown by Wong and Sellers,56 palmitic and oleic acid affect both the number of binding sites and the binding constants of diazepam to HSA. Thus, S could have a similar effect on the binding site number value of EL and ED.
1 are much smaller. We found that EL and ED bind to the diazepam binding site (given below). As shown by Wong and Sellers,56 palmitic and oleic acid affect both the number of binding sites and the binding constants of diazepam to HSA. Thus, S could have a similar effect on the binding site number value of EL and ED.
3.5. Site selective binding of enterolactone and enterodiol on HSA
Diazepam has been considered as a stereotypical ligand for site II.57 Therefore, information about binding of EL and ED to drug site II could be obtained by monitoring the fluorescence changes of FA-free HSA-EL and FA-free HSA-ED after titration with diazepam. As shown in Fig. 7, with the addition of diazepam, the fluorescence intensity decreased gradually and only FA-free HSA-EL had an obvious blue shift (λmax from 340 to 336 nm) (Fig. 7b) indicating that Trp could be placed in a more hydrophobic environment after the replacement of enterolactone with diazepam.To facilitate the comparison of the influence of S, EL and ED on the binding of diazepam to HSA, the diazepam Ka were calculated using eqn (3) (Table 4). The results suggested that the binding of S or EL to FA-free HSA led to a significant decrease in the Ka values for diazepam compared to the obtained Ka values for FA-free HSA. In comparison to EL and S, ED showed a smaller influence on the diazepam Ka value. These results suggest that the binding site of EL and ED may be located within site II of HSA. The high affinity binding site for FA is located in subdomain IIIA and FA can influence EL and ED binding as they occupy the same binding place on HSA.7
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 25 °C and pH 7.4
1 at 25 °C and pH 7.4
| Complex | K a × 104 M−1 |
|---|---|
| Diazepam+ | |
| FA-free HSA | 18.28 |
| FA-free HSA-EL | 2.68 |
| FA-free HSA-ED | 12.56 |
S/HSA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6.05 |
3.6. Correlation between binding constants and increases of Cys34 reactivity
The highest Ka was obtained for FA-free HSA-EL (Table 3), and this complex also led to the greatest increase of Cys34 reactivity (Table 1). A high correlation (r = 0.974) between Ka values and increases of HSA-SH reactivity was found (Fig. 8). This result indicates that conformational changes in HSA which are induced upon enterolignan binding are an important factor affecting the increase of Cys34 reactivity.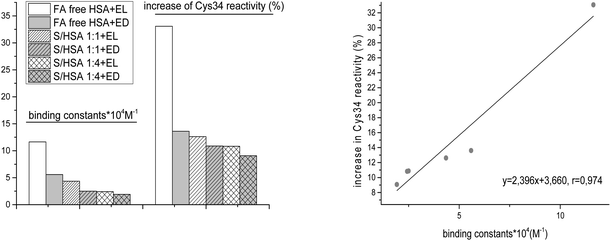 | ||
Fig. 8 Correlation between binding constants (Ka) and increases of HSA-SH reactivity (%) for FA-free HSA, S/HSA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4 1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and enterolignan (EL or ED) complexes. 1, and enterolignan (EL or ED) complexes. | ||
4. Conclusion
In conclusion, ED and EL increase HSA-SH reactivity, due to HSA conformational changes. They bind moderately to drug site II in subdomain IIIA, and Ka values are approximately two times higher for EL than for ED. A high correlation was found between enterolignan binding constants and changes in the HSA-SH reactivity. This finding could be important for the expression of the enterolignan antioxidant effect in vivo after an intake of lignan rich food. The binding of S to HSA modulates the effect of EL on Cys34 reactivity, as well as enterolignan binding to HSA even at the S/HSA molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Therefore, in some physiological conditions (intensive exercise, fasting) and some diseases which are accompanied with an increase of non-esterified FA in serum, enterolignan binding to HSA and its effect on HSA-SH reactivity (i.e. antioxidant activity) may be significantly modified.
1. Therefore, in some physiological conditions (intensive exercise, fasting) and some diseases which are accompanied with an increase of non-esterified FA in serum, enterolignan binding to HSA and its effect on HSA-SH reactivity (i.e. antioxidant activity) may be significantly modified.
Abbreviations
| DMSO | Dimethyl sulfoxide |
| DTNB | 5,5′-Dithiobis-(2-nitrobenzoic acid) |
| DTT | Dithiothreitol |
| ED | Enterodiol |
| EL | Enterolactone |
| FA | Fatty acid |
| FA-free HSA | Defatted HSA |
| FA-bound HSA | Complex of HSA with FA |
| HSA | Human serum albumin |
| HSA-EL and HSA-ED | HSA complexes with enterolactone and enterodiol, respectively |
| HSA-SH | The Cys34 free thiol group of HSA |
| PUFA | Polyunsaturated FA |
| S | Stearic acid |
| S/HSA | Complex of HSA with stearic acid |
Acknowledgements
The Ministry of Education, Science and Technological Development of Serbia supported this work with Grant No. 172049 and III 41030. The authors acknowledge the support of the FP7 RegPot project FCUB ERA GA No. 256716. The EC does not share responsibility for the content of the article.References
- U. Kragh-Hansen, Pharmacol. Rev., 1981, 33, 17 CAS.
