Diffusion layer characteristics for increasing the performance of activated carbon air cathodes in microbial fuel cells
Xiaoyuan
Zhang
 *ab,
Weihua
He
c,
Wulin
Yang
b,
Jia
Liu
b,
Qiuying
Wang
a,
Peng
Liang
a,
Xia
Huang
a and
Bruce E.
Logan
*b
*ab,
Weihua
He
c,
Wulin
Yang
b,
Jia
Liu
b,
Qiuying
Wang
a,
Peng
Liang
a,
Xia
Huang
a and
Bruce E.
Logan
*b
aState Key Joint Laboratory of Environment Simulation and Pollution Control, School of Environment, Tsinghua University, Beijing 100084, PR China. E-mail: zhangxiaoyuan@tsinghua.edu.cn; Fax: +86 10 62771472; Tel: +86 10-62787866
bDepartment of Civil & Environmental Engineering, Penn State University, 231Q Sackett Building, University Park, PA 16802, USA. E-mail: blogan@psu.edu; Fax: +1 814 863 7304; Tel: +1 814 863 7908
cState Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, No.73 Huanghe Road, Nangang District, Harbin 150090, PR China
First published on 15th December 2015
Abstract
The characteristics of several different types of diffusion layers were systematically examined to improve the performance of activated carbon air cathodes used in microbial fuel cells (MFCs). A diffusion layer of carbon black and polytetrafluoroethylene (CB + PTFE) that was pressed onto a stainless steel mesh current collector achieved the highest cathode performance. This cathode also had a high oxygen mass transfer coefficient and high water pressure tolerance (>2 m), and it had the highest current densities in abiotic chronoamperometry tests compared to cathodes with other diffusion layers. In MFC tests, this cathode also produced maximum power densities (1610 ± 90 mW m−2) that were greater than those of cathodes with other diffusion layers, by 19% compared to Gore-Tex (1350 ± 20 mW m−2), 22% for a cloth wipe with PDMS (1320 ± 70 mW m−2), 45% with plain PTFE (1110 ± 20 mW m−2), and 19% higher than those of cathodes made with a Pt catalyst and a PTFE diffusion layer (1350 ± 50 mW m−2). The highly porous diffusion layer structure of the CB + PTFE had a relatively high oxygen mass transfer coefficient (1.07 × 10−3 cm s−1) which enhanced oxygen transport to the catalyst. The addition of CB enhanced cathode performance by increasing the conductivity of the diffusion layer. Oxygen mass transfer coefficient, water pressure tolerance, and the addition of conductive particles were therefore critical features for achieving higher performance AC air cathodes.
Water impactA microbial fuel cell simultaneously treats and generates electricity from wastewater, but its performance is mainly limited by the cathode. Different diffusion layers were evaluated to prevent water leakage and allow oxygen transfer to the catalyst. Cathode performance was enhanced by adding conductive carbon black particles into a polytetrafluoroethylene polymer to optimize conductivity, porosity and mechanical strength. |
1. Introduction
A microbial fuel cell (MFC) is an innovative technology for conversion of organic and inorganic substrates in wastewater to electricity.1–5 In an MFC, exoelectrogenic bacteria grown on the anode oxidize substrates and release electrons that travel through an external circuit to the cathode.6,7 Various chemicals can be used as electron acceptors for the cathode reaction, but ambient oxygen is commonly used since it is readily available, it has a high reduction potential, and its reduction product is water. Air cathodes are more practical than liquid-immersed cathodes for MFC applications since this configuration avoids the need for water aeration and thus saves energy.8,9Air cathodes used for MFCs are typically constructed with three layers: a catalyst layer, a current collector, and a diffusion layer. Platinum (Pt) is an excellent oxygen reduction catalyst10–16 as MFC performance is mainly limited by oxygen reduction kinetics at the cathode.17,18 Activated carbon (AC) catalysts have emerged as the most promising alternatives to Pt due to their low cost and comparable or even improved performance compared to Pt.14,19–22 Several methods have recently been developed to improve AC cathode performance, for example, by selecting certain types of AC base materials or through modification of the carbon chemistry.23–26 These AC-based cathodes have shown much better performance in long-term operation (17 months) than cathodes made with Pt.27
Diffusion layers in air cathodes play an important role in MFC power generation,8,9,28 but there are few reports that have focused on diffusion layer characteristics relative to performance. The two main functions of the diffusion layer are enabling oxygen transfer and preventing water leakage out of the reactor. The ideal diffusion layer for an air cathode facilitates oxygen transfer to the catalyst layer while providing excellent integrity to maintain performance at a high water pressure. Uniformity and flexibility of diffusion layers could be important for achieving a high water pressure tolerance.29 One commercially available AC cathode with good MFC performance (VITO CORE™, VITO; Mol, Belgium) uses a highly porous polytetrafluoroethylene (PTFE) diffusion layer prepared by a proprietary process.27,30 However, it has not been compared to other types of diffusion layers in terms of oxygen mass transfer and water head tolerance. PTFE diffusion layers with carbon black (CB), which is used to increase the electrical conductivity of the cathode, can be made using a continuous roller method.19,20 Cathodes with this type of CB + PTFE diffusion layer also have good performance in MFC tests, but this type of cathode also has not been examined relative to other cathodes in terms of diffusion layer performance. Other approaches used to add a diffusion layer to a cathode are to place a poly(dimethylsiloxane) (PDMS) solution and CB solution onto a cloth wipe (textile) material,21 or a poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP)/CB solution directly onto the AC catalyst layer.31 These latter two types of diffusion layers were shown to have a high oxygen mass transfer coefficients, and they produced maximum power densities similar to cathodes made with a Pt catalyst; however, they did not have good integrity relative to water pressure, as they leaked at water pressures higher than 0.45 m.31 Thus, there are several different types of diffusion layers that are being examined for use with MFCs, but their properties relative to oxygen diffusion and water pressure have not been systematically examined.
