Membrane materials for water purification: design, development, and application
Anna
Lee
*a,
Jeffrey W.
Elam
b and
Seth B.
Darling
*ac
aCenter for Nanoscale Materials, Argonne National Laboratory, 9700 South Cass Avenue, Lemont, Illinois 60439, USA. E-mail: leea@anl.gov, darling@anl.gov
bEnergy Systems Division, Argonne National Laboratory, 9700 South Cass Avenue, Lemont, Illinois 60439, USA
cInstitute for Molecular Engineering, University of Chicago, 5801 South Ellis Avenue, Chicago, Illinois 60637, USA
First published on 9th September 2015
Abstract
Water purification for human use, ecosystem management, agriculture, and industry is emerging as a leading global priority. Access to sufficient clean water ultimately requires improvements over the current state of water filtration technology. Membrane technologies for water purification have been actively pursued for decades, but with recent innovation of both analytical and fabrication tools, more advanced membrane technologies are surfacing. Here, we review the design, development, and application of new membrane materials, fabrication methods for controlling the filtration size regime, analytical tools for performance testing, and molecular modeling for transport and separation. Membrane chemical stability, fouling, and environmental impact as open questions are also presented.
Water impactPurifying water using today's technology is expensive and energy-intensive; there is a pressing need for new research to identify novel approaches to purify water at lower cost, using less energy, and—importantly—minimizing environmental impact. Membrane technologies, in particular, have proven viable in water purification with decades of productive use. Membrane processes have distinct advantages, including high water quality with easy maintenance, stationary parts with compact modular construction, low chemical sludge effluent, and excellent separation efficiency. With recent innovation of both analytical and fabrication tools, more advanced membrane technologies are surfacing in a multitude of water purification applications. |
1. Introduction
Increasing demand for and shortage of clean water as a result of rapid urbanization, population growth, misuse, and climate disruption have become unprecedented urgent global issues. Nearly two in ten people in the world do not have access to safe drinking water, and according to the World Health Organization, 3900 children die every day due to diseases communicated by unsafe water or poor hygiene. According to the U.N. World Water Development Report, this troubling predicament is projected to worsen substantially by 2050, when at least a quarter of the people on Earth will live in a country suffering from chronic or recurring freshwater shortages.1 Statistics such as these are likely to worsen further as water contamination from waterborne pathogens and discharge of pollutants (e.g. heavy metals, arsenic, pharmaceutical derivatives, agricultural chemicals, endocrine disrupters) increases.2 Beyond direct human consumption and use, water for agriculture and irrigation accounts for approximately 70% of fresh water use globally, reaching as much as 90% in some industrialized nations. Ever-increasing water scarcity directly threatens an already strained global food supply. Moreover, compounding existing geopolitical pressures, lack of access to water can foster regional conflicts that threaten global peace and stability.3Our planet does not have a water shortage. The world's oceans are vast, and bodies of fresh water are essentially as voluminous as they have been throughout human history. We as a society are contaminating and misusing our precious fresh water resources. Purifying water using today's technology is expensive and energy-intensive; there is a pressing need for new research to identify novel approaches to purify water at lower cost, using less energy, and—importantly—minimizing the impact on the environment. Membrane technologies, in particular, have proven viable in water purification with decades of productive use. Membrane processes have distinct advantages, including high water quality with easy maintenance, stationary parts with compact modular construction, low chemical sludge effluent, and excellent separation efficiency. With recent innovation of both analytical and fabrication tools, more advanced membrane technologies are surfacing in a multitude of water purification applications.
In this review, recent progress in membrane science and technologies for water purification is presented. Topics include design, development, and application of water filters with a specific emphasis on emerging new membrane materials. To provide sufficient background for readers, we further discuss fabrication methods for controlling the filtration size regime, analytical tools for performance testing, and molecular modeling for transport and separation. Membrane chemical stability, fouling, and environmental impact as open questions are also presented. Desalination and related technologies including reverse osmosis and thermal distillation, though of central importance in addressing water scarcity, are beyond the scope of this review; readers are directed to several excellent reviews on this topic in the literature.4–9
2. Classes of membranes
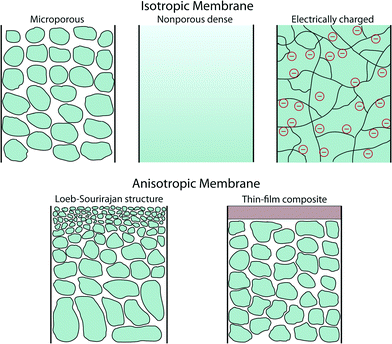