- D. Carter and J. Ho, Adv. Protein. Chem., 1994, 45, 153 CrossRef CAS PubMed.
- S. Sugio, A. Kashima, S. Mochizuki, M. Noda and K. Kobayashi, Protein Eng., 1999, 12, 439 CrossRef CAS PubMed.
- F. Zsila, Z. Bikadi, D. Malik, P. Hari, I. Pechan, A. Berces and E. Hazai, Bioinformatics, 2011, 27, 1806 CrossRef CAS PubMed.
- A. Bhattycharya, T. Grune and S. Curry, J. Mol. Biol., 2000, 303, 721 CrossRef PubMed.
- X. Li and S. Wang, New J. Chem., 2015, 39, 389 Search PubMed.
- J. Simard, P. Zunszain, J. Hamilton and S. Curry, J. Mol. Biol., 2006, 361, 336 CrossRef CAS PubMed.
- Y. Xu, M. Zhang, T. Wu, S. Dai, J. Xu and Z. Zhou, Food Funct., 2016, 6, 296 Search PubMed.
- S. Hooshmand, A. Kumar, J. Zhang, S. Johnson, S. Chai and B. Armjmardi, Food Funct., 2015, 6, 1719 CAS.
- K. Setchell, N. Brown, L. Zimmer-Nechemias, B. Wolfe, P. Jha and J. Heubi, Food Funct., 2014, 5, 491 CAS.
- J. Peñalvo, B. Moreno-Franco, L. Ribas-Barba and L. Serra-Majem, Eur. J. Clin. Nutr., 2012, 66, 795 CrossRef PubMed.
- D. Ristic-Medic, G. Perunicic-Pekovic, Z. Rasic-Milutinovic, M. Takic, T. Popovic, A. Arsic and M. Glibetic, Sci. World J., 2014, 2014, 563576 Search PubMed.
- C. Xu, Q. Liu, Q. Zhang, Z. Y. Jiang and A. Gu, Br. J. Nutr., 2015, 114, 91 CrossRef CAS PubMed.
- P. Pietinen, K. Stumpf, S. Männistö, V. Kataja, M. Uusitupa and H. Adlercreutz H, Cancer Epidemiol. Biomarkers Prev., 2001, 10, 339 CAS.
- M. Hedelin, A. Klint, E. T. Chang, R. Bellocco, J. E. Johansson, S. O. Andersson, S. M. Heinonen, H. Adlercreutz and H. O. Adami, Cancer, Causes Control, Pap. Symp., 2006, 17, 169 CrossRef PubMed.
- M. Vanharanta, S. Voutilainen, T. H. Rissanen, H. Adlercreutz and J. T. Salonen, Arch. Intern. Med., 2003, 163, 1099 CrossRef CAS PubMed.
- A. Hoikkala, E. Schiavoni and K. Wahala, Br. J. Nutr., 2003, 89, S5 CrossRef CAS PubMed.
- J. Xiao and G. Kai, Crit. Rev. Food Sci. Nutr., 2012, 52, 85 CrossRef CAS PubMed.
- S. Carbllal, R. Radi, M. C. Kirk, S. Barnes, B. A. Freeman and B. Alvarez, Biochemistry, 2003, 42, 9906 CrossRef PubMed.
- M. Torres, L. Turell, H. Botti, L. Antmann, S. Carballal, G. Ferrer-Sueta, R. Radi and B. Alvarez, Arch. Biochem. Biophys., 2012, 521, 102 CrossRef CAS PubMed.
- L. Turell, R. Radi and B. Alvarez, Free Radicals Biol. Med., 2013, 65, 244 CrossRef CAS PubMed.
- J. Simard, P. Zunszain, C. Ha, J. Yang, N. Bhagavan, I. Petitpas, S. Curry and J. Hamilton, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 17958 CrossRef CAS PubMed.
- S. Curry, Adv. Mol. Cell Biol., 2003, 33, 29 Search PubMed.
- L. Turell, S. Carballal, H. Botti, R. Radi and B. Alvarez, J. Med. Biol. Res., 2009, 42, 305 CAS.
- I. Pavićević, V. Jovanović, M. Takić, A. Penezić, J. Aćimović and L. J. Mandić, Chem. – Biol. Interact., 2014, 224, 42 CrossRef PubMed.