In this study, different types of diffusion layers were examined using the same AC air cathodes in terms of physical and electrochemical properties. These diffusion layers included custom-made PTFE, PTFE/CB or PDMS/CB21 layers applied to a cloth wipe; a PTFE film; a CB + PTFE diffusion layer prepared on a stainless steel mesh using a batch press process, similar to the process previously used for cathode preparation using a rolling-press;20 a highly porous PTFE diffusion layer on a commercially available AC cathode (VITO CORE™ electrodes);27 and a layer of Gore-Tex fabric that is manufactured primarily for clothing.28 For these different AC air cathode diffusion layers, we systematically examined oxygen mass transfer coefficient, water pressure tolerance, electrochemical performance, and maximum power production in MFCs.
2. Materials and methods
2.1 Air cathode diffusion layer materials and fabrication
All diffusion layers were added to homemade cathodes containing AC catalysts, except for one commercially available cathode (VITO CORE™) that already contained a porous PTFE diffusion layer (30% porosity, 0.13 kg m−2) and an AC catalyst on a stainless steel mesh.Several diffusion layers were made by applying a polymer or polymer–CB solution to a cloth wipe (textile) material (Amplitude Prozorb, Contec Inc., USA). A wipe with two layers of PDMS (WP-PDMS) was prepared as previously reported32 in order to provide a benchmark for the other preparation methods. Different amounts of a 60% PTFE dispersion were applied on a wipe 11 cm2 in size, with a working projected area of 7 cm2: 200 μL (WP-PTFE 200), 300 μL (WP-PTFE 300), and 800 μL (WP-PTFE 800). To increase the electrical conductivity of the diffusion layer, CB (18 mg) was mixed into PTFE (300 μL) and also applied to a wipe (WP-PTFE 300 + CB). These wipe-based materials with PTFE were heated at 80 °C for 30 min. A heating temperature of 340 °C used in previous studies to produce a PTFE diffusion layer on carbon cloth could not be used here as this would destroy the integrity of the wipe that contained cellulose (∼50%).
A diffusion layer made with PTFE, CB (CB + PTFE)20 and a stainless steel mesh with improved electrical conductivity was fabricated using a batch press. The mass ratio of CB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PTFE was 2
PTFE was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (CB loading of 25 mg cm−2 and PTFE loading of ∼37.5 mg cm−2). CB was mixed with a PTFE suspension and ethanol and stirred in an 80 °C bath with ultrasonification for 30 min. The paste that was formed was kneaded several times and pressed at 1.5 MPa and 80 °C for 10 s (Model 4386, Carver Inc., USA). The final CB + PTFE diffusion layer was pressed onto a stainless steel mesh (42 × 42, type 304, McMaster-Carr, USA) at 4.5 MPa and 80 °C for 1 min and then heat treated at 340 °C for ∼15 min.
3 (CB loading of 25 mg cm−2 and PTFE loading of ∼37.5 mg cm−2). CB was mixed with a PTFE suspension and ethanol and stirred in an 80 °C bath with ultrasonification for 30 min. The paste that was formed was kneaded several times and pressed at 1.5 MPa and 80 °C for 10 s (Model 4386, Carver Inc., USA). The final CB + PTFE diffusion layer was pressed onto a stainless steel mesh (42 × 42, type 304, McMaster-Carr, USA) at 4.5 MPa and 80 °C for 1 min and then heat treated at 340 °C for ∼15 min.
Other commercial materials were also tested as diffusion layers without additional modification: a PTFE film (VIRGIN PTFE ROLLS .005′′ × 12′′, DuPont, USA) and a commercial nylon fabric (Gore-Tex® Nextec Microfiber Nylon Water & Wind Repellant, Breathable Fabric, USA).
