Open Access Article
Open Access ArticleHigh spatial resolution (1.1 μm and 20 nm) FTIR polarization contrast imaging reveals pre-rupture disorder in damaged tendon
Richard
Wiens
a,
Catherine R.
Findlay
 a,
Samuel G.
Baldwin
b,
Laurent
Kreplak
b,
J. Michael
Lee
cd,
Samuel P.
Veres
ce and
Kathleen M.
Gough
*a
a,
Samuel G.
Baldwin
b,
Laurent
Kreplak
b,
J. Michael
Lee
cd,
Samuel P.
Veres
ce and
Kathleen M.
Gough
*a
aDepartment of Chemistry, University of Manitoba, Winnipeg, MB R3T 2N2, Canada. E-mail: Kathleen.Gough@umanitoba.ca
bDepartment of Physics and Atmospheric Science, Dalhousie University, Halifax, NS B3H 4R2, Canada
cSchool of Biomedical Engineering, Dalhousie University, Halifax, NS B3H 3J5, Canada
dDepartment of Applied Oral Sciences, Dalhousie University, Halifax, NS B3H 4R2, Canada
eDivision of Engineering, Saint Mary's University, Halifax, NS B3H 3C3, Canada
First published on 11th January 2016
Abstract
Collagen is a major constituent in many life forms; in mammals, collagen appears as a component of skin, bone, tendon and cartilage, where it performs critical functions. Vibrational spectroscopy methods are excellent for studying the structure and function of collagen-containing tissues, as they provide molecular insight into composition and organization. The latter is particularly important for collagenous materials, given that a key feature is their hierarchical, oriented structure, organized from molecular to macroscopic length scales. Here, we present the first results of high-resolution FTIR polarization contrast imaging, at 1.1 μm and 20 nm scales, on control and mechanically damaged tendon. The spectroscopic data are supported with parallel SEM and correlated AFM imaging. Our goal is to explore the changes induced in tendon after the application of damaging mechanical stress, and the consequences for the healing processes. The results and possibilities for the application of these high-spatial-resolution FTIR techniques in spectral pathology, and eventually in clinical applications, are discussed.
1. Introduction
We are using FTIR polarization contrast imaging (PCI) to examine mechanically damaged mammalian tendon. Characteristic features in the polarized FTIR spectra of collagen were recognized early in the development of infrared biospectroscopy,1,2 even before the true triple helix molecular architecture had been discovered.3–5 The polarization dependence results from the highly ordered arrangement of the basic units (tropocollagen) and their alignment within the aggregated structures of the microfibrils, fibrils and fibres.6 The complex, hierarchical architecture of collagen facilitates a multitude of specialized, highly localized functions in tissues including tendon, skin, cartilage, ligament, scar tissue and bone. Under polarized infrared light, functional groups are vibrationally excited only when the alternating dipole change of the vibration is aligned with the polarization of the incident field. Vibrational spectroscopy has been an important technique for studying these tissues, providing molecular information on composition, orientation, post-translational modification and disease.7–11Collagen fibrils are the fundamental load-bearing constituent of mammalian tissues. They are approximately 50–500 nm in diameter but can be mm in length.12 Fibrils form as self-assembled linear aggregates of tropocollagen molecules (300 nm long × 1.5 nm in diameter); the latter consist of three helical chains wound together in a triple super-helix, stabilized by interchain hydrogen bonds.13 Our knowledge of the molecular changes occurring within overload-damaged fibrils remains sparse. Using very high magnification SEM (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×), Veres and Lee showed that both ruptured14 and cyclically overloaded15,16 bovine tendons contain collagen fibrils with “discrete plasticity”: a characteristic damage morphology where discrete strain-induced kinks appear serially along the length of the nanoscale fibrils. Enzymatic probes and differential scanning calorimetry showed that some collagen molecules had undergone denaturation, i.e. uncoiling of the triple helix within fibrils that experienced discrete plasticity. This work led to the hypothesis that a molecular-based toughening mechanism is implicit in the structure of the mammalian collagen fibril. Our current hypothesis is that under discrete plasticity a designated subset of molecules within fibrils denature on overload, absorbing mechanical strain energy at many discrete locations along each fibril's length. Discrete plasticity in tendons may represent a naturally evolved, built-in, toughening design that is ultimately protective against actual tendon rupture.
000×), Veres and Lee showed that both ruptured14 and cyclically overloaded15,16 bovine tendons contain collagen fibrils with “discrete plasticity”: a characteristic damage morphology where discrete strain-induced kinks appear serially along the length of the nanoscale fibrils. Enzymatic probes and differential scanning calorimetry showed that some collagen molecules had undergone denaturation, i.e. uncoiling of the triple helix within fibrils that experienced discrete plasticity. This work led to the hypothesis that a molecular-based toughening mechanism is implicit in the structure of the mammalian collagen fibril. Our current hypothesis is that under discrete plasticity a designated subset of molecules within fibrils denature on overload, absorbing mechanical strain energy at many discrete locations along each fibril's length. Discrete plasticity in tendons may represent a naturally evolved, built-in, toughening design that is ultimately protective against actual tendon rupture.
Our goal is to obtain a molecular spectroscopic basis for understanding changes to the molecular structure of collagen fibrils, under mechanical overload injury. To that end, using a bovine tail tendon model in which overload-induced discrete plasticity damage has been well characterized,14–16 we have studied matched-pair control and overloaded tendons with high-magnification FTIR PCI, confirming results with SEM and AFM. The highest spatial resolution far-field FTIR was attained at the IRENI beamline (Synchrotron Radiation Center, WI, now decommissioned) employing a Focal Plane Array (FPA) detector, to give 0.54 × 0.54 μm2 pixels.17 The Agilent high-magnification system used in the present work achieves the next best spatial resolution of 1.1 μm pixel dimension,18 considered to be the practical limit for mid-IR with FPA.19 This scale was sufficient to capture the orientation of bundled fibrils with fibres, including generalized sub-micron disorder, but could not provide sub-micron detail. To address the detection of finer details, single fibrils of the order of 300 nm in diameter were prepared from control tendon and examined with infrared near-field scattering scanning microscopy IR s-SNOM, at 20 nm spatial resolution. These experiments were performed with synchrotron source IR at the Advanced Light Source (ALS), Berkeley CA20 and with a broadband mid-infrared nano-FTIR spectroscopy laser at Neaspec, GmbH, DE.21–24 Additionally, near-field images were recorded at Neaspec using a mid-IR Quantum Cascade Laser (QCL). We show here that, regardless of source and system, PCI can be exploited in each case to reveal crucial aspects of organization in fibres and in fibrils. The potential for spectropathological and clinical applications will be discussed.