A membrane is a thin physical interface that moderates certain species to pass through depending on their physical and/or chemical properties. In general, there are two classes of membranes (often defined as cross-section): isotropic and anisotropic membranes as shown in Fig. 1.Isotropic membranes are chemically homogenous in composition. Examples include microporous membranes, nonporous dense films, and electrically charged membranes.10 Porous membranes generally separate solutes based on the size of particulate and the size of pore. Microporous membranes are similar to a conventional filter, but the pore diameter typically ranges from 0.1–5 μm (conventional filters are used for particles larger than 1–10 μm in size, so their pore diameter is typically larger than 5 μm). Microporous membranes are often polymer-based track-etched (nonporous polymeric films are irradiated with heavy ions to form tracks through the film), phase inversion (controlled transformation from a homogeneous polymeric solution to a solid state induced by immersion precipitation), or stretched polymer films (solvent-free technique whereby polymers are heated above the melting point and extruded into thin films followed by stretching techniques) (Fig. 2).11 In the case of nonporous dense films, transport of permeants is due to diffusion driven by an applied force such as pressure, concentration, or electric-field gradients. Therefore, the separation of solutes is governed by their relative transport rates. Electrically charged membranes can be either nonporous dense films or microporous structures consisting of positively or negatively charged ions decorated on the membrane walls (known as anion-exchange or cation-exchange membranes, respectively). Separation of solutes is primary achieved by analyte ion concentration and charge exclusion (i.e., solute with the same charge as the ions on the membrane walls is rejected by Coulombic repulsion).10
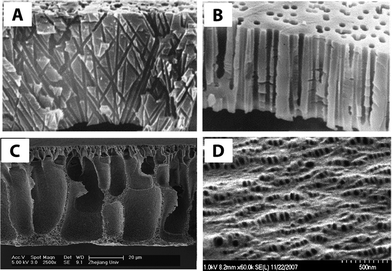 | ||
| Fig. 2 Cross-sectional SEM images of (A) track-etched cylindrical, non-parallel pore channels of polycarbonate and (B) polypropylene track-etched membrane with slightly conical parallel pores. Reprinted from ref. 12 Copyright (2001), with permission from Elsevier. (C) Polyester-g-methoxyl polyethylene glycol (HPE-g-MPEG) blended with poly vinylidene fluoride (PVDF) membrane via phase inversion process. Reprinted from ref. 13 Copyright (2008), with permission from Elsevier. (D) SEM image of the surface of two polypropylenes (PP28 and PP08) having 10 wt% PP08 and both cold and hot stretched. Reprinted from ref. 14 Copyright (2008), with permission from Elsevier. | ||
There are two main types of anisotropic membranes: phase-separation membranes (Loeb–Sourirajan membranes) and composite membranes such as thin-film, coated films, and self-assembled structures. Loeb–Sourirajan membranes are homogenous in chemical composition similar to isotropic microporous membranes, but pore sizes and porosity (cf. 5.2) vary across the membrane thickness. Development of such anisotropic membranes in the early 1960s represented a major breakthrough in the field of membrane technology.15 Composite membranes such as thin-film membranes are chemically and structurally heterogeneous. A thin surface layer is supported by a much thicker porous structure (functioning as a mechanical support), and these structures are traditionally made of different polymeric materials. This class of membranes, prepared by methods such as interfacial polymerization, solution coating, and plasma polymerization, have been established for various filtration processes as shown in cf. 3. The separation of solutes and permeation rates of the membrane are exclusively determined by the thin surface layer, which results in a high flux. Typical polymeric materials for industrially established filtration membranes include cellulose acetates, polyacrylonitrile, polyetherimides, polyethersulfones, polyamide, polycarbonates, cross-linked polyether, polypropylene, and polyvinylidenefluoride (see more details on polymeric membranes in cf. 4.2 and other materials in cf. 4).16
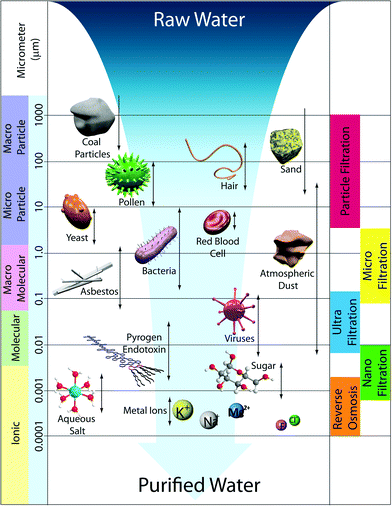
3. Membrane size regimes
Water treatment processes employ several types of membranes based on their pore sizes: reverse osmosis (RO), nanofiltration (NF), ultrafiltration (UF), microfiltration (MF), and particle filtration. Fig. 3 summarizes various membrane filtration processes relative to common materials that would be filtered out through each process.UF and MF follow a similar process in that the mode of separation is particle sieving through membrane pores. MF membranes have larger pore sizes (approx. 0.1–5 μm) and typically reject particles, asbestos, and various cellular materials such as red blood cells and bacteria from 0.1–10 μm in diameter. UF membranes have smaller pores (approx. 0.01–0.1 μm) than MF membranes such that, in addition to filtering out large particles and microorganisms, they can filter dissolved bio-macromolecules, such as pyrogens, proteins, and viruses (sizes ranging from 0.01–0.2 μm). UF has various applications in wastewater treatment, water remediation, recovery of surfactants in industrial cleaning, food processing, protein separation, gene engineering, and beyond.17 Commonly, particle sizes are characterized by their molecular weight cut-off (MWCO), a concept introduced by Amicon Corporation in the 1960s. MWCO value is defined by rejection of organic solutes (90% rejection by the membrane), and the particle retention is evaluated by converting MWCO to the membrane pore size.18Table 1 shows the relation between MWCO and pore sizes for UF processes. A general guideline for designing UF membranes is that the MWCO must be about half of the lowest molecular weight species to be retained. As a more direct, quantitative means, monodisperse nanoparticles were also recently used to determine pore size distributions of a variety of UF membranes.19
| MWCO [Daltons] | [nm] |
|---|---|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100 |
500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
20 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
10 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.0 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.5 |
| 5000 | 1.5 |
In general, UF membranes are fashioned in an anisotropic Loeb–Sourirajan structure. The first UF membranes were made of nitro cellulose in the early 1900s.20 A key breakthrough in this field was the development of anisotropic membranes made of cellulose acetate in the 1960s, and UF application of these membranes was realized in the 1970s.15 Cellulose derivatives, inorganic materials (TiO2, Al2O3, ZrO3, etc.), and various polymeric species (polyacrylonitrile (PAN), polysulfone amides (PSA), polyether sulfone (PES), polyvinylidene fluoride (PVDF), etc.) are typical materials for UF; the advancement of implementing these membrane materials will be discussed in later sections (cf. 4).