2.2 Activated carbon air cathode fabrication
Cathodes were fabricated using a peat-based AC powder (Norit SX plus, Norit Americas Inc., USA) that was previously shown to have the highest performance among different types of carbons.24 The AC binder solution was prepared by combining 37.8 μL of 60% PTFE and 700 μL of DI water and mixing by ultrasonication for 1 min. AC powder (300 mg) was added to the binder to form a paste, followed by 1 min of ultrasonication. The paste was then spread with a spoon onto a stainless steel mesh (11 cm2 in size, with a working projected area of 7 cm2, 50 × 50, type 304, McMaster-Carr, USA) to form the catalyst layer, and then the diffusion layer prepared as described above was placed on the catalyst layer.21,25 The catalyst layer used in these cathodes therefore was situated in between the stainless steel mesh current collector and supporting layer and the diffusion layer.For the wipe-based diffusion layers, the conductive diffusion layer (CB + PTFE) and the PTFE film, the cathodes were pressed at 40 MPa and 80 °C for 20 min (Model 4386, Carver Inc., USA). For the Gore-Tex fabric diffusion layer, cathodes were pressed at 40 MPa and 150 °C (ref. 28) for 20 min. These AC cathodes with different diffusion layers were benchmarked against a Pt/C air cathode previously used in many studies that contained Pt (5 mg cm−2 10%) held with a binder (Vulcan XC-72 and 5 wt% Nafion) on a 30% wet-proofed carbon cloth that was made with four PTFE diffusion layers.8 After fabrication, the AC cathode diffusion layers were photographed (Canon 5D Mark II, Japan) and examined using a scanning electron microscope (SEM, FEI Quanta 200, Netherlands).
2.3 MFC experiments
MFCs were cube-shaped 4 cm blocks (Lexan) containing a single cylindrical chamber with an inner diameter of 3 cm as previously described.33 Anodes were heat-treated (at 450 °C for 30 min) graphite fiber brushes made with titanium wires as current collectors. The anode was placed in the middle of the reactor horizontally. The air cathode was placed on the other side of the reactor with the diffusion layer facing the air. The distance between the air cathode and the edge of the anode was ~1 cm to avoid short- circuiting through direct electrode contact.MFCs were inoculated with the effluent from other acetate-fed MFCs that were operated for over one year. The substrate was 1 g L−1 sodium acetate amended with 12.5 mL L−1 minerals and 5 mL L−1 vitamins,34 in a 50 mM phosphate buffer solution (PBS) that contained 4.57 g L−1 Na2HPO4, 2.45 g L−1 NaH2PO4·H2O, 0.31 g L−1 NH4Cl and 0.13 g L−1 KCl. All the MFCs were operated with a 1000 Ω external resistor (except polarization tests) in fed-batch mode at 30 °C.
Voltage (U) across an external resistor (R) was recorded every 20 min using a data acquisition system (2700, Keithley Instrument, USA) connected to a computer. Polarization tests were conducted using the multi-cycle method by varying the external resistances from 1000 Ω to 20 Ω, with each resistance applied for one complete cycle. Current density (J) and power density (P) were normalized by the projected area of air cathode (A = 7 cm2), using J = U/RA and P = JU.35 Anode and cathode potentials were reported versus an Ag/AgCl reference electrode (+0.21 V versus a standard hydrogen electrode; RE-5B, BASi, West Lafayette, USA). Coulombic efficiency (CE) was calculated at each external resistance as previously described.35
2.4 Electrochemical analyses
Air cathodes were tested in an abiotic cell with a 50 mM PBS medium for electrochemical performance. This reactor consisted of two cylindrical chambers each 2 cm in length, with an anion exchange membrane (AMI-7001, Membrane International Inc., USA) placed in the middle. A platinum plate with 1 cm2 area was placed in one chamber as a counter electrode, and a Ag/AgCl reference electrode was placed adjacent to the air cathode (working electrode).9 Chronoamperometry tests were conducted using a potentiostat (VMP3 Workstation, BioLogic Science Instruments, USA). After operation at an open circuit condition for 3 h, the cathode potential was set with a potential applied for 1 h in a stepwise manner (0.2, 0.1, 0, −0.1 and −0.2 V versus Ag/AgCl).2.5 Air cathode water pressure tolerance, oxygen mass transfer coefficient and diffusion layer resistivity
An air cathode water pressure tolerance test system was set up as previously described.29 An MFC reactor was used with the cathode facing upwards, with a tube connected to the reactor that could be filled with water up to a height of 2.4 m. The height of water in the tube was raised up via pumping water into the tube from a container until water droplets were observed on the surface of the air cathode diffusion layer. This static water head was recorded as the maximum water pressure.Air cathode oxygen mass transfer coefficient tests were conducted using an MFC reactor without an anode to calculate an oxygen mass transfer coefficient (k, cm s−1) as previously described.8 The reactor was filled with oxygen-free 50 mM PBS and dissolved oxygen concentrations in the solution were monitored over time using a non-consumptive DO probe (Foxy-18G, Ocean Optics Inc., USA). The resistivity of the diffusion layer was measured using a commercial four-probe sheet resistance system (model SX1944, Jiangsu Telecommunication, China).