2. Methods
2.1 Tissue collection & mechanical damage procedure
Four tendons were dissected from the tails of two young adult steers killed for food at a local abattoir (Reid's Meats, Wolfville, NS, Canada). Once removed, tendons were immediately stored at 4 °C in phosphate-buffered saline (PBS) containing 1% antibiotic/antimycotic solution (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units penicillin, 10 mg streptomycin, and 25 μg amphotericin B per mL; product A5955, Sigma-Aldrich Canada). Prior to the mechanical damage procedure, a 15 mm-long sample was removed from each tendon to serve as an unloaded control. Using a servo-hydraulic materials testing system, the remainder of each tendon was subjected to 5 cycles of subrupture overload at a strain rate of 1% per s, as described previously.15 After the mechanical damage procedure, both the control and overloaded portions of each tendon were cut in half longitudinally yielding two sets of matched-pair samples, one set for SEM, and one set for FTIR. The tendon segments, approximately 10 mm long by 4 mm in diameter, were stored at room temperature for 24 hours in PBS containing 1% antibiotic/antimycotic solution and 1% protease inhibitor (2 mM 4-(2-aminoethyl)benzenesulfonyl fluoride, 14 μM E-64, 130 μM bestatin, 1 μM leupeptin, 0.3 μM aprotinin, and 1 mM EDTA; product S8820, Sigma-Aldrich Canada) prior to preparation for SEM and FTIR spectroscopy.
000 units penicillin, 10 mg streptomycin, and 25 μg amphotericin B per mL; product A5955, Sigma-Aldrich Canada). Prior to the mechanical damage procedure, a 15 mm-long sample was removed from each tendon to serve as an unloaded control. Using a servo-hydraulic materials testing system, the remainder of each tendon was subjected to 5 cycles of subrupture overload at a strain rate of 1% per s, as described previously.15 After the mechanical damage procedure, both the control and overloaded portions of each tendon were cut in half longitudinally yielding two sets of matched-pair samples, one set for SEM, and one set for FTIR. The tendon segments, approximately 10 mm long by 4 mm in diameter, were stored at room temperature for 24 hours in PBS containing 1% antibiotic/antimycotic solution and 1% protease inhibitor (2 mM 4-(2-aminoethyl)benzenesulfonyl fluoride, 14 μM E-64, 130 μM bestatin, 1 μM leupeptin, 0.3 μM aprotinin, and 1 mM EDTA; product S8820, Sigma-Aldrich Canada) prior to preparation for SEM and FTIR spectroscopy.
2.2 Scanning electron microscopy
In preparation for SEM, matched-pair control and overloaded tendon samples were fixed for one hour in 2.5% SEM-grade glutaraldehyde, rinsed in deionized water, dehydrated in graded ethanol, and critical point dried. The tendon samples were mounted on stubs using carbon tape with their exposed internal surfaces facing upward, and then sputter-coated with gold–palladium. Imaging was conducted with a Hitachi S-4700 scanning electron microscope operating at 3 kV, 15 μA.2.3 FTIR sample preparation and imaging at normal (5.5 μm) and high (1.1 μm) magnification pixel size
The other matched-pair set, comprising 4 matched pairs of control and mechanically overloaded tendon, that is two pairs from each animal, were shipped overnight to the University of Manitoba, immediately removed from PBS medium, covered with OCT [Sakura Finetek Inc. USA], frozen in isopentane cooled in liquid N2, and stored at −80 °C. Cryosections were cut to 5 or 8 μm thickness at −21 °C, mounted on BaF2 windows and refrigerated overnight at 4 °C.25Nine sections from the 4 control tendon samples and ten sections from the matching 4 overloaded tendon samples were imaged with an Agilent Cary 670 interferometer and 620 infrared microscope, equipped with a 64 × 64 FPA. Normal and high magnification optics (5.5 × 5.5 μm2 and 1.1 × 1.1 μm2 pixels, respectively)18 were used to explore fibril organization. Sections were typically aligned on the stage such that the fibril direction was horizontal in the field of view. Spectral acquisition and data processing were accomplished with the ResolutionsPro FTIR spectroscopy software (Agilent Inc. version 5.2.0). All spectra were acquired as sums of 128 scans ratioed against a background of 512 scans. Initial survey images with normal magnification were conducted on one matched pair, with no, 0° or 90° polarization, where 90° corresponds to the horizontal direction of the overall fiber orientation. The majority of the study was conducted with high magnification optics. Of these, 46, 40 and 124 individual FPA tiles were acquired from control tendon sections with no, 0° and 90° polarization, respectively; similarly, 36, 64 and 212 tiles were acquired from overload tendon sections. Some sections on BaF2 windows were stored in a sealed container at 4 °C pending re-imaging with FTIR PCI or other analyses.
The amide I and II bands differ in intensity, as they arise from different vibrational modes that produce different intrinsic changes in molecular dipole. Moreover, they change with orientation relative to the polarization of the incident IR light. The FTIR PCI false colour images were created from the ratio of amide II to amide I integrated band areas, to compensate for differences in tissue thickness across a section. Integrated band areas for amide I were taken between 1675 and 1645 cm−1, and for amide II between 1567 and 1537 cm−1. The baseline was established from the average in the non-absorbing region between 1790 and 1760 cm−1 for both. Instrumental illumination differs under the different polarizations and a new background had to be recorded in each case, necessitating slightly different colour scales. Display limits were chosen to consistently illustrate variation in orientation: 0.1 to 1.3 for all 0° images; 0.6 to 2.0 for all 90° images, 0.6 to 1.2 for images acquired without the polarizer accessory. These scales were chosen such that a ratio of 1.0 represented spectra wherein the amide I and II bands also had approximately equal height, while the range of the false colours was primarily aqua-blue to yellow-green for normal tissue. Extremes are reached only when fibers within the field of view are strongly aligned with or against the polarization.