NF membranes exhibit performance between RO and UF membranes. NF membranes are porous and can filter species ranging from 0.001–0.01 μm in size. This includes most organic molecules, viruses, and a range of salts. Further, NF membranes can reject divalent ions, so NF is often used to soften hard water.21
In contrast to NF, UF, and MF membranes, RO membranes are so dense that the “pores” are considered as non-porous (approx. 0.0001–0.001 μm), and they are within the range of thermal motion of the polymer chains that form the membrane. Therefore, RO membranes can even filter low-molecular-weight species such as aqueous inorganic solids (including salt ions, minerals, and metal ions) and organic molecules. The accepted mechanism of transport by RO is via diffusion through statistically distributed free volume areas. Solutes pass through the membrane by dissolving in the membrane material and diffusing down a concentration gradient under applied pressure exerted in excess of osmotic pressure. Separation occurs because of the difference in solubilities and mobilities of different solutes within the membrane. The most common applications of RO are desalination of brackish groundwater or seawater and the production of potable water. This is the subject of several useful reviews in the literature.5,22–26
4. Membrane materials and processes
The following factors should be considered in order to design effective membranes: choice of membrane materials, high water flux, high solute rejection, module configuration, mechanical/chemical/thermal/temporal stability, system design including processibility at large scale, and operating conditions for cost-effectiveness. The performance of a membrane is mainly governed by the structure of its pores and the physical/chemical properties of the material. Intensive effort has been invested both in exploring new membrane materials/processes and in modifying traditionally used materials. Most commonly used commercial NF, UF, and MF membrane materials are synthetic polymers (such as polyvinylidene fluoride, polysulfone, polyacrylonitrile and poly(acrylonitrile)-poly(vinyl chloride) copolymers).16UF and MF membranes are often prepared from the same materials, but preparation methods may be different to produce various pore sizes. Membranes can also be made from inorganic materials such as ceramics or zeolites. However, large-scale applications of these inorganic materials are limited due to high operation cost and inherent mechanical fragility thus far. In the following sections, we survey recently developed membrane materials, their characteristics, synthesis/fabrication, and relevant analytical methods. Details of traditional membrane formation and operation processes can be found in the literature.10,27–29
4.1. Inorganic membranes
Inorganic membranes have recently received considerable attention due to their relative thermal, chemical, and mechanical robustness as well as their reusability and often photocatalytic ability. In many wastewater treatment applications, for example, ceramic membranes exhibit greater fouling-resistance and chemical stability than current polymeric membranes. Materials developed recently are typically in a nanocrystalline form, and these membranes include porous ceramics (e.g., Al2O3, TiO2, ZrO2, ZnO, and SiO2,),30 composites containing two or more materials (e.g., TiO2–SiO2, TiO2–ZrO2, and Al2O3–SiC), and various nanoparticle composites (e.g., Ag–TiO2, Zn–CeO2, and zeolites).31,32In the case of ceramic membranes, photocatalytic materials such as TiO2 and composites containing TiO2 have been actively studied due to their multi-functionality and wide applications including remediation of ground water and wastewater.3,33–36 Along with a separation function, TiO2 offers photocatalytic ability for decomposition of organic species/microorganisms/pollutants, photolysis, and superhydrophilicity, which reduces unwanted adsorption of organic/biological species to the membrane surface.37–41 Also, TiO2 is a non-toxic, readily available, and inexpensive material.
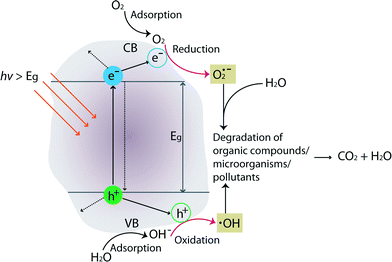
The mechanism of TiO2 photocatalysis is based on photo-induced charge separation on the surface of the oxide. Excellent reviews on TiO2 photocatalysis can be found in these ref. 42–45. Briefly, when incoming photon energy (hν) is greater than or equal to the band-gap energy (Eg) of TiO2 (3.0 eV and 3.2 eV for rutile and anatase crystal structures, respectively), an electron (e−) will be photoexcited to the conduction band (CB) of TiO2 leaving an empty unfilled valence band (VB), resulting in the formation of an electron–hole pair (e−–h+). Fig. 4 schematically illustrates degradation of organic compounds, microorganisms, or pollutants by formation of photo-induced charge carriers (e−/h+) on the surface of TiO2 upon hν irradiation.
A series of oxidative/reductive reactions take place on the surface. Specifically, photo-induced electrons in the CB typically reductively react with adsorbed O2 in air to produce superoxide radicals (O2˙), which are unstable. Similarly, the photo-induced holes in the VB diffuse to the surface and likely react with adsorbed water and hydroxyl ions to form hydroxyl radicals (OH˙), which are strong oxidizing agents. These species (O2˙ and OH˙) react with organics/microorganisms/pollutants adsorbed on the surface of TiO2 resulting in hydroxylation, oxidation, and finally mineralization to carbon dioxide and water. In general, photocatalytic activities are governed by various factors such as pH, oxidizing agents, amount of catalyst/surface coverage, calcination temperature/crystal structures, doping level/content, and composition of the membrane materials.