3. Results and discussion
3.1 Electrochemical performance of air cathodes with different diffusion layers
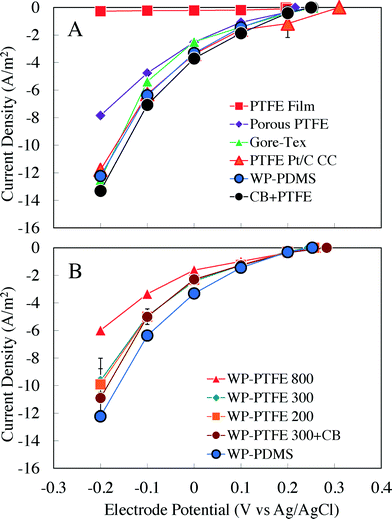
Chronoamperometry tests in abiotic reactors demonstrated that the different diffusion layers significantly affected air cathode performance (Fig. 1). Among the cathodes made with diffusion layers without a wipe, the conductive diffusion layer (CB + PTFE) with a stainless steel mesh that was applied directly to the cathode produced the highest current density (Fig. 1A). For example, at a set potential of −0.2 V vs. Ag/AgCl, the CB + PTFE cathode produced a current density of 13.3 ± 0.3 A m−2. This current density was 6% higher than that of the Gore-Tex fabric (12.5 ± 0.1 A m−2), 9% higher than that of a wipe-based PDMS diffusion layer (WP-PDMS, 12.2 ± 0.1 A m−2), 13% higher than that of the Pt cathode (PTFE Pt/C CC, 11.8 ± 0.1 A m−2), and 71% higher than that of the 30% porosity PTFE diffusion layer (porous PTFE, 7.8 ± 0.1 A m−2) (Fig. 1A). The cathode with only a plain PTFE film produced very little current (0.3 ± 0.1 A m−2).Among the wipe-based diffusion layers, cathodes with the PDMS coatings produced the highest densities at potentials of 0 to −0.2 V (vs. Ag/AgCl). The performance of the cathodes decreased inversely with the amount of PTFE added. For example, at a set potential of −0.2 V vs. Ag/AgCl, the cathode with 200 μL of 60% PTFE (WP-PTFE 200) produced a current density of 9.9 ± 1.9 A m−2, which was slightly higher (3%) than the 300 μL (WP-PTFE 300, 9.6 ± 0.9 A m−2), and 65% higher than the 800 μL (WP-PTFE 800, 6.0 ± 0.1 A m−2) (Fig. 1B). However, when less PTFE was used (100 μL), the cathode leaked water with only the water pressure of the small reactor. The addition of CB with PTFE (300 μL of 60% PTFE) increased the current density by 14% to 10.9 ± 0.1 A m−2 compared to cathodes made only with 300 μL of 60% PTFE (Fig. 1B).
3.2 MFC performance of MFCs with different diffusion layers
The use of the different diffusion layers affected air cathode performance and maximum power densities in MFCs (Fig. 2). In general, the performance based on polarization data for the MFCs was consistent with that observed in half-cell electrochemical tests, suggesting that the anodes were not responsible for the different performance of the MFCs. The measured anode potentials in these reactors were also very similar, confirming that the different performance of the MFCs was due to the diffusion layers and cathodes (Fig. 2C and D).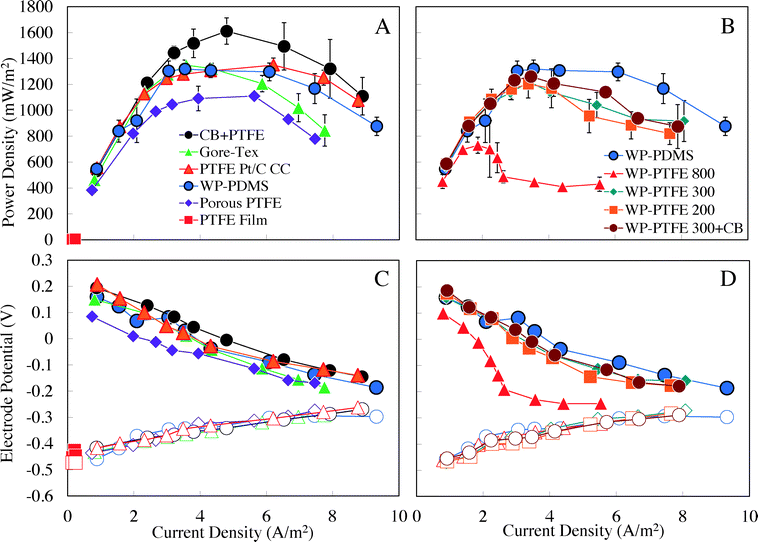 | ||
| Fig. 2 (A and B) Power density and (C and D) electrode potential as a function of current density in the MFCs using cathodes with different diffusion layers. | ||
The cathode with a conductive diffusion layer (PTFE + CB) generated the highest maximum power density of 1610 ± 90 mW m−2 in MFCs (Fig. 2A), which was 19% larger than that with the Gore-Tex fabric (1350 ± 20 mW m−2), 19% higher than that of the Pt cathode (PTFE Pt/C CC, 1350 ± 50 mW m−2), 22% higher than that of the wipe-based PDMS diffusion layer (WP-PDMS, 1320 ± 70 mW m−2), and 45% higher than that of the porous PTFE diffusion layer (porous PTFE, 1110 ± 20 mW m−2) (Fig. 2A). As expected from the electrochemical data, the MFC with the PTFE film diffusion layer had very poor performance (10 ± 1 mW m−2). The similar performance of the Gore-Tex (1350 ± 20 mW m−2) and Pt cathodes (1350 ± 50 mW m−2) (Fig. 2A) in MFC tests was consistent with the chronoamperometry tests when the comparisons are made at potentials relevant to those in MFC tests (0 to −0.2 V vs. Ag/AgCl) (Fig. 2). At a set potential of −0.2 V vs. Ag/AgCl, the Gore-Tex cathode produced a current density (12.5 ± 0.1 A m−2) that was higher than that of the Pt cathode (PTFE Pt/C CC, 11.8 ± 0.1 A m−2), but at 0 V vs. Ag/AgCl, the Gore-Tex cathode produced a lower current density (2.5 ± 0.1 A m−2) than the Pt cathode (3.4 ± 0.1 A m−2) (Fig. 1A). Thus, in MFC tests, the performance of the two cathodes was similar due to cathode potentials in this range.