2.4 AFM imaging
Following the FTIR imaging experiments, two of the overload tendon sections were returned for AFM surface analysis at the Structural Nanomechanics Lab (Dalhousie University). Each section was imaged in air with a Bioscope Catalyst AFM (Bruker, USA) mounted on an Olympus IX71 inverted microscope (Olympus, USA). ScanAsyst Fluid probes (Bruker, USA) with a nominal cantilever spring constant of 0.7 N m−1 were used. The AFM was operated in peak force quantitative nanomechanical mapping mode at a scan rate of 0.5 to 1 Hz. The images were processed in Nanoscope Analysis (Bruker, USA) or SPIP (Image Metrology, Denmark). To ensure that areas being measured corresponded to those imaged with FTIR PCI, a stack of survey images were taken at 25 nm spatial resolution over 40 or 50 μm regions, and the morphology was matched to the IR and visible images. Higher resolution images (10 μm region, 10 nm spatial resolution) were obtained on selected regions.2.5 Fibril preparation for AFM and nanoscale near-field IR
In order to conduct preliminary nanoscale near-field IR analysis of collagen fibrils, an additional tail tendon was acquired from a different young adult steer. The tendon was laid flat between sheets of gauze moistened with PBS, double bagged, and stored at −86 °C. After thawing at room temperature in its sealed bag, the tendon was rinsed in deionized water and dissected further using tweezers and a fine-tipped glass rod in 1 mL of deionized water until the mixture appeared cloudy. A 50 μL drop of the suspension was deposited on a 1 cm2 gold-coated silicon wafer, allowed to settle for 10 min, rinsed with deionized water and dried with nitrogen. Visible images at 100× and 50× were used to identify single collagen fibrils prior to the first nano-FTIR experiments at the ALS, Berkeley CA.| σ2(ω) = s2(ω)ei[φ(ω)] | (1) |
The near-field nano-FTIR absorbance spectrum is obtained by normalizing the signal on a fibril against a signal from on a flat, non-absorbing surface, here, a nearby point on the gold-coated wafer:
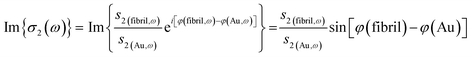 | (2) |
Single wavelength images recorded with the QCL source yielded amplitude and phase results that could be displayed in several formats: individual, normalized, or as the ratio of the near-field absorbance signals at 1655 and 1850 cm−1.
3. Results
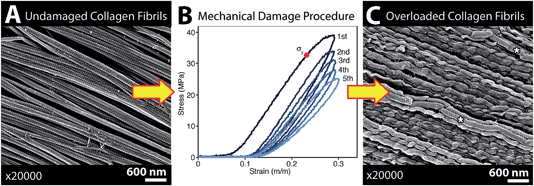
The infrared spectrum of normal, undamaged tendon (Fig. 1A) displays the numerous well-known spectral features that are unique to collagen assemblies,26–28 and derive from the unusual molecular composition and superhelical structure. In each of the three α-chains that form a tropocollagen molecule, the repeat amino acid sequence is Gly–X–Y, where X and Y are often proline and hydroxyproline, respectively.29 The alignment is such that the carbonyls are nearly perpendicular to the direction of the backbone; this orientation is retained to a significant extent in the assemblies. The intensity of the amide I band at 1660 cm−1 is enhanced when light is polarized parallel to carbonyl (0°); all other bands, particularly the amide II at 1545 cm−1, are enhanced under light polarized parallel to the fibril direction (90°), Fig. 1B. These spectral features can be easily exploited to reveal fibril orientation under polarized infrared light, provided that the internal organization has been retained at a molecular level. The contrast would be reduced by fibril disorganization and/or molecular denaturation.The SEM images of collagen fibrils before and after tendon overload, and a typical series of stress–strain curves for tendon during sub-rupture overload, are shown in Fig. 2. Fibrils from control tendon exhibit the expected D-banding5,14,30,31 that arises from the regular staggered arrangement of tropocollagen molecules within the fibrils, Fig. 2A. The interpretation of such stress–strain curves (Fig. 2B) is provided in detail elsewhere.6,15,32 Briefly, under the application of low force to the tendon sample, out to ∼0.1 strain in Fig. 2B, the 100 μm-scale collagen fibre crimp in the tissue straightens (see Fig. 3–5). If the extending force were removed, this crimp would reform as the tissue retracted. Beyond 0.1 strain, the collagen fibres are in direct tension, loading more or less elastically, and the stress–strain curve is linear. Sub-rupture overload is achieved by application of sufficient force to extend the tendon into the final non-linear region, beyond the yield stress point, (σy, Fig. 2B). In our sub-rupture experiments, the tendon sample was extended until the slope of the stress/strain curve became zero (∼0.3 strain in the first overload cycle), and the extension was reversed to zero. This process was performed 5 times. Under cyclic overload, plastic damage accumulates in the tendon and greater final strain is achieved—with a smaller maximum force—in the four subsequent overload cycles.
SEM imaging of matched-pair control and overload samples confirmed that discrete plasticity damage was produced by repeated stretching beyond the tendon's yield point. As previously observed,14,15 fibrils with discrete plasticity were characterized by the presence of kinks that repeated along the length of fibrils with nanoscale frequency, while some kinked fibrils also had a surface layer of denatured collagen, masking the fibrils' native D-banding (Fig. 2C).
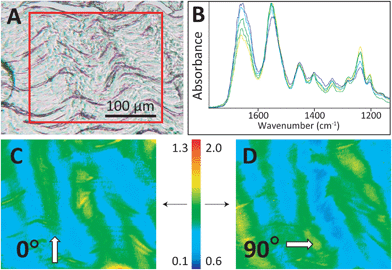
The four control/overload sample pairs matching the set imaged with SEM were cryosectioned and imaged in FTIR transmission mode with normal and high magnification optics. The fibres of all control tendons exhibited the expected microscale crimps under white light (Fig. 3A), with an undulation repeat of about 100–150 μm. Note that this naturally occurring crimp pattern is 1000-fold greater than the nanokinks that are produced by mechanical damage within the individual fibrils that comprise the microscopic fibre bundles. Spectra were extracted from mosaic images, acquired as FTIR PCI with high magnification optics, and displayed at full scale to clarify the variation in the ratio of the integrated band areas of amide I and II with fibril orientation (Fig. 3B). The false colour scales in the processed images were adjusted separately for parallel and perpendicular polarizations to yield complementary images for perpendicular (0°) and parallel (90°) polarizations (Fig. 3C and D), as described in the Methods section. The high magnification optics images have sufficient spatial resolution to show that control tendon exhibits the expected polarization dependence, wherein the visible crimped appearance of the well-organized fibres is reflected in the FTIR PCI images. The colours in Fig. 3B match the colours in the processed images. For the example shown, the crimps only become parallel to the 90° light at the top of the wave, yellow spectrum and yellow regions in (Fig. 3B and C).