Pioneering work demonstrating separation function along with photocatalytic activity was published in 2006 and involved titania prepared via a sol–gel dip-coating method.46 Since then, the sol–gel method is the most studied technique due to its versatility and simplicity.33 TiO2 is prepared by hydrolysis of TiO2 precursors (e.g., tetra-n-butyl titanate or tetra-isopropoxide titanium) to form a sol. The TiO2 sol is often used to decorate the surface of commercially available porous membranes such as Al2O3 or ZrO2 through dip-coating.47,48 In addition to the sol–gel technique, various synthetic fabrication methods have been developed: liquid phase deposition,49 hydrothermal synthesis for freestanding or grafted TiO2 nanowires,50,51 anodization for nanotube formation from Ti foil,52 chemical vapor deposition, and sputter methods (Fig. 5).3,53,54
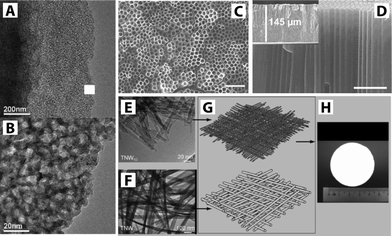 | ||
| Fig. 5 (A–B) TEM images of nanostructured TiO2via sol–gel method. Reprinted from ref. 47 Copyright (2007), with permission from Elsevier. (C–D) SEM images of TiO2 nanotubes via anodization showing top and cross-sectional views, respectively. The scale bar corresponds to 1 μm. Reprinted with permission from ref. 52 Copyright (2007) American Chemical Society. (E–H) TEM images showing components of a hierarchical layer of a TiO2 nanowire membrane. TNW10 and TNW20 refer to TiO2 nanowires with 10 nm and 20 nm diameters. The TNW10 layer serves as the functional layer while the TNW20 layer is laid as the supporting layer shown in schematic (G), and photograph of the TiO2 nanowire membrane is shown in (H). Reprinted with permission from ref. 50. Copyright (2009) John Wiley and Sons. | ||
Two of the most extensively studied application areas of photocatalytic materials are disinfection (E. coli bacteria is the most common model system studied as shown in Fig. 6A–D).50,55,56 and removal of targeted organic pollutants (e.g., methyl orange, methylene blue, Rhodamine B, humic acid, phenol, aniline, benzylamine).57–61
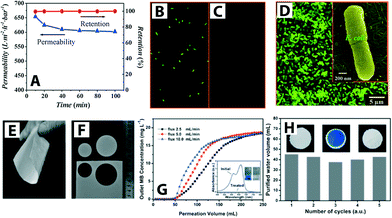 | ||
| Fig. 6 (A) Titanate nanotube membrane (TNM) performance with a permeability of 608 L m−2 h−1 at 1 bar (initial concentration of E. coli ∼4 × 106 CFU mL−1). (B) and (C) Fluorescent microscopic images of E. coli feed (shown in green) and permeate, respectively. Complete E. coli removal (i.e., 100% retention) was observed (D) SEM image of retained E. coli on the TNM after filtration (low magnification) and inset shows a high magnification SEM image. Reprinted from ref. 55 Copyright (2009), with permission from Elsevier. (E–F) Optical images of SiO2–TiO2 composite porous nanofibrous membrane (STPNM-6) showing flexibility and ease of cutting. (G) Breakthrough curves for permeation of methylene blue (MB) solutions through the STPNM-6 with various fluxes. UV-vis spectra of the MB solution and purified water are shown with the corresponding digital photographs in inset (H) cyclic test of STPNM-6 with a flux of 5 mL h−1. Optical images of STPNM-6 before use (left), used (middle) and after calcination (right), respectively, are shown in insets. Reproduced from ref. 62 with permission from The Royal Society of Chemistry. | ||
The efficiency of the photocatalytic membrane system is measured by the degradation rate of the targeted pollutants and the membrane flux. TiO2 nanoparticles deposited on the surface of an Al2O3 membrane by a layer-by-layer chemical vapor deposition method showed efficient photocatalytic degradation of azo-dye pollutant and high water permeability in a continuous flow process.53 A TiO2–SiO2 composite membrane (Al2O3 as a substrate; TiO2 sol as an intermediate layer; TiO2–SiO2 as a membrane top layer) exhibited enhanced pollutant removal efficiency by coupling the separation and photocatalytic activity; removal efficiency was improved up to 94% compared to 63% and 60% for independent photocatalysis and separation processes, respectively (methyl orange dye removal efficiency was determined after 60 min of UV irradiation).63 Moreover, the common concern of fragility associated with inorganic ceramic membranes can be overcome. Wen et al. reported flexible TiO2–SiO2 composite nanofibrous membranes obtained via electrospinning.62 The nanofibrous composite membrane is flexible, can be easily cut, and also showed fast water-spreading (i.e., hydrophilicity) (Fig. 6E–H).
Photocatalytic activity of TiO2 membranes with various morphologies can generally only be activated by irradiation with UV light. Sunlight, however, contains only 2–3% UV light, and strong UV irradiation of a few W cm−2 is required in order to induce sufficient charge carriers. Further, typical interior room light contains UV light intensity of only a few μW cm−2 thereby necessitating external UV power in order to achieve usable activity. Exploitation of solar energy instead of UV light sources would dramatically reduce energy consumption and facilitate off-grid applications.
In order to have more efficient utilization of photocatalytically driven devices including membranes, recently many studies have been conducted to increase the sensitivity of the devices by broadening the solar absorption bands into the visible-light range.64 Typically, absorption shifting can be achieved by doping TiO2 with metal (e.g., Fe, Cr, Co, Mn, V, Mo), nonmetal (e.g., N, F, S, B, or various carbon materials), or introducing more than one dopant (e.g., N-doped TiO2 can be further modified with various metal species).65 An overview of underlying mechanisms, design, and development of visible-light-active photocatalysts used in many different fields including environmental applications is provided in previous reviews.59,64–69 Recently, a few examples of visible-light-active membrane studies have been reported. Core (TiO2)–shell (carbonaceous-type) nanoparticles (visible light absorption up to 2.19 eV) synthesized via a sol–gel dip-coating method on Al2O3 membranes were incorporated in a water purification photocatalytic reactor shown in Fig. 7A.70
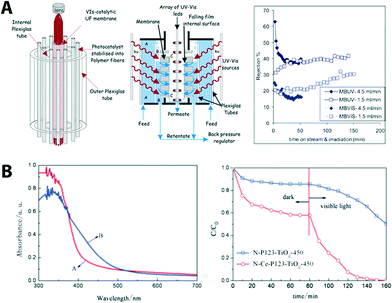 | ||
| Fig. 7 (A) Schematic of the photocatalytic membrane reactor cell showing its basic components (left), cross section of the membrane unit cell indicating the flow paths of the polluted water stream (middle), and the membrane performance (right). Reprinted from ref. 70 Copyright (2014), with permission from Elsevier. (B) UV-VIS absorption spectra of N, C co-doping (red, N-P123- TiO2-450) and N, C, Ce co-doped TiO2 (blue, N-Ce-P123 TiO2-450). Photo-induced degradation of methyl orange under visible light is shown (right). Reproduced from ref. 71 with permission from The Royal Society of Chemistry. | ||
The reactor was operated in a continuous-flow filtration mode (cf. 5.1 for classification of filtration mode) and was tested for photocatalytic degradation of azo-dyes (methyl orange and methylene blue in this case). Results showed an increase of water permeability. This improvement was attributed to the photo-induced hydrophilicity effect of the membrane under solar illumination serving as the only energy input without significant fouling problems. Recently, carbon-based nanostructured materials like graphene oxide received much attention due to their large surface area, flexible structure, excellent charge-carrier mobility, and good electrical and thermal conductivities72,73 (see more examples in later section). Graphene-containing composite materials (graphene/TiO2) also have been recently developed as photocatalysts for the treatment of pollutants and prevention of microorganisms in water and air.74 An UF composite membrane containing graphene oxide sheets decorated with TiO2 that is deposited into UF mono-channel monoliths using a dip-coating method was developed for photocatalytic/UF water treatment.75 The composite membrane showed photocatalytic activity (removal of organic dye) under UV as well as visible light due to the charge-transfer effect as the graphitic surface is coupled to the surface of the photocatalyst. C, N, and Ce co-doped TiO2 membranes (cut-off molecular weight of 3300 Da with narrowing the bandgap from 2.14 eV from 2.65 eV) via a sol–gel method showed an improved photocatalytic activity under visible light (Fig. 6B).71 Improved photocatlytic activity is due to co-doping of nitrogen and carbon resulting in narrowing of the band gap of TiO2 and, therefore, enhanced visible light absorption; moreover, cerium doping provided electron trap sites for the reaction.