The maximum power density obtained here using the CB + PTFE diffusion layer was higher than that previously reported for a CB + PTFE diffusion layer AC cathode fabricated by a roller process (1360 ± 30 mW m−2).19 The difference might be due in part to the use of an additional stainless steel mesh here for making the CB + PTFE cathode, which would have increased the cathode conductivity. In previous tests, only one stainless steel mesh was used between the CB + PTFE layer and the AC catalyst layer.19 The performance of the CB + PTFE cathodes was also higher than that obtained using cathodes made using a PVDF-HFP diffusion layer (1430 ± 90 mW m−2), where the AC catalyst, reactor and solution conditions were the same as those used here.31 The commercial cathodes with the porous PTFE diffusion layer (porous PTFE, 1110 ± 20 mW m−2) did not achieve the highest maximum power density, but it was previously shown that they had much improved longevity compared to Pt cathodes in these types of MFCs (over 17 months).27 In this current study, polarization tests were conducted after the MFCs generated repeatable current profiles over multiple batch cycles, with all tests completed within 30 days. While the performance of the cathode over a longer period of time was not examined here, it was previously shown in a 17 month study that the catalytic activity of the AC decreased, but it was also demonstrated that activity could be nearly completely (>85%) restored by removing the cathode biofilm and soaking the cathode in a weak acid (60 mM HCl).27 All the materials used here for the CB + PTFE diffusion layer AC cathodes were the same as those used in a previous study of cathode performance over time, and thus it is expected that the CB + PTFE cathode performance would change over time and that it could be restored by chemical cleaning. Longer-term performance of the optimized diffusion layer (CB + PTFE) cathodes will be examined in the future.
In tests with MFCs using cathodes with the wipe-based diffusion layers, the PDMS (WP-PDMS) generated a relatively high maximum power density of 1320 ± 70 mW m−2 (Fig. 2B). By using PTFE (200 and 300 μL), the maximum power densities were reduced to 1200 ± 100 mW m−2 for WP-PTFE 200 and 1220 ± 30 mW m−2 for WP-PTFE 300. These power densities were ∼65% higher than that of the cathode with a high PTFE loading of 800 μL for the wipe diffusion layer (WP-PTFE 800, 730 ± 60 mW m−2). When carbon black was added to increase the diffusion layer conductivity, the maximum power density increased to 1260 ± 10 mW m−2 (WP-PTFE 300 + CB) compared to that of the same cathode without CB (WP-PTFE 300, 1220 ± 30 mW m−2) (Fig. 2B).
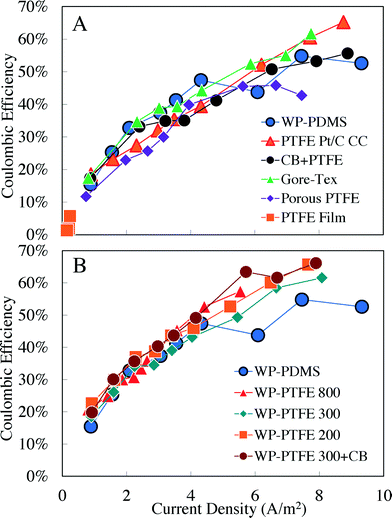
In general, CEs of MFCs increased in the same order as the maximum power densities measured in the MFC tests. This increase in CE with power was consistent with a previous report that showed that increasing the current density results in a greater efficiency of transfer of the organic matter into current due to less loss of substrate to aerobic decomposition of the substrate facilitated by oxygen transfer through the cathode.36 In general, the cathodes had similar CEs when compared on the basis of the current, with a CE of ∼15% at a current density of ∼0.8 A m−2, and ∼55–65% CEs at the current density of ∼9 A m−2 (Fig. 3). The only exception was the PTFE film, which had very low CEs of <6% due to very little current generation.