All tendons subjected to sub-rupture overload stress lost the characteristic undulating crimps, but not uniformly. In some regions, the fibres were visibly straightened under white light (Fig. 4A), but other sections appeared to have retained crimped regions (Fig. 4B). FTIR PCI at 90° mirrored the organization in the visible images in the first case (Fig. 4C and D). Spectra uniformly showed significantly enhanced amide II C–N stretch backbone modes across the section; the amide II to amide I ratio reached the high end of the false colour scale, yellow-red, as illustrated in the images (Fig. 4A, C and E). In the second example, where some crimp structure was retained, the ratios of the band areas were within normal bounds, translating as blue-green-yellow (Fig. 4B, D and F), implying that the tendon had undergone less, albeit uneven, stress during overload. However, closer analysis of the spectra from this FTIR PCI revealed differences strongly suggestive of disorder at a molecular scale: the enhancement of the amide II was much less than expected in regions that visibly appeared to be straight and uncrimped, along the deeper blue horizontal swaths (vide infra).
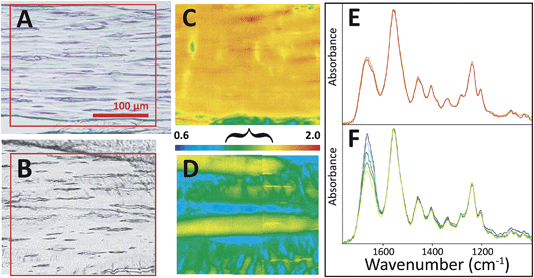 | ||
| Fig. 4 FTIR PCI revealed sub-micron disorder in some stressed tendons. Visible images of tendon sections on BaF2 windows show (A) loss of crimp structure on some sections and (B) residual crimp structure in other sections. FTIR PCI at 90° showed (C) a high degree of molecular alignment with fibrils in the case of uniform stretch and (D) alternating regions of alignment and crimp in the case of non-uniform stretch. Spectra extracted from FTIR images corresponding to the range of colours in FTIR PCI are shown in (E) and (F), normalized to amide II band. False colour scale bar as in Fig. 3 for 90°; the bracket indicates a range typical of normal crimped tendon. Spectra shown represent the full range of relative intensities observed. | ||
To confirm this interpretation, the two overload samples shown in Fig. 4 were returned for AFM imaging of the regions of interest in the tissue. Results of this correlated imaging are shown in Fig. 5 and 6. Part of the FTIR PCI false colour image has been rendered semi-transparent to allow visual inspection of the macroscopic axial alignment. All the AFM pictures are peak-force error channels, chosen to emphasize the finest details in the topography as well as the edges of individual fibrils. As seen in Fig. 5A, the orientation of the AFM images of the first sample matched that of the FTIR PCI. AFM images acquired at 25 nm (data not shown) and 10 nm resolution allowed detection of discrete plasticity, with the kinks along individual fibrils, spaced at ∼800 nm intervals.
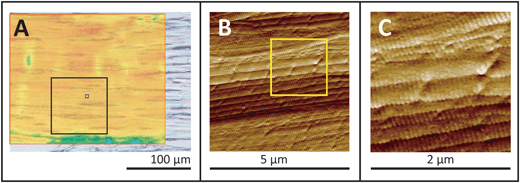 | ||
| Fig. 5 Uniformly stressed tendon is lightly kinked. (A) The semi-transparent FTIR PCI false colour image from Fig. 4C is superimposed on a visible image. Black boxes outline the locations of 100 × 100 μm2 (results not shown) and 5 × 5 μm2 AFM images; (B) AFM 5 × 5 μm2 image recorded at 10 nm resolution. (C) 2 × 2 μm2 region as outlined by yellow box (B), enlarged to facilitate identification of low kink density in the otherwise normal, intact D-banded fibrils. | ||
The second sample was not precisely aligned to match the FTIR PCI and AFM images, Fig. 6A, but this does not impact the analysis. A series of 40 μm survey AFM images were acquired at 25 nm spatial resolution inside the yellow rectangle (data not shown). Three of six higher-magnification AFM images are shown at two enlargement scales (Fig. 6B and C), showing aligned, moderately kinked fibrils in the top image and a progressively more disordered arrangement of kinked fibrils in the middle and lower images. The uppermost AFM image in Fig. 6C is similar to those in Fig. 5. The progressively disordered regions correspond to the regions revealed by FTIR PCI to contain submicron disorder, that is, a loss of axial alignment at the fibril or molecular scale (blue-green regions in false colour image, Fig. 6A).
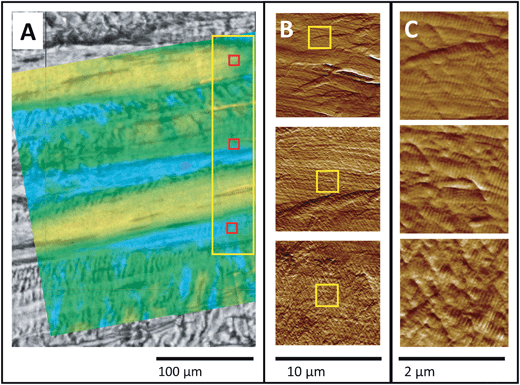 | ||
| Fig. 6 Non-uniformly stressed tendon. (A) Visible image of tendon with semi-transparent FTIR PCI false colour image from Fig. 4C superimposed. The large yellow rectangle outlines the location of lower-resolution AFM images, taken as a series of 40 × 40 μm2 areas (not shown). (B) Three 10 × 10 μm2 AFM images, recorded at 10 nm spatial resolution at red squares in (A). (C) Excerpts from (B), outlined by yellow squares, enlarged to facilitate identification of kinking in the otherwise normal, intact D-banded fibrils. | ||
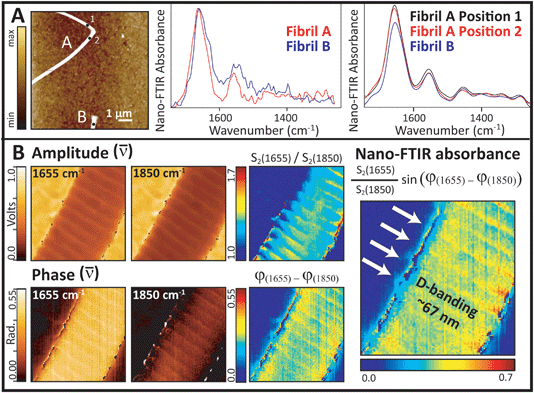
The first Synchrotron Infrared NanoSpectroscopy (SINS) spectra from a control collagen fibril, recorded at ALS, are presented in Fig. 7A. The AFM image on the left was acquired rapidly in order to locate a useful target, and shows parts of two normal fibrils. Following Fourier transform of the second order scattered near-field light,20 the nano-FTIR absorption spectra, Im σ2(ω) are obtained. These match well with the far-field IR spectrum of collagen shown in Fig. 1. The proline ring mode (∼1465 cm−1) is just barely visible. Similar results were obtained on the same fibrils and locations from second order backscattering using the broadband nano-FTIR illumination source from Neaspec (Neaspec, GmbH, DE), shown beside the synchrotron spectra. The nano-FTIR absorption spectra are very similar to the results from ALS; the proline ring mode is clearly identifiable. The ALS spectra are noisier but should not be compared directly, since the Neaspec data were zero-filled.