Multifunctional photocatalytic membranes have shown promise in addressing biofouling, which is one of the major hurdles in both water purification research and the water treatment industry. For example, mixed-phase particles consisting of both rutile and anatase TiO2-coated ZrO2 ceramic membrane discs (47 mm in diameter, 2.5 mm in thickness, supporting layer consisting of Al2O3–TiO2–ZrO2, ZrO2 being an active layer with 0.01 μm pore diameter with a water flow rate of 450–600 L h−1 m−2 at 1 bar) showed a reduction of bacterial cell (Pseudomonas putida) attachment (as compared to a control system – no TiO2 coating and dark), an initial stage of biofilm formation, and increase of cell kill on the membrane surface.77 Building from this, the same group studied the effect of fouling caused by biofilm formation over a 10 day period. As shown in Fig. 8A and B, mixed-phase TiO2 particles coated on UF ZrO2 ceramic membranes exhibit biofouling resistance and, therefore, suffer less from water-flux decline as compared to a control system (bare ceramic membranes).76
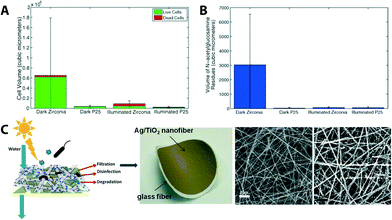 | ||
| Fig. 8 (A) Comparison of live (green) and dead (red) cell volume in biofilms formed on zirconia (ZrO2) ceramic membranes after 10 days of exposure to cell culture. P25 is a sample name for mixed-phased TiO2 particles coated on ZrO2 membranes. (B) Average flux decline of membranes after 10 days of biofilm growth. Reprinted from ref. 76 Copyright (2014), with permission from Elsevier. (C) Scheme and photograph of a multifunctional (filtration and solar photocatalytic disinfection/degradation) Ag/TiO2 nanofiber membrane. SEM images of bareTiO2 fibers and Ag NP decorated TiO2 fibers. Reprinted from ref. 56 Copyright (2012), with permission from Elsevier. | ||
Aside from the most commonly used commercial ceramic materials (TiO2, ZrO2, and Al2O3; these membranes are mainly used in MF and UF processes), recent studies were carried out using a SiC membrane support. Examples include coating, drying, and calcination of a boehmite sol to produce γ-Al2O3 nanocrystals onto SiC membranes showing a reduction of defect density on the SiC membrane surface78 and one-step production of SiC membranes by pyrolysis of allylhydrido polycarbosilane in the presence of α-SiC particles to address chemical stability and cost issues associated with SiC UF membranes.79
With the advancement of nanoscience and technology, applications of inorganic nanoparticles (NPs) for water purification and remediation have progressed rapidly.80–84 Various NPs such as gold, silver, copper, and core–shell nanocomposites offer preferential adsorption to heavy metals (e.g., arsenic, mercury, lead, chromium, and cadmium), disinfection (e.g., biological toxins including waterborne pathogens),83,85–89 and degradation of pollutants as seen with the photocatalytic membranes discussed above. Typical NPs are metal oxide species such as magnesium oxide (MgO), iron oxides (Fe3O4), aluminum oxide (Al2O3), and titanium oxide (TiO2).31,32,86,90 Silver NPs have been frequently used in membrane development (generally decorated on membrane surfaces cf.Fig. 14) because of their antibacterial properties and their ability to reduce bio-film adhesion.91–93 Ag NPs encapsulated in positively charged polyethyleneimine on a UF membrane surface94 and Ag/TiO2 nanofiber membranes (Fig. 8C) showed strong antimicrobial activity (Ag NPs on the surface of TiO2 showed 99.9% E. coli bacteria inactivation and 80.0% dye degradation under solar irradiation within 30 min).56 Also, Ag NP stacks achieved through a layer-by-layer dip-coating process showed comparable permeability and separation performance to commercially available UF membranes.95 Examples of other NPs used in membrane technology are shown in later sections.