3.3 Air cathode diffusion layer characteristics
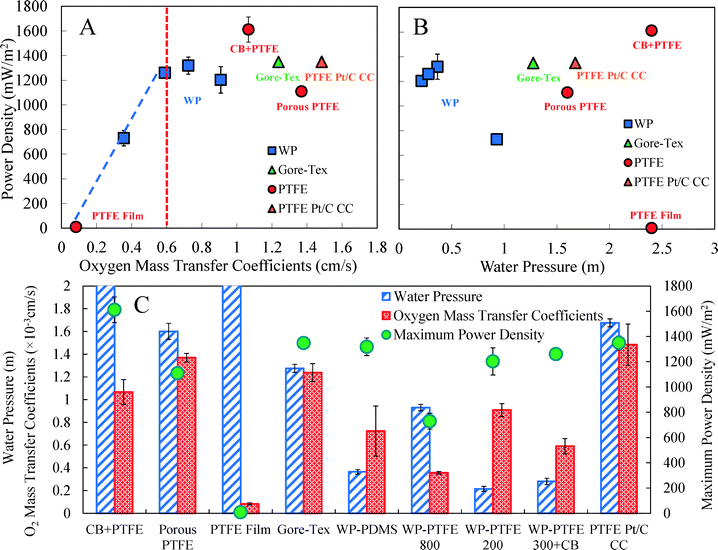
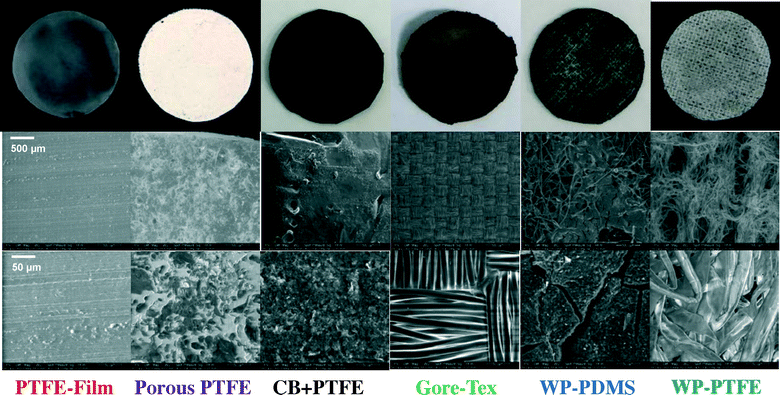
The oxygen mass transfer coefficient was a key factor in air cathode performance relative to power generation. When the oxygen mass transfer coefficient was below 0.6 × 10−3 cm s−1, the maximum power densities directly increased with oxygen transport coefficients (Fig. 4A). The PTFE film had a dense surface (Fig. 5), as shown by a very low oxygen mass transfer coefficient of 0.08 ± 0.01 × 10−3 cm s−1, and thus a small maximum power density (10 ± 1 mW m−2) (Fig. 4). When the oxygen mass transfer coefficient was 0.36 ± 0.01 × 10−3 cm s−1 (WP-PTFE 800), the power densities were increased to 730 ± 60 mW m−2. By using less PTFE, the oxygen mass transfer coefficient was further increased to 0.60 ± 0.07 × 10−3 cm s−1 (WP-PTFE 300 + CB), and the maximum power density was 1260 ± 10 mW m−2.When the oxygen mass transfer coefficients were above ∼0.6 × 10−3 cm s−1, all these cathodes generated maximum power densities larger than 1100 mW m−2 (Fig. 4A). However, further increasing the oxygen mass transfer coefficient did not directly increase the maximum power density (Fig. 4A). Other factors, such as electrical conductivity, appeared to be a critical factor when the oxygen mass transfer coefficient was >0.6 × 10−3 cm s−1. By adding CB and an additional stainless steel mesh layer to increase the cathode conductivity, the CB + PTFE cathode produced the highest maximum power density of 1610 ± 90 mW m−2 in MFCs among all these cathodes, with an oxygen mass transfer coefficient of 1.07 ± 0.08 × 10−3 cm s−1. The porous PTFE cathodes had a highly porous PTFE diffusion layer as shown by a large oxygen mass transfer coefficient of 1.37 ± 0.04 × 10−3 cm s−1, but they produced a maximum power density (1110 ± 20 mW m−2) that was lower than that of the cathode that had CB in the diffusion layer (CB + PTFE) (Fig. 4A). The CB + PTFE diffusion layer had a very low resistivity of 0.17 Ω cm compared to the porous PTFE without any CB (1990 Ω cm), demonstrating that adding CB could substantially improve the conductivity of the diffusion layer. This would suggest that adding CB and making the diffusion layer more electrically conductive helped to improve the cathode performance. It is known that electric conductivity is a critical feature of diffusion layers in proton exchange fuel cells and good electrical conductivity of gas diffusion layers directly correlates with fuel cell performance.37 Here in the MFC system, the conductive CB + PTFE diffusion layer with an additional stainless steel mesh might be helpful for facilitating electron conduction to and from the catalyst layer, reducing the contact resistance, and thus reducing ohmic loss, resulting in an enhancement of the cathode performance. However, it is also possible that when a PTFE solution was added directly to the cathode, without additional material (such as CB), it soaked into the AC catalyst layer and reduced its effectiveness as a catalyst. Gore-Tex, a commercial material that is marketed as a breathable fabric (non-conductive), had a very high oxygen mass transfer coefficient of 1.24 ± 0.08 × 10−3 cm s−1 (Fig. 4), but it also produced less power than the diffusion layer made with CB (CB + PTFE). These oxygen mass transfer coefficient and maximum power results demonstrate that both a high oxygen mass transfer coefficient (>0.6 × 10−3 cm s−1) and good electrical conductivity of the diffusion layer are important relative to AC air cathode performance.