Single wavelength images were acquired with the Neaspec QCL at the amide I maximum (1655 cm−1), and at a non-absorbing wavelength (1850 cm−1), Fig. 7B. The area was scanned in 5 nm steps, where the expected spatial resolution is 20 nm. All amplitude and phase images show the fibril features. The on- and off-resonance contrast in amplitude is much less than the contrast in phase, but both parts are required to construct the relative near-field FTIR absorbance image. The highest contrast seen at the edges of the fibril probably arises from artefacts in the edge scatter. The D-banding at ∼67 nm spacing is well-resolved in all images, indicating a strong difference in optical response. The alteration in intensity arises from the gap-overlap regions caused by molecular stagger in the fibrils, just as would be seen in an SEM image.
4. Discussion
A little over a decade ago, a Faraday Discussion meeting was held to consider the state-of-the-art applications of spectroscopy in biomedicine. At that time, the state-of-the-art for FTIR spectroscopic imaging required a synchrotron source, and the highest spatial resolution possible was defined by the wavelength-dependent diffraction limit, from 10 to 2.5 μm.33 Recent advances have made dramatic improvements on those limitations: benchtop FTIR instruments that can be used for spectropathology, with the necessary speed, sensitivity and high spatial resolution, are now commercially available. The diffraction limit to spatial resolution has been broken with near-field IR, allowing us to probe areas of the order of a few nanometers, in several different instrumental designs.20,22 Here, we present results that employ all of these advances: (1) far-field diffraction-limited FTIR microscopy with a thermal bench source and improved optics that yield 1.1 μm pixels,18 further enhanced with polarization contrast and (2) near-field FTIR with 20 nm resolution, with a synchrotron source FTIR20 and with broadband nano-FTIR illumination source.21–24 Our purpose is to illustrate the advantages these technological advances can bring to spectropathology, and eventually to clinical pathology in the context of our on-going study of mechanical damage and healing in tendons. We will first summarize what we have learned from high-magnification transmission FTIR PCI and from near-field IR imaging in the present study, and then consider larger implications for spectropathological and clinical applications.4.1 What does high-magnification far-field infrared imaging tell us?
FTIR imaging analysis of control and mechanically overloaded tendon section, with polarization contrast, allows us to identify regions where axial alignment of the fibrils has been lost. We interpret this as a spectroscopic proxy for discrete plasticity kinking. These results demonstrate that we can now evaluate mechanically-induced damage in tendon sections, at an unprecedented, physically relevant length scale. We can thus study the changes in terms of molecular structure, in parallel with physico-structural (SEM, TEM, AFM) and biological methods. In the longer view, with the development of new instrumentation, rapid spectropathological screening and classification of tendon damage in a clinical setting can be envisioned.Veres and Lee first demonstrated the existence of nanoscale serial kinking in collagen fibrils within ruptured and cyclically overloaded tendons,14 co-articulating what we now term the discrete plasticity mechanism as a protective mechanism in tendon biomechanics. Recently, the overload-induced collagen kink morphology was shown to be a possible cue to cellular recognition of injury.34 FTIR PCI is eminently suitable to monitor fibril organization, particularly with diffraction-limit optics that enable imaging at 1.1 μm pixel dimension, since this is a good match with natural fibril width. FTIR spectra provide molecular spectroscopic evidence of fibril orientation in control tendon, Fig. 1. Contrast is easily displayed, given the enormous difference in relative intensities of the amide I and II bands when the polarized infrared light is aligned either with the amide carbonyls (0°) or with the backbone (90°), as well as large differences in many of the other backbone modes.
In control tendon, fibrils are well organized; D-bands were clearly evident in SEM images (Fig. 2A), whereas mechanical overload introduced the expected serial nanoscale kinks characteristic of discrete plasticity (Fig. 2B and C). Some undulating crimps were easily seen in the visible images (Fig. 3A) and also in lower magnification SEM (not shown). Spectra extracted from a mosaic represent the typical variation that accompanies the undulating orientation of crimps in normal fibrils (Fig. 3B). Relative intensities are not directly comparable under different polarizations, but the FTIR PCI false colour scale in the processed images was adjusted to achieve the same, relatively narrow range of colour contrast, from yellow to blue (Fig. 3C and D) in control images. These ranges were optimized to allow for the full range observed in all tissues, including those that had been mechanically damaged.
In the mechanically damaged tendons, FTIR PCI revealed extreme alignment in uncrimped tendon fibrils in some cases, and an uneven loss of crimp, with concomitant molecular level disorder in others. The amide II band dominates in all spectra throughout the uncrimped region imaged in Fig. 4A, C and E. The calculated ratio for our chosen imaging parameters is consistently of the order of 1.7 to 2.0. Fibrils are visibly aligned; false colour FTIR images capture this alignment. The amide I band profile is well-known to be a good indicator of protein conformation. In normal self-assembled collagen, the typically observed profile is an asymmetric amide I band, with a maximum at around 1660 cm−1 and a lower energy shoulder at around 1635 cm−1.7–10 We note that spectral band shapes were unaltered from the norm and only the relative intensities had changed, indicating a high degree of physical order, but no evidence of denaturation.
Some of the spectral variations that were observed (Fig. 4B, D and F) suggested that despite their physical alignment, the fibrils were internally disorganized, i.e., that nanoscale kinking had occurred. Undulations matching normal crimped tissue were evident in the lower part of these images. As before, the FTIR PCI results indicated fibre alignment without much nanoscale disorder in some regions where crimps were lost, seen here as yellow stripes in the false colour image Fig. 4D and yellow spectra in Fig. 4F. Interestingly, blue-green swaths were found between the yellow bands in Fig. 4D. In these regions, while the visible tissue image suggested that the crimps were lost and fibres were axially straightened, the amide II to amide I band area ratio was much lower than that expected for intact but well-ordered, aligned fibrils (Fig. 4F, blue and green spectra). In fact, the amide II band was frequently less intense than the amide I; the range of values for the amide II to amide I ratio was 0.9 to 1.2. Given the physical alignment of the fibre bundles with the polarization of the IR light, the most plausible explanation is that these regions underwent much greater, localized, tension, leading to significant kinking of fibrils within the fibre bundle.