Graphene-based materials are one of the most recent material developments in the field.96,97 Among this class of materials, graphene oxide (GO) is the most common. GO nanosheets are hydrophilic due to the presence of oxygen-containing functional groups (e.g., hydroxyl, carboxyl, carbonyl, and epoxy groups). Two-dimensional GO sheets offer mechanical stability, tunable physicochemical properties, and well-defined nanometer-scale pores, making them promising for water filtration applications (especially for NF and desalination).98,99 When layered in a membrane (Fig. 9),100 a permeation channel network is formed for water to migrate between the sheets. This tortuous path travels preferentially over nonoxidized (hydrophobic) surfaces that exhibit virtually no friction for the water molecules as opposed to the hydrophilic oxidized regions. Ultrafast ion permeation (≈10−3 cm2 s−1 for 1 M solution as compare to ≈10−5 cm2 s−1 for a typical diffusion coefficient of ions in water) through a micrometer-thick GO membrane has recently been reported.101 Dip coating provides a simple fabrication method for GO composite membranes on a silane-modified ceramic support.102 Silane modification on ceramic supports (Al2O3) resulted in better adhesion of GO membranes, which are hydrophilic (desirable as it reduces the adsorption of undesired organic/biological molecules on the membrane surfaces and also enhances water permeability). The separation of water from ethanol/water mixtures by pervaporation showed a water concentration enhancement (from 5 wt% to 39.92 wt% at 40 °C), which indicates promise for small-molecule separation. Another property of GO nanosheets is electro- and magneto-controlled ion transport (e.g., KCl, MgCl2, CaCl2 and FeCl3).103 It was observed that the applied electric field can influence ion migration whereas magnetic fields altered the structure of nanocapillaries in GO membranes. Also, electric fields can be used to control the selectivity of ions toward the GO membrane while magnetic fields enhance the ion transport.
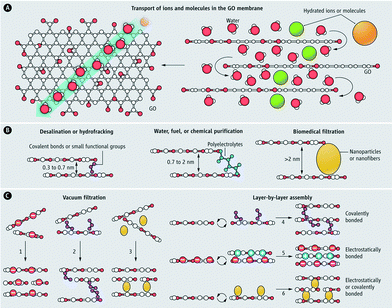 | ||
| Fig. 9 Graphene oxide (GO) membrane (A) water, ions, and molecules, which are smaller than the void spacing between stacked GO nanosheets permeate superfast in the GO membrane (B) nanochannel size can be tuned for different separation applications (C) various methods for the synthesis of GO membranes. Reprinted from ref. 100 Copyright (2014), with permission from The American Association for the Advancement of Science. | ||
Another carbon-based nanomaterial recently adopted in membrane technology is carbon nanotubes (CNTs). Along with removal of biological impurities (e.g., bacterial pathogens and viruses), CNT-based membranes offer cost-effectiveness, robustness, size exclusion, and reduction of biofouling.104–106 Baek et al. recently demonstrated fast water transport using a vertically aligned CNT membrane (4.8 nm of pore diameter) also featuring antimicrobial properties.107
It should be noted that GO, CNT-based membranes as well as boron nitride nanotubes have been actively studied, especially in the field of desalination, which is beyond the scope of this review. Readers interested in this topic should refer to these ref. 96, 106, 108–111. Table 2 summarizes some of the most recent development of inorganic membranes.
| Membrane material | Performance (L (m2 h bar)−1) | Key features |
|---|---|---|
| Microfiltration | ||
| TiO2 nanowire | TiO2 nanowire growth via hydrothermal processing photocatalytic under UV degradation of pharmaceuticals51 | |
| Al2O3/ZrO2 | 118–1698 (UF-MF) | Conformal, thickness-controllable coating and pore size reducing via ALD112 |
| Ultrafiltration | ||
| TiO2 | Max 20 | Photocatalysis decomposition of azo-dye pollutant under UV irradiation53 |
| SiC–SiC | 0.06 | Free and uniform membranes in a single coating procedure nearly defect-free SiC (ref. 79) |
| Ag NPs | 9.5 m3 (m2 day atm)−1 | Ag NP deposition via layer-by-layer method95 |
| Ag/TiO2 nanofiber | 5–20 at 1–4 bar | 99.9% bacteria inactivation and 80.0% dye degradation under solar irradiation within 30 min (ref. 56) |
| TiO2 | 4.05 | Visible-light responsive C, N and Ce co-doped TiO2 (ref. 71) |
| TiO2 nanowire | 12.2 | Anti-fouling, anti-bacterial, concurrent separation, and photocatalytic oxidation50 |
| Al2O3/SiC | 10–3000 at 10 bar | Reduction of defect density on the surface78 |
| Modified TiO2/Al2O3 | 12 | Photocatalytic degradation of azo-dye model modification of TiO2 with urea70 |
| TiO2–SiO2/Al2O3 | 8.37 at 5 bar | Nanostructured TiO2–SiO2via sol–gel synthesis multifunctional (photocatalytic and physical separation) capabilities113 |
| TiO2 nanotube | 15–33 L m−2 h−1 | Controllable inner tube diameter of TiO2via liquid-phase deposition grafting time photocatalytic membrane, pollutant removal (humic acid) under UV57 |
| Nanofiltration | ||
| TiO2–SiO2 | 2.5, 5.0 and 10.0 ml min−1 | Flexible nanofibrous membranes via electrospinning can be recycled by calcination and chemical and thermal stability pollutant (dye) adsorption capacity62 |
| TiO2 hollow fiber | 12.2 L m−2 h−1 | Hollow fiber fabrication via spinning–sintering method calcination temperature (900 °C) an important factor for membrane properties Acid Orange 7 (AO7) and raw sewage as pollutants were tested and 90.2% organic removal rate114 |
| Graphene oxide (GO) | 80–276 Lmh MPa−1 | One of the first examples of a GO-based membrane 4–10 times higher flux range than commercial NF cross-linked GO sheets made by layer-by-layer process115 |
| Graphene oxide (GO) | 4 mol m−2 h−1 | Fast ion permeation rate101 |
| Graphene | 21.8 | Ultrathin sheets high retention (>99%) for organic dyes and for ion salts retention rate is moderate (approximate to 20–60%)98 |
4.2. Organic membranes
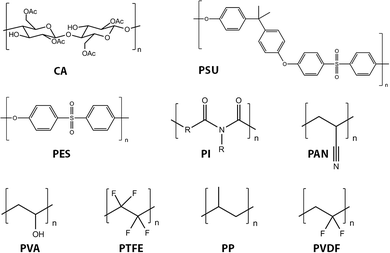
Virtually all organic membranes explored to date have been made of polymeric materials. Although inorganic membranes are gaining more attention, the majority of membranes are made of polymeric materials. Polymer materials in general offer a wide variety of structures and properties. Cellulose acetate (CA) and cellulose nitrates,116–118 polysulfone (PSU),119 polyethersulfone (PES),120 polyacrylonitrile (PAN),121,122 polyvinylidene fluoride (PVDF),123,124 polypropylene (PP), poly vinyl alcohol (PVA), polytetrafluoroethylene (PTFE), and polyimide (PI) represent the most widely used current (first generation) organic membrane materials (Fig. 10).10PSU and PES are among the most commonly used for UF and also as supporting substrates for NF and RO processes. These materials exhibit excellent permeability, selectivity of permeate, mechanical stability, and chemical resistance. For example, the glass transition temperature (Tg) of PES is 225 °C, and PSU exhibits pH stability and oxidation resistance.120 Modification of PES membranes has been summarized in several recent reviews.120,125–127 For MF applications, PP and PVDF are the most frequently used materials.128
The main drawback of most of these polymeric membranes is their inherent hydrophobicity resulting in a high fouling tendency, which often leads to higher operation cost, shorter lifetime, irreproducible separation performance, and smaller application range. Fouling is primarily caused by buildup of proteins, organics, inorganics, microorganisms, and microbial communities on the membrane surface.129 As such, the next generation of these membrane materials focus on an improvement of synthesis/processing to develop novel polymeric membranes and novel functionality—the current trends in this field are summarized in Fig. 11.