High water pressure tolerance of air cathodes is critical for MFC wastewater treatment applications as reactors will be much larger than those used in the laboratory. Although it could be expected that greater resistance to water pressure might reduce oxygen mass transfer into the cathode, there was no clear correlation between water pressure tolerance and either the oxygen mass transfer coefficient or the maximum power density. This lack of a correlation suggests that the CB + PTFE diffusion layer air cathode could achieve both desirable high water pressure tolerance and high oxygen mass transfer coefficient (Fig. 4). The wipe-based diffusion layers all had low water pressure tolerance. The WP-PDMS cathode produced a maximum power density of 1320 ± 70 mW m−2 (Fig. 4), but the water pressure tolerance was less than 0.4 m, probably due to cracks in the surface as these were noticeable as shown in Fig. 5. Water pressure tolerance was increased from 0.21 ± 0.02 m to 0.93 ± 0.02 m by using more PTFE (from 200 μL to 800 μL), but the maximum power density decreased from 1200 ± 100 mW m−2 (WP-PTFE 200) to 730 ± 60 mW m−2 (WP-PTFE 800) (Fig. 4). Heat treatment (at 340 °C) is typically used to melt the PTFE diffusion layer and create a porous but more watertight surface, but this could not be done here as heating would have destroyed the wipe. Gore-Tex cathodes had a water pressure tolerance of 1.28 ± 0.04 m, but it was noticed that the Gore-Tex fabric separated from the AC catalyst layer after the water head reached ∼0.5 m.
All PTFE-based diffusion layers without a wipe achieved a relatively high water pressure tolerance (>1.5 m) (Fig. 4). The Pt cathode with PTFE diffusion layer tolerated a relatively high water pressure of 1.68 ± 0.04 m. Cathodes made without a wipe, but made using an additional stainless steel mesh layer, CB and PTFE, had both a strong and porous diffusion layer (Fig. 5). The water head reached the maximum height tested here of 2.4 m and still did not show any visible leakage. Thus the conductive CB + PTFE diffusion layer was the most promising approach used here to make the diffusion layer, as this cathode had both the highest maximum power density and the highest water tolerance.
4. Conclusions
Diffusion layer characteristics significantly affected cathode performance in both abiotic electrochemical tests and in MFCs. The conductive diffusion layer (CB + PTFE) for AC cathodes had the best performance, with the highest current densities in the abiotic chronoamperometry tests, the highest maximum power densities (1610 ± 90 mW m−2) in the MFC polarization tests, and the highest water pressure tolerance (>2 m). Wipe-based diffusion layers had low water pressure tolerance (in most cases, <0.4 m), and thus they are useful for small-scale tests but not for larger-scale systems. A porous cathode structure resulted in a higher oxygen mass transfer coefficient that increased power production. When the oxygen mass transfer coefficients were >0.6 × 10−3 cm s−1, all these cathodes generated maximum power densities larger than 1100 mW m−2. Enhancing the conductivity of the diffusion layer by adding CB and a stainless steel mesh was also helpful for producing better cathode performance.Acknowledgements
The authors thank David Jones for laboratory support, John J. Cantolina for help with SEMs, and Dr. Deepak Pant for providing the porous PTFE diffusion layer AC cathodes. This research was supported by the Strategic Environmental Research and Development Program (SERDP), Award KUS-I1-003-13 from the King Abdullah University of Science and Technology (KAUST), National Natural Science Foundation of China (Grant No. 51408336) and the International Program of MOST (No. 2013DFG92240).References
- W.-W. Li, H.-Q. Yu and Z. He, Energy Environ. Sci., 2014, 7, 911–924 CAS.
- B. E. Logan and K. Rabaey, Science, 2012, 337, 686–690 CrossRef CAS PubMed.
- D. R. Lovley, Curr. Opin. Biotechnol., 2008, 19, 564–571 CrossRef CAS.
- K. Rabaey and W. Verstraete, Trends Biotechnol., 2005, 23, 291–298 CrossRef CAS PubMed.
- B. E. Logan, M. J. Wallack, K.-Y. Kim, W. He, Y. Feng and P. E. Saikaly, Environ. Sci. Technol. Lett., 2015, 2, 206–214 CrossRef CAS.
- F. Harnisch and U. Schröder, Chem. Soc. Rev., 2010, 39, 4433–4448 RSC.
- H. Wang and Z. J. Ren, Biotechnol. Adv., 2013, 31, 1796–1807 CrossRef CAS.
- S. Cheng, H. Liu and B. E. Logan, Electrochem. Commun., 2006, 8, 489–494 CrossRef CAS.
- X. Zhang, H. Sun, P. Liang, X. Huang, X. Chen and B. E. Logan, Biosens. Bioelectron., 2011, 30, 267–271 CrossRef CAS PubMed.
- Y. Chen, Z. Lv, J. Xu, D. Peng, Y. Liu, J. Chen, X. Sun, C. Feng and C. Wei, J. Power Sources, 2012, 201, 136–141 CrossRef CAS.