Subsequent AFM surface imaging of these regions confirmed the existence of nanoscale kinking. AFM surface images of the well-aligned fibres in the visible image and FTIR PCI (Fig. 5A) show well-ordered fibrils with clear D-banding at the sub-micron scale, Fig. 5B and C. Light kinking is observed, corresponding to only a few kinks per 1.1 × 1.1 μm2 pixel area sampled by a high magnification FTIR field of view. As noted, the spectra in the FTIR PCI are nearly uniform throughout (Fig. 4E). From the AFM results, we may conclude that the massive amide II enhancement in axially aligned tendon indicates nearly linear orientation of well-organized fibrils. FTIR PCI gives molecular evidence that the stress was sufficient to eliminate the microscale waveform crimp that fibrils normally follow, but, with only a few kinks per pixel, did not produce sufficient molecular disorder to cause a change in the PCI spectra.
In stark contrast, the AFM surface images in Fig. 6 exhibit alternating regions of uncrimped fibrils (yellow strips in FTIR image Fig. 6A; upper AFM images in Fig. 6B and C) and regions of disordered fibrils and with nanokinks in regions with low polarization contrast (blue-green strips in FTIR image Fig. 6A; middle and lower AFM images in Fig. 6B and C). Together, the results in Fig. 5 and 6 show that in some cases, the mechanical damage was heterogeneous, producing higher stress leading to regions of disorder immediately adjacent to regions that experienced relatively lower stress, such that the crimp structure was regained upon release of the mechanical force. This might be an artefact arising from an uneven grip in the experimental mechanical damage procedure. However, it has recently been shown that interfibrillar shear stresses occur under notch tension testing of rat tail tendon.35 Both uneven tension and natural variations in the tendon architecture may contribute to this result, though the degree to which each is involved remains a question yet to be resolved.
4.2 What does nanoscale near-field infrared imaging give us?
With the FTIR imaging at the 1.1 micron scale, fibril damage at the nanometer scale may be suspected but cannot be proved. The SINS experiment at BL 5.4.1 is the first synchrotron site in the world to combine the high chemical specificity of FTIR with the nanoscale spatial resolution of AFM.20 This facility was designed on the same basic principles as the broad band DFG laser s-SNOM, in that both employ an asymmetric Michelson interferometer with the sample located under the AFM tip in the extended fixed-mirror arm of the interferometer.21–24 The brilliant broad band source has greater source intensity, but must be tuned through three spectral ranges to get the entire mid-IR spectrum. With the QCL, a fibril may be scanned at a single wavelength; the relative absorbance at different wavelengths can be used to create spectral contrast images.The results shown in Fig. 7 demonstrate that near-field IR gives the required nanoscale resolution to image fibrils and even to identify differences in optical response across the D-banding structure. Several important features can now be exploited. First, comparison of the absorbance spectra with those in Fig. 1 illustrates the importance of polarization: spectra have an enhanced amide I band, relative to the amide II and the backbone modes. Here, however, it is the orientation of the electric field, which is polarized parallel to the tip and to some of the amide carbonyl groups, which are perpendicular to the fibril lying on the slide, that causes the spectra to be the same as that in the far-field 0° polarization spectrum.23 While the far-field FTIR PCI experiments allowed us to evaluate polarization in the (x, y) plane of the tendon section, the near-field IR experiments allow us to evaluate polarization in the z-direction. The phenomenon is observed in both the synchrotron source and broad band source spectra, Fig. 7A. The absence of characteristic bands in the amide III region, especially those at 1337, 1284, 1240 and 1204 cm−1, is understandable given that they are enhanced only for light polarized parallel to the fibril. From this, we can deduce that these other modes would become active if disorder caused the molecular backbone of fibrils to be aligned parallel to the tip.
The single wavelength images (Fig. 7B) present a dramatic example of the power of this technique for the study of oriented materials. The distinct D-band pattern is apparent in all of the images. The D-band defines a region that includes a gap between staggered tropocollagen molecules and the telopeptide sequences at the ends of the three individual chains in each triple helix.15,32 Since the spectral contrast is based on the intensity of the carbonyl band, we can conclude that the amide group orientation is changing with local changes in molecular orientation down the length of the fibril. The D-band gap has been found to increase slightly under tension.36 Although the contrast in the amplitude response is much lower than that of the phase response, the amplitude image is somewhat smoother. Since this is the first demonstration of a near-field nano-FTIR image of a single collagen fibril, and is taken only at two wavelengths, it is premature to speculate on the reasons for this difference in signal quality. Further work is planned to address these questions and to explore semi-quantitative analysis of the full spectral differences along the length of normal and damaged fibrils.
Band position is of interest, since different conformations might be anticipated in normal and in damaged tendon. Amide I and II band shapes and positions are sensitive to molecular conformation, thus, if disorder is at a molecular scale, i.e., molecular denaturation, then we should also be able to see this in nearfield spectra. Subtle changes in molecular dynamics were detected with AFM-based nanomechanical mapping of single hydrated collagen fibrils at indentation speeds around 1 mm s−1, at temperatures above 50 °C.37 Using the same technique with overloaded fibrils, they have shown the presence of a poorly organized surface shell,38 as seen in the many fibrils lacking D-banding in the SEM image presented here (Fig. 2C), and previously.15 In this context, it will be critical to compare near-field IR results with the first surface and tip-enhanced Raman (SERS and TERS) analysis of the surface of collagen fibrils,39 which showed the presence of different protein conformations at the surface of the fibrils. By permitting examination of polarization (orientation) dependence and band positions, near-field nano-FTIR thus opens the door into the spectroscopic evaluation of fibril structure at a molecular scale. This knowledge will be a unique window into what it means for soft tissues to be damaged in terms of structural change at the most fundamental level.
4.3 Potential and implications
With regard to the problem investigated here, the discrete plasticity response may be a fundamental feature of collagen's evolutionary design that helps to determine the remarkable toughness of connective tissues and the inflammatory and healing responses to injury. Spectropathological understanding of this key structural feature of soft tissue injuries can serve the development of rational strategies for modulation of injury recognition and optimized healing. This particular health issue is one example of damage occurring in an ordered system, damage that is amenable to analysis with FTIR PCI at the micron scale. The hierarchical, oriented structure is typical of many collagen-based materials, as is the fact that disease may often be equated with disorder. In the case of tendon, a spatial resolution of 1 μm is fortuitously a good match for fibre dimension. The polarization-dependent nature of the IR spectra of collagen fibres was recognized at the dawn of FTIR microscopy.1 With the spatial resolution capability of current commercial FTIR microscopes, this approach can be applied to the study of disease-induced disorder in a broad range of tissues from extracellular matrix to bone, to monitor disease state and healing, and to evaluate synthetic collagen matrices and scaffolds for bioengineered tissue replacements.FTIR PCI spectropathology as presented here has been performed in transmission, on tendon sections mounted on relatively expensive salt windows. This is perceived as a necessity, versus the much less expensive transflectance substrates, notably the low-emissivity Keveley MirrIR substrates, which cost about 1% that of a BaF2 window, because of the standing wave artefacts associated with transflectance spectra of thin sections.40,41 Despite these artefacts, valid biomarkers for transflectance FTIR PCI have been identified in cartilage sections, though mineralized tissues were more problematic.42 Provided that reference spectra are understood and samples are prepared consistently, the less expensive transflectance substrates could be a cost-effective option for tissue studies.
Infrared fibre optic probes have been used in the clinical evaluation of connective tissue,43,44 with spectral results that show evidence of cartilage degeneration in surgically excised human tissue. The probe contact surface was a ZnS ATR crystal, 1 mm in diameter, and tissue grading was achieved through PLS analysis of some 150 spectra taken at different points. The classification was based on changes in several spectral parameters, but general tissue orientation could only be assessed in tissue sections imaged with a standard bench microscope and IR polarizer, at 6.25 μm pixel resolution.44 Nevertheless, instrumental capabilities are evolving and it may eventually be possible to include polarization and tissue orientation as part of a clinical probe.
Near-field nanoscale infrared imaging capabilities are evolving swiftly, raising the question not of whether but of how such tremendously detailed information can be exploited for spectropathology. As seems evident from this work, the fact that the near-field nano-FTIR spectra are z-polarized is an important aspect that can be exploited in the study of the molecular basis of disease, particularly those diseases involving the formation or destruction of protein aggregates. The results of the experiments presented here demonstrate that we have the capability of studying the spectra of collagen fibrils at the level of their molecular organization. In future work, we will study the changes that occur at the fibril and molecular levels in mechanically damaged tendon. With near-field nano-FTIR, we hope to obtain confirmation that collagen molecules are locally denatured, perhaps via specific mechanisms, at specific sites within overload-damaged fibrils. In future work, we plan to explore the question of when and how this mechanism appears in the human life cycle, from youth to old age.
5. Conclusions
Collagen is the critical load-bearing constituent of mammals and performs innumerable functions throughout the body, all of which are enabled by the highly ordered structures that scale from molecular tropocollagen molecules and fibrils, up to fibres, fasicles, tendons and the matrices of bones and teeth. In tendon, cartilage, and certain other tissues, disorder may often be equated with damage and disease. The first high-magnification FTIR PCI spectra of control and mechanically damaged tendons show spectral differences in the relative intensity of the conformationally sensitive amide I and II bands. Different degrees of mechanical damage and disorder within individual tendons can be detected spectroscopically at the fibre length scale of 1 μm. SEM images of parallel matched-pair tendons confirm the presence of mechanical damage, in the form of serial kinking in collagen fibrils. AFM surface images of the sections imaged with FTIR PCI confirm the presence of fibril disorder and the presence of kinks at the nanoscale. With near-field nano-FTIR spectroscopy, it has been possible to obtain the first ever spatially resolved infrared spectra and images of collagen fibrils, including remarkably distinct spectral variations with D-bands. These techniques offer significant advantages for fundamental research, spectropathology and clinical applications.Acknowledgements
This research is funded by a grant from the Canadian Institutes for Health Research, MOP-133453. S. Baldwin acknowledges the support of an ASPIRE CREATE NSERC PhD Fellowship; C. Findlay acknowledges the support of a Research Manitoba Health Studentship (PhD). The authors thank Reid's Meats, NS, Canada, for their assistance with the collection of tendon samples and Pat Scallion, Institute for Research in Materials at Dalhousie University for assistance with scanning electron microscopy. We are grateful to Dr Hans Bechtel and Dr Michael C. Martin for assistance with data collection at Beam Line 5.4.1 at the Advanced Light Source, Berkeley CA, USA. The authors are very grateful to Neaspec GmbH DE, and in particular to Dr Max Eisele of Neaspec, for the nano-FTIR spectra and the phase and amplitude-resolved near-field images of collagen fibrils and for many helpful discussions.References
- R. D. B. Fraser, Discuss. Faraday Soc., 1950, 9, 379–383 RSC.
- R. M. Badger and A. D. E. Pullin, J. Chem. Phys., 1954, 22, 1142 CAS.
- J. T. Randall, R. D. B. Fraser, S. Jackson, A. V. W. Martin and A. C. T. North, Nature, 1952, 169, 1029–1033 CrossRef CAS PubMed.
- F. O. Schmitt, Proc. Am. Philos. Soc., 1956, 100, 476–486 CAS.
- A. J. Hodge and F. O. Schmitt, Proc. Natl. Acad. Sci. U. S. A., 1960, 46, 186–197 CrossRef CAS.
- P. Fratzl, K. Misof, I. Zizak, G. Rapp, H. Amenitsch and S. Bernstorff, J. Struct. Biol., 1998, 122, 119–122 CrossRef CAS PubMed.
- K. Esmonde-White, Appl. Spectrosc., 2014, 68, 1203–1218 CrossRef CAS PubMed.
- C. Eklouh-Molinier, T. Happillon, N. Bouland, C. Fichel, M.-D. Diébold, J.-F. Angiboust, M. Manfait, S. Brassart-Pasco and O. Piot, Analyst, 2015, 140, 6260–6268 RSC.
- A. Boskey and N. P. Camacho, Biomaterials, 2007, 28, 2465–2478 CrossRef CAS PubMed.
- R. Wiens, M. Rak, N. Cox, S. Abraham, B. H. J. Juurlink, W. M. Kulyk and K. M. Gough, Anal. Bioanal. Chem., 2007, 387, 1679–1689 CrossRef CAS PubMed.
- A. Masic, L. Bertinetti, R. Schuetz, S.-W. Chang, T. H. Metzger, M. J. Buehler and P. Fratzl, Nat. Commun., 2015, 6, 5942 CrossRef CAS PubMed.
- N. S. Kalson, Y. Lu, S. H. Taylor, T. Starborg and D. F. Holmes, eLife, 2015 DOI:10.7554/eLife.05958.001.
- B. Brodsky and A. V. Persikov, Adv. Protein Chem., 2005, 70, 301–339 CrossRef CAS PubMed.
- S. P. Veres and J. M. Lee, Biophys. J., 2012, 102, 2876–2884 CrossRef CAS PubMed.
- S. P. Veres, J. M. Harrison and J. M. Lee, J. Orthop. Res., 2013, 31, 731–737 CrossRef CAS PubMed.
- S. P. Veres, J. M. Harrison and J. M. Lee, Matrix Biol., 2014, 33, 54–59 CrossRef CAS PubMed.
- M. J. Nasse, M. J. Walsh, E. C. Mattson, R. Reininger, A. Kajdacsy-Balla, V. Macias, R. Bhargava and C. J. Hirschmugl, Nat. Methods, 2011, 8, 413–416 CrossRef CAS PubMed.
- C. R. Findlay, R. Wiens, M. Rak, J. Sedlmair, C. J. Hirschmugl, J. Morrison, C. J. Mundy, M. Kansiz and K. M. Gough, Analyst, 2015, 140, 2493–2503 RSC.
- R. K. Reddy, M. J. Walsh, M. V. Schulmerich, P. S. Carney and R. Bhargava, Appl. Spectrosc., 2013, 67, 93–105 CrossRef PubMed.
- H. A. Bechtel, E. A. Muller, R. L. Olmon, M. C. Martina and M. B. Raschke, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 7191–7196 CrossRef CAS PubMed.
- T. Taubner, R. Hillenbrand and F. Keilmann, Appl. Phys. Lett., 2004, 85, 5064–5066 CrossRef CAS.
- F. Huth, A. Govyadinov, S. Amarie, W. Nuansing, F. Keilmann and R. Hillenbrand, Nano Lett., 2012, 12, 3973–3978 CrossRef CAS PubMed.
- I. Amenabar, S. Poly, W. Nuansing, E. H. Hubrich, A. A. Govyadinov, F. Huth, R. Krutokhvostov, L. Zhang, M. Knez, J. Heberle, A. M. Bittner and R. Hillenbrand, Nat. Commun., 2013, 4, 1–9 Search PubMed.
- S. Mastel, A. A. Govyadinov, T. V. A. G. de Oliveira, I. Amenabar and R. Hillenbrand, Appl. Phys. Lett., 2015, 106, 023113 CrossRef.
- D. M. Stitt, M. Z. Kastyak-Ibrahim, C. R. Liao, J. Morrison, B. Albensi and K. M. Gough, Vib. Spectrosc., 2012, 60, 16–22 CrossRef CAS.
- W. H. Moore and S. Krimm, Biopolymers, 1976, 15, 2439–2464 CrossRef CAS PubMed.
- G. S. Young, Mater. Res. Soc. Symp. Proc., 1992, 267, 859–867 CrossRef CAS.
- N. P. Camacho, P. West, P. A. Torzilli and R. Mendelsohn, Biopolymers, 2001, 62, 1–8 CrossRef CAS PubMed.
- M. D. Shoulders and R. T. Raines, Annu. Rev. Biochem., 2009, 78, 929–958 CrossRef CAS PubMed.
- B. B. Doyle, D. W. L. Hukins, D. J. S. Hulmes, A. Miller, C. J. Rattew and J. Woodhead-Galloway, Biochem. Biophys. Res. Commun., 1974, 60, 858–864 CrossRef CAS PubMed.
- J. P. R. O. Orgel, T. C. Irving, A. Miller and T. J. Wess, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 9001–9005 CrossRef CAS PubMed.
- M. J. Buehler, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12285–12290 CrossRef CAS PubMed.
- M. J. Tobin, M. A. Chesters, J. M. Chalmers, F. J. M. Rutten, S. E. Fisher, I. M. Symonds, A. Hitchcock, R. Allibone and S. Dias-Gunasekara, Faraday Discuss., 2004, 126, 27–39 RSC.
- S. P. Veres, E. P. Brennan-Pierce and J. M. Lee, J. Biomed. Mater. Res., Part A, 2015, 103, 397–408 CrossRef PubMed.
- S. E. Szczesny, J. L. Caplan, P. Pedersen and D. M. Elliott, Sci. Rep., 2015, 5, 14649–14658 CrossRef CAS PubMed.
- M. P. E. Wenger and P. Mesquida, Appl. Phys. Lett., 2011, 98, 163707 CrossRef.
- S. J. Baldwin, A. S. Quigley, C. Clegg and L. Kreplak, Biophys. J., 2014, 107, 1794–1801 CrossRef CAS PubMed.
- S. J. Baldwin, in prep.
- C. Gullekson, L. Lucas, K. Hewitt and L. Kreplak, Biophys. J., 2011, 100, 1837–1845 CrossRef CAS PubMed.
- P. Bassan, A. Sachdeva, A. Kohler, C. Hughes, A. Henderson, J. Boyle, J. H. Shanks, M. Brown, N. W. Clarke and P. Gardner, Analyst, 2012, 137, 1370–1377 RSC.
- J. Filik, M. D. Frogley, J. K. Pijanka, K. Wehbe and G. Cinque, Analyst, 2012, 137, 853–861 RSC.
- A. Hanifi, C. McGoverin, Y.-T. Oua, F. Safadi, R. G. Spencer and N. Pleshko, Anal. Chim. Acta, 2013, 779, 41–49 CrossRef CAS PubMed.
- A. Hanifi, H. McCarthy, S. Roberts and N. Pleshko, PLoS One, 2013, 8, e64822 CAS.
- A. Hanifi, X. Bi, B. Kavukcuoglu, P. C. Lin, E. DiCarlo, R. G. Spencer, M. P. G. Bostrom and N. Pleshko, Am. J. Sports Med., 2012, 40, 2853–2861 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2016 |

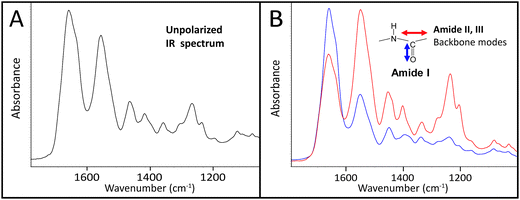
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch at 1660 cm−1) and 90° (red, enhanced C–N stretch at 1545 cm−1 and backbone modes). Spectra are normalized to the stronger band in each case.
O stretch at 1660 cm−1) and 90° (red, enhanced C–N stretch at 1545 cm−1 and backbone modes). Spectra are normalized to the stronger band in each case.