 | ||
| Fig. 11 Current trends in the field of organic membrane materials to develop next-generation membrane materials. | ||
In general, no single polymeric membrane material mentioned above simultaneously exhibits chemical/thermal stability, oxidation/pH resistance, and mechanical strength. Significant effort has been directed toward enhancing permeation flux, pollution resistance, operation pressure stability, and membrane service life. One such method is surface modification,130 for example, by making these polymeric membranes hydrophilic. It is generally accepted that increasing the hydrophilicity of the membrane may reduce fouling issues because many foulants including proteins and organics are hydrophobic in nature. There are various methods to fabricate hydrophilic polymeric membranes. These include homogeneous blending,131–134 plasma treatment,135–139 surface grafting,140,141 cross-linking,142–144 gamma ray and UV irradiation,145,146 surfactant methods, surface coatings with hydrophilic polymers,147–151 and surface segregation methods with amphiphilic block copolymers.152 For example, amphiphilic copolymer (Pluronic F127) was used as a surface modifier and pore-forming agent to prepare antifouling polyethersulfone (PES) ultrafiltration membranes.153Fig. 12 illustrates Pluronic F127 in the membrane-formation process, a cross-sectional SEM image, and a plot of flux variation during three cycles of UF experiments.
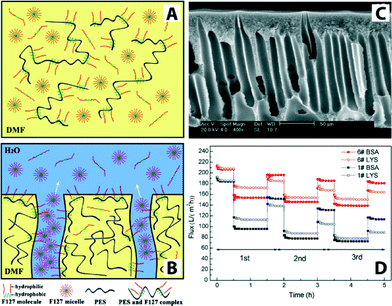 | ||
| Fig. 12 Schematic illustrations of PES/Pluronic F127 membrane formation process. (A) Initially there is a homogenous casting solution. (B) Immersion of the film in water leads to phase separation and formation of ordered structures containing pores. (C) Cross-sectional SEM image of PES/Pluronic F127 membrane. (D) Flux comparison of PES/Pluronic F127 membrane in three cycles of UF experiment. Bovine serum albumin (BSA, Mw = 67 kDa) and lysozyme (LYS, Mw = 14 kDa) were used as model foulants to evaluate antifouling property. Reprinted from ref. 153 Copyright (2008), with permission from Elsevier. | ||
Similar to Pluronic F127 membrane, polyvinyl chloride (PVC, one of the most widely used polymer materials for UF and MF membranes due to its robust mechanical strength, low-cost, and chemical stability) and polyvinyl formal (PVF) blends were synthesized by the non-solvent induced phase separation method. Results showed an increase of hydrophilicity (thus enhanced antifouling property) due to the presence of surface-segregated PVF.131 PVC/PVF membranes displayed good flux recovery in three cycles (three cycles were 85.5%, 94.8%, and 95.6%, respectively). Incorporation of PVF is potentially a low-cost, economic solution for scale-up production.
Other factors affecting fouling properties include surface roughness/morphology, pore size, and surface charge.131,154–157 Rana and Matsuura in their review paper reported extensive tabulated data for reduction of fouling by increasing the surface hydrophilicity, increasing surface charge, decreasing surface roughness, making biomimetic surfaces, and forming surface thin-film layers.129 Aside from surface modifications and novel fabrication techniques mentioned above, another processing method to improve membrane surface hydrophilicity and smoothness is a solvent-induced microswelling technique. (Higher roughness is thought to exacerbate fouling.) This technique was developed using commercially available membranes (PSU UF membrane and PVDF UF and MF membranes). The process is driven by minimization of interfacial free energy such that reassembly of microporous membranes occurs when solvency is changed.158
To realize tunable membranes, functional polymers such as polyacrylic acid (PAA) and polymethyl methacrylate (PMMA) are mixed with polymeric membranes in order to improve pH sensitivity. By changing the conformational state of the polymer (these additives induce shrinking–swelling of the pores of the membranes by deionization of carboxyl group (–COOH) and ionization (–COO−) around their pKa values), the permeability of the membranes are affected by pH, ions, and solute concentrations.159–161 Typical preparation of such membranes involves directly blending PAA with other polymers, so that elution of PAA is possible due to its water dissolubility. In order to address this issue, Wei et al. prepared tunable membranes by blending cross-linked PAA gel with PES solution using a phase-separation technique.162 As the PAA gels were cross-linked before introduction to the PES substrate, small pores in the skin layer prevented microgel leakage. The blended PES membranes showed pH sensitivity and pH reversibility between 3 and 8.