- J. M. Morris, S. Jin, J. Wang, C. Zhu and M. A. Urynowicz, Electrochem. Commun., 2007, 9, 1730–1734 CrossRef CAS.
- X. Shi, Y. Feng, X. Wang, H. Lee, J. Liu, Y. Qu, W. He, S. M. S. Kumar and N. Ren, Bioresour. Technol., 2012, 108, 89–93 CrossRef CAS PubMed.
- Y. Yuan, S. Zhou and L. Zhuang, J. Power Sources, 2010, 195, 3490–3493 CrossRef CAS.
- F. Zhang, S. Cheng, D. Pant, G. V. Bogaert and B. E. Logan, Electrochem. Commun., 2009, 11, 2177–2179 CrossRef CAS.
- F. Zhao, F. Harnisch, U. Schröder, F. Scholz, P. Bogdanoff and I. Herrmann, Electrochem. Commun., 2005, 7, 1405–1410 CrossRef CAS.
- B. Erable, N. Duteanu, S. M. S. Kumar, Y. Feng, M. M. Ghangrekar and K. Scott, Electrochem. Commun., 2009, 11, 1547–1549 CrossRef CAS.
- Y. Fan, E. Sharbrough and H. Liu, Environ. Sci. Technol., 2008, 42, 8101–8107 CrossRef CAS PubMed.
- F. Zhao, F. Harnisch, U. Schröder, F. Scholz, P. Bogdanoff and I. Herrmann, Environ. Sci. Technol., 2006, 40, 5193–5199 CrossRef CAS PubMed.
- H. Dong, H. Yu and X. Wang, Environ. Sci. Technol., 2012, 46, 13009–13015 CrossRef CAS PubMed.
- H. Dong, H. Yu, X. Wang, Q. Zhou and J. Feng, Water Res., 2012, 46, 5777–5787 CrossRef CAS PubMed.
- B. Wei, J. C. Tokash, G. Chen, M. A. Hickner and B. E. Logan, RSC Adv., 2012, 2, 12751–12758 RSC.
- X. Zhang, J. Shi, P. Liang, J. Wei, X. Huang, C. Zhang and B. E. Logan, Bioresour. Technol., 2013, 142, 109–114 CrossRef CAS PubMed.
- X. Wang, N. Gao, Q. Zhou, H. Dong, H. Yu and Y. Feng, Bioresour. Technol., 2013, 144, 632–636 CrossRef CAS PubMed.
- V. J. Watson, C. N. Delgado and B. E. Logan, Environ. Sci. Technol., 2013, 47, 6704–6710 CAS.
- X. Zhang, X. Xia, I. Ivanov, X. Huang and B. E. Logan, Environ. Sci. Technol., 2014, 48, 2075–2081 CrossRef CAS PubMed.
- B. Zhang, Z. Wen, S. Ci, S. Mao, J. Chen and Z. He, ACS Appl. Mater. Interfaces, 2014, 6, 7464–7470 CAS.
- X. Zhang, D. Pant, F. Zhang, J. Liu, W. He and B. E. Logan, ChemElectroChem, 2014, 1, 1859–1866 CrossRef CAS.
- Y. Luo, F. Zhang, B. Wei, G. Liu, R. Zhang and B. E. Logan, Biochem. Eng. J., 2013, 73, 49–52 CrossRef CAS.
- W. He, J. Liu, D. Li, H. Wang, Y. Qu, X. Wang and Y. Feng, J. Power Sources, 2014, 267, 219–226 CrossRef CAS.
- D. Pant, G. Van Bogaert, C. Porto-Carrero, L. Diels and K. Vanbroekhoven, Water Sci. Technol., 2011, 63, 2457–2461 CrossRef CAS.
- W. Yang, F. Zhang, W. He, J. Liu, M. A. Hickner and B. E. Logan, J. Power Sources, 2014, 269, 379–384 CrossRef CAS.
- F. Zhang, T. Saito, S. Cheng, M. A. Hickner and B. E. Logan, Environ. Sci. Technol., 2010, 44, 1490–1495 CrossRef CAS.
- B. E. Logan, S. Cheng, V. Watson and G. Estadt, Environ. Sci. Technol., 2007, 41, 3341–3346 CrossRef CAS PubMed.
- D. R. Lovley and E. J. P. Phillips, Appl. Environ. Microbiol., 1988, 54, 1472–1480 CAS.
- B. E. Logan, B. Hamelers, R. Rozendal, U. Schröder, J. Keller, S. Freguia, P. Aelterman, W. Verstraete and K. Rabaey, Environ. Sci. Technol., 2006, 40, 5181–5192 CrossRef CAS PubMed.
- X. Zhang, S. Cheng, X. Wang, X. Huang and B. E. Logan, Environ. Sci. Technol., 2009, 43, 8456–8461 CrossRef CAS PubMed.
- A. Arvay, E. Yli-Rantala, C. H. Liu, X. H. Peng, P. Koski, L. Cindrella, P. Kauranen, P. M. Wilde and A. M. Kannan, J. Power Sources, 2012, 213, 317–337 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |