DOI: 10.1039/C5EW90026C
(Highlight)
Environ. Sci.: Water Res. Technol., 2016, 2, 13-16
Research highlights: visible light driven photocatalysis and photoluminescence and their applications in water treatment
Qinmin
Zheng
a,
David T.
Tan
b and
Danmeng
Shuai
*a
aDepartment of Civil and Environmental Engineering, The George Washington University, 800 22nd St NW Suite 3530, Science and Engineering Hall, Washington, DC 20052, USA. E-mail: danmengshuai@gwu.edu
bDepartment of Civil, Environmental, and Geo-Engineering, University of Minnesota, 500 Pillsbury Drive SE, Minneapolis, Minnesota 55455, USA
First published on 28th October 2015
Photocatalysis holds great promise for sustainable water treatment due to the generation of reactive radicals for efficient contaminant removal, minimized chemicals consumption, and the utilization of renewable, inexhaustible solar energy (DOI: 10.1021/cr00033a004, DOI: 10.1021/cr00018a003). The most widely used photocatalyst for water treatment, titanium dioxide (TiO2), requires UV excitation. However, UV only accounts for 4% of solar energy, and the dependence of current photocatalysts on UV compromises the efficiency and feasibility of solar powered water treatment. Disinfection, an important water treatment process for the inactivation of pathogenic microorganisms, also requires UV radiation with a wavelength of 250–260 nm. Therefore, the development of novel photocatalysts, and photophysical and photochemical processes for the use of optical radiation with a longer wavelength, such as visible light that accounts for 40% of solar energy, would present a major breakthrough for solar powered water treatment. A unique photoluminescence process, upconversion, has recently drawn attention for its ability to convert low energy photons (e.g., visible light) into high energy photons (e.g., UV light) for antimicrobial purposes (DOI: 10.1021/es200196c, DOI: 10.1021/es405229p). In this research highlight, we discuss three innovative materials used in photocatalysis and photoluminescence for water treatment applications, including graphitic carbon nitride (g-C3N4), red phosphorus, and upconversion phosphors (Y2SiO5 doped with Pr and Li).Graphitic carbon nitride (g-C3N4)
g-C3N4 has emerged as a novel metal-free polymeric photocatalyst in water splitting, CO2 reduction, organic synthesis, and environmental remediation because of its response to visible light (<460 nm), chemical and thermal stability, facile synthesis from earth abundant materials, low to non-existent toxicity, and highly tunable properties (DOI: 10.1002/adma.201500033). However, the major hurdle of applying bulk g-C3N4 in photocatalytic reactions is its low surface area (<10 m2 g−1) and fast charge recombination (DOI: 10.1002/adma.201500033). In environmental applications, these limitations reduce reactive sites for contaminant adsorption and reaction and lower the production of reactive oxygen species (ROS) for contaminant transformation. Recently, Zhao et al. (DOI: 10.1039/C3RA45776A) fabricated a new type of atomic single layer g-C3N4 (SL g-C3N4) with enhanced photocatalytic activity for the degradation of an organic dye, rhodamine B (RhB). Bulk g-C3N4 shares a similar layered structure as graphite, and the SL g-C3N4 was synthesized by the liquid exfoliation of bulk g-C3N4 under ultrasonication. The thickness of g-C3N4 was reduced to 0.4–0.5 nm after ultrasonication, which is confirmed as a single layer (Fig. 1a). The photocatalytic activity for the degradation of RhB was conducted under visible light irradiation (λ > 400 nm) from a xenon lamp source. The reaction rate constant of SL g-C3N4 was 1.96 h−1, which is much higher than its counterpart photocatalysts, including bulk g-C3N4, cadmium sulfide (CdS), and TiO2 P25 (Fig. 1b). Zhao et al. attributed the superior photocatalytic activity of SL g-C3N4 to the efficient charge separation and transport that are reflected by its unique features: a long lifetime for the photogenerated charges, a low charge transfer resistance, and a high charge carrier density. Meanwhile, Wang et al. (DOI: 10.1021/es503073z) also observed improved photocatalytic activity of SL g-C3N4 for the oxidation of waterborne ammonia due to efficient charge separation (Fig. 1c). The scavenger test showed that the excited holes and hydroxyl radicals were the main reactive species for ammonia oxidation.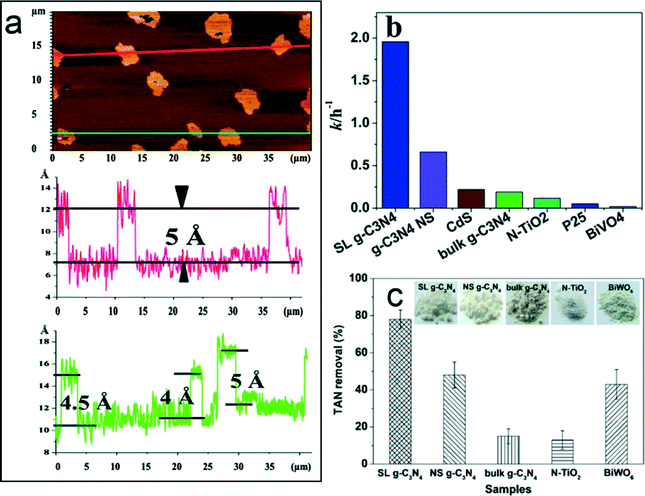 | ||