Recent reports addressing functional polymeric membranes include use of conductive polymers,163 demonstration of temperature- and pH-sensitive membranes with improved antifouling property by phase separation of blends of PVF/poly(N-isopropylacrylamide-co-acrylic acid) microgels/DMF in water,164 blending of copolymer poly(acrylic acid-co-polyethylene glycol methyl ether methacrylate) (p(AA-co-EGMA)) with PSU to prepare flat-sheet polymeric membranes by the phase-inversion method,165 one-pot in situ cross-linked copolymerization of poly(methyl methacrylate-co-acrylic acid) (P(MMA-AA)) and poly(methyl methylacrylate-co-4-vinyl pyridine) (P(MMA-4VPy)) on PES membrane exhibiting pH-response, anti-fouling property, and Cu2+ adsorption capacity,166 and track-etched polyethylene terephthalate (PET) membranes by grafting 2-hydroxyethyl-methacrylate (HEMA) via atom transfer radical polymerization showing reversible pH-response permeation to environmental pH values by controlling the PHEMA chain lengths.167 Similar to pH-responsive membranes, Lohokare et al. observed 230% increase in water flux (90% rejection for BSA and 90–110 L m−2 water flux) upon base treatment (NaOH) on a PAN-based UF membrane.168 This dramatic improvement was attributed to a change in the membrane pore size caused by swelling of the pores and increased hydrophilicity.
Temperature-modulated water filtration is another example of the use of functional polymers. Poly(N-vinylcaprolactam)-based microgels were employed to coat commercially available hollow-fiber membranes used for MF and UF applications.169 The main advantages of this method are that microgel functionalization (via adsorption) can be applied to almost all types of membranes and that the membrane modification is simple. In this instance, the membrane showed reversible thermoresponsive permeability and rejection (previously reported that thermoresponsive polymer also exhibited less fouling).170
Another widely used class of organic membranes are cellulose-based membrane materials including cellulose acetate (CA). CA membranes were among the first polymeric membrane materials, and, historically, Loeb and Sourirajan's asymmetric CA membrane in 1963 exhibited high salt rejection and flux values (5 to 11 gallons of 0.05% NaCl water per sq. foot per day from a brine containing 5.25% NaCl at 1500 to 2000 psi for RO applications).171 Since then, CA has been applied in a wide range of filtration processes from RO to MF driven by CA's relative low cost (cellulose is the most abundant natural biomaterial on earth, ~700 billion tons per year)172 and hydrophilicity.173 However, CA membranes lack long-term chemical, thermal, and biological stability (e.g., intolerant of chlorine, have limited operational temperature and pH ranges, and have a tendency to hydrolyze over time). CAs are often blended with other widely used hydrophobic polymers to produce composite membranes such as PES/CA, PVDF/CA, and PSF/CA.174–176 Due to the high hydrophilicity of CA, for example, hydrophobic PVDF (widely used membrane exhibiting thermal stability, solvent and chemical resistance) is blended with CA to address PVDF's low permeability and poor antifouling ability. Among cellulose-based materials, cellulose nanofibers177 extracted from various natural sources (wood and cotton pulps) or under culturing conditions (bacterial cellulose) were found unique in that they offer network structure with excellent mechanical properties (e.g., Young's modulus of bacterial cellulose >15 GPa).178 Examples of recent cellulose nanofiber membrane studies include the fabrication of CA nanofibrous composite UF membranes (CA MF membrane was used as a support) with ultrahigh water permeability and efficient separation (3540 L m−2 h−1 flux and 90.7% ferritin rejection)118 and cellulose nanofiber (fabricated from wood pulp by using the TEMPO/NaBr/NaClO system followed by a mechanical treatment) composite UF membranes (cellulose nanofiber as a top layer, polyacrylonitrile (PAN) as a mid-layer, and polyethylene terephthalate (PET) as a supporting layer) exhibiting five times higher flux than that of commercial UF membranes (e.g., PAN10 produced with the same polymer components without the cellulose nanofiber barrier layer) and higher rejection ratio (>99.9%) of microspheres.178
The use of self-assembly of block copolymers potentially offers high selectivity due to their narrow pore-size distribution, high permeability resulting from high porosity, and controllable dimensions, surface properties, and chemistries.179–184 Block copolymers with hexagonally packed cylinders oriented perpendicular to the membrane surface offer an ideal pore morphology.185–187 Phillip et al. prepared an UF membrane using self-assembled poly(styrene-block-lactide) block copolymer.188 By controlling the solvent (toluene) evaporation rate (fast solvent evaporation kinetically traps the cylindrical formation in a nonequilibrium morphology), polylactide cylinders are formed perpendicular to the substrate (commercially available microporous membrane was used as a support layer). Fig. 13 shows a top view SEM image of hexagonally arranged arrays of polylactide cylinders and an experimental rejection curve along with the predicted curve.
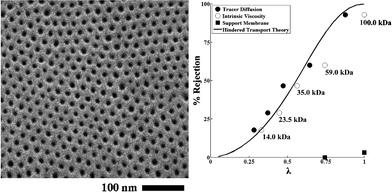 | ||
| Fig. 13 Self-assembled poly(styrene-block-lactide) block copolymer. (A) Top view SEM image of perpendicularly oriented polylactide cylinders via fast solvent (toluene) evaporation. (B) Rejection curve (filled and open circle) along with the predicted values (solid line). The rejection increases as solute molecular weight of solute increases from 14 kDa to 100 kDa (>93%). Reprinted with permission from ref. 188 Copyright (2010) American Chemical Society. | ||
Common methods to fabricate polymeric membranes include phase inversion, interfacial polymerization, stretching, track-etching, and electrospinning (cf.Fig. 2).11 Atomic layer deposition (ALD) has recently been adopted to modify and functionalize organic membranes (ALD modification of inorganic membranes has been explored in greater depth – some examples are shown in Table 4).189,190 A brief example includes a conformal and uniform thin layer deposition of polyimide (PI).191 Pyromellitic dianhydride (PMDA) and ethylenediamine (EDA) were used as precursors for the ALD of PI on the pore walls of PES membranes. PI-coated membranes showed improved mechanical and thermal