- V. Jovanovic, I. Pavicevic, M. Takic, A. Penezic-Romanjuk, J. Acimovic and L. J. Mandic, Anal. Biochem., 2014, 448, 50 CrossRef CAS PubMed.
- R. Chen, J. Biol. Chem., 1967, 242, 173 CAS.
- A. Penezic, V. Jovanovic, I. Pavicevic, J. Acimovic and L. J. Mandić, Metallomics, 2015, 7, 1431 RSC.
- B. Garreau, H. Vallette, H. Adlercreutz, K. Wahala, T. Makela, C. Brnassayang and E. Nunez, Biochim. Biophys. Acta, 1991, 1094, 339 CrossRef CAS.
- H. Robinson and C. Hogden, J. Biol. Chem., 1940, 135, 707 CAS.
- J. Aćimović, A. Penezić, I. Pavićević, V. Jovanović and L. J. Mandić, Mol. BioSyst., 2014, 10, 2166 RSC.
- C. K. Reiner, G. Kada and H. J. Gruber, Anal. Bioanal. Chem., 2002, 373, 266 CrossRef PubMed.
- M. van de Weert and L. Stella, J. Mol. Struct., 2011, 998, 144 CrossRef CAS.
- J. R. Lakovicz, Principles of Fluorescence Spectroscopy, Springer, New York, 2006 Search PubMed.
- S. Bi, L. Ding, Y. Tian, D. Song, X. Zhou, X. Liu and H. Zhang, J. Mol. Struct., 2001, 703, 37 CrossRef.
- X. He and D. Carter, Nature, 1992, 358, 209 CrossRef CAS PubMed.
- X. Li and Z. Yang, Chem. – Biol. Interact., 2015, 232, 77 CrossRef CAS PubMed.
- Q. Li, W. Yang, L. Qu, H. Qi, Y. Huang and Z. Zhang, J. Spectroscopy., 2014, 2014, 834501 Search PubMed.
- H. Adlecreutz, Environ. Health Perspect., 1995, 103, 103 CrossRef.
- M. Martin, M. Haourigui, C. Pelissero, C. Benassayag and E. Nunez, Life Sci., 1996, 58, 429 CrossRef CAS PubMed.
- V. Sinisi, C. Fortzato, N. Cefarin, L. Navarini and F. Berti, Food Chem., 2015, 168, 332 CrossRef CAS PubMed.
- M. Kamran Khan, N. Rakotomanomana, C. Dufour and O. Dangles, Food Funct., 2011, 2, 617 Search PubMed.
- A. Pastukhov, L. Levchenko and A. Sadkov, J. Mol. Struct., 2007, 842, 60 CrossRef CAS.
- H. Mahesha, S. Singh, N. Srinivasan and R. Appu Rao, FEBS J., 2006, 273, 451 CrossRef CAS PubMed.
- C. Longcope, H. Feldman, J. McKinlay and A. Araujo, J. Clin. Endocrinol. Metab., 2000, 85, 293 CrossRef CAS PubMed.
- N. Latruffe, M. Menzel, D. Delmas, R. Buchet and A. Lançon, Molecules, 2014, 19, 17066 CrossRef PubMed.
- S. Galland, N. Rakotmanomana, C. Dufour, N. Mora and O. Dangles, Org. Biomol. Chem., 2008, 6, 4253 CAS.
- C. Dufour and O. Dangles, Biochim. Biophys. Acta, 2005, 1721, 164 CrossRef CAS PubMed.
- M. Untergehrer, B. Kraus, J. Heilmann and G. Jurgenliemk, Planta Med., 2013, 79, PJ48 Search PubMed.
- B. Bojko, A. Sulkowska, M. Maciazek, J. Rownicka, F. Njau and W. Sulkowski, Int. J. Biol. Macromol., 2008, 42, 314 CrossRef CAS PubMed.
- H. Vorum and B. Horne, J. Pharm. Pharmacol., 1996, 48, 870 CrossRef CAS PubMed.
- B. Bojko, A. Sulkowska, M. Maciazek-Jurczyk, J. Rownicka and W. Sulkowski, J. Mol. Struct., 2009, 924–926, 332 CrossRef CAS.
- M. Pantusa, L. Sportelli and R. Bartucci, Eur. Biophys. J., 2012, 41, 969 CrossRef CAS PubMed.
- R. Brodersen, S. Andersen, H. Vorum, S. Nielsen and A. Pedersen, Eur. J. Biochem., 1990, 189, 343 CrossRef CAS PubMed.
- D. Cistola and D. Small, J. Clin. Invest., 1991, 87, 1431 CrossRef CAS PubMed.
- G. Wong and E. Sellers, Biochem. Pharmacol., 1979, 28, 3265 CrossRef CAS PubMed.
- Y. Ni, R. Zhu and S. Kokot, Analyst, 2011, 136, 4794 RSC.
| This journal is © The Royal Society of Chemistry 2016 |