| Fig. 1 (a) Atomic force microscopic image of SL g-C3N4; (b) the reaction rate constants of rhodamine B photocatalytic degradation on different photocatalysts; and (c) comparison of the photocatalytic performance of total ammonia nitrogen (TAN) removal on different photocatalysts. SL and NS represent atomic single layer and nanosheet, respectively. Adapted from DOI: 10.1039/C3RA45776A with permission of The Royal Society of Chemistry, and reprinted with permission from DOI: 10.1021/es503073z. Copyright 2014 American Chemical Society. | ||
Red phosphorus
Red phosphorus is a metal-free, elemental photocatalyst that has unique advantages of low cost, earth abundance, and easy availability. However, the study of this elemental photocatalyst is still in its infancy. For the first time, Xia et al. (DOI: 10.1021/acs.est.5b00531) explored the application of red phosphorus for disinfection. Commercial red phosphorus was purified with hydrothermal treatment, and it is expected to utilize even infrared (IR) light with a wavelength up to 870 nm (due to the narrow band gap of 1.42 eV). The authors conducted inactivation tests of E. coli using visible light irradiation from a xenon lamp (λ > 400 nm), sunlight (including both UV and visible light), and visible light emitting diodes (LEDs). The inactivation rate constant was 0.72 min−1 under solar irradiation and 0.33 min−1 under visible light irradiation from the xenon lamp, but no apparent inactivation was observed in the control experiment with red phosphorus alone (dark control) or light alone (light control) (Fig. 2). Visible light contributed substantially to the inactivation of E. coli in the presence of red phosphorus, though solar irradiation was more effective than visible light-only irradiation due to higher irradiance and the presence of UV. In LED tests, the inactivation kinetics decreased as the wavelength of the light source increased from blue to red, in agreement with the optical absorbance of red phosphorus. To investigate the roles of various reactive species for disinfection, scavenger tests were performed. The results showed that the photogenerated electrons and the derived reactive species, ˙O2−, were the dominant effective species in the inactivation of E. coli. The proposed mechanism of E. coli inactivation is shown in Fig. 3. The radicals generated by red phosphorus first oxidize cell membrane associated proteins, including enzymes necessary for energy production. Consequently, affected cells have insufficient energy to maintain the cell membrane potential, resulting in an increase in cell permeability followed by leakage and rapid decay of cytoplasmic contents such as proteins and DNA, and ultimately cell death.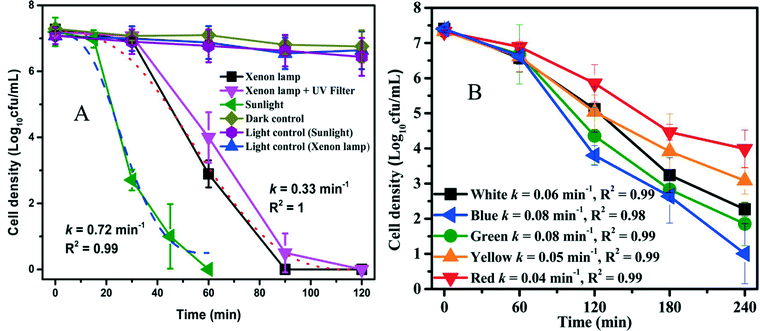 | ||
| Fig. 2 (A) Photocatalytic inactivation kinetics under xenon lamp and sunlight irradiation in the presence of red phosphorus; (B) photocatalytic inactivation efficiencies under different monochromatic LED irradiation (red 610–650 nm, yellow 570–620 nm, green 470–570 nm, and blue 440–490 nm). Reprinted with permission from DOI: 10.1021/acs.est.5b00531. Copyright 2015 American Chemical Society. | ||
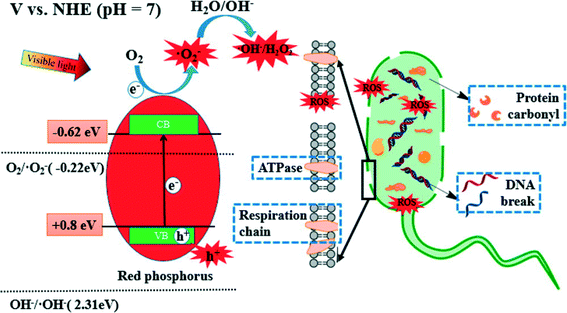 | ||
| Fig. 3 Proposed bactericidal mechanism of red phosphorus under visible light irradiation. Red phosphorus generates ROS under irradiation, and ROS subsequently inhibit bacterial metabolism on cell membranes and oxidize intracellular components. Reprinted with permission from DOI: 10.1021/acs.est.5b00531. Copyright 2015 American Chemical Society. | ||
Upconversion phosphors (Y2SiO5 doped with Pr and Li)
There is an increasing interest in upconversion phosphors (UCPs) because of these materials' unique capability to convert low energy photons into high energy photons, which are more useful for a variety of applications. In past decades, IR-to-visible and visible-to-visible upconversion have been extensively studied for applications in anti-counterfeiting devices, advanced solar cells, bioimaging, and photodynamic therapy. Cates et al. (DOI: 10.1021/es200196c) were the first to introduce an innovative material, namely Y2SiO5:Pr,Li dip-coated film, for upconverting visible light to germicidal UVC radiation for antimicrobial purposes (Fig. 4). The authors (DOI: 10.1021/es405229p) recently further tailored the synthesis of UCPs and successfully fabricated Y2SiO5-based upconversion ceramics with increased thickness and density, and in turn the ceramics showed a significant improvement in upconversion intensity under laser excitation: the UVC emission intensity of ceramics is 34 times higher than that of Y2SiO5:Pr,Li dip-coated films. Even under low-power visible excitation (commercial fluorescent bulb), the Y2SiO5-based upconversion ceramics exhibited a 3.9 times greater inactivation rate of Bacillus subtilis spores compared to that of the dip-coated Y2SiO5:Pr,Li films (Fig. 5). Specifically, the ceramics were synthesized by high temperature sintering of compacted Y2SiO5 powders doped with Pr3+ and Li+ (1300 °C for 3 hours, pressed at 100 MPa). The synthesis of Y2SiO5 powder was optimized by calcining the sol–gel precursor at 600 °C instead of 1000 °C to prevent the crystallization of Y2SiO5 prior to pressing, which was a key step to prepare ceramics because the non-crystalline particles were easier to fuse together.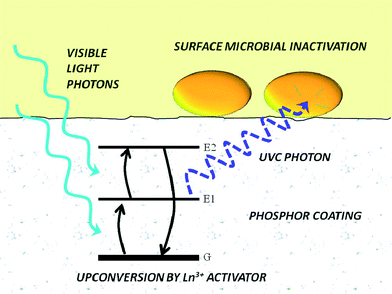 | ||
| Fig. 4 Utilization of visible-to-ultraviolet upconversion phosphor coating for light-activated antimicrobial materials. Energy diagram depicts excited-state absorption of visible light from the ground-state configuration, G, to the excited states, E1 and E2, to emit a UVC photon upon relaxation. Reprinted with permission from DOI: 10.1021/es200196c. Copyright 2011 American Chemical Society. | ||
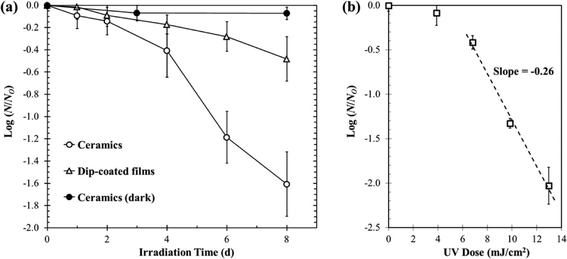 | ||
| Fig. 5 (a) Inactivation of Bacillus subtilis spores on ceramic and dip-coated film surfaces with exposure to visible fluorescent light. (b) UVC dose–response curve showing Bacillus subtilis spore inactivation under low intensity UVC irradiation (0.04 mW cm−2). Reprinted with permission from DOI: 10.1021/es405229p. Copyright 2014 American Chemical Society. | ||
Outlook
The highlighted studies pave new avenues for improving the performance of photocatalysis and photoluminescence for water treatment and efficient utilization of solar energy. The development of earth abundant materials (e.g., g-C3N4, red phosphorus) is promising for addressing the challenges of practical application of photocatalysis for water treatment; however, further improvements are needed in enhancement of charge separation, surface area, and low energy photon harvesting (i.e., visible and IR light) to achieve the necessary performance to make this a viable technology. Upconversion materials are unique and promising for water treatment but their energy conversion efficiency is currently low (0.0012–0.0019%) (DOI: 10.1021/es200196c, DOI: 10.1021/es405229p). More importantly, the utilization of UV light in solar radiation should not be overlooked even as the development of visible/IR-light-responsive materials takes place. A hybrid material with UV-, visible-, and IR-light-responsive components could provide the necessary structure to harvest the full spectrum of solar irradiation. Future studies should focus on the optimization of current photoactive materials and processes, the exploration of novel photoactive materials and processes, and the mechanistic understanding of photophysical and photochemical processes.| This journal is © The Royal Society of Chemistry 2016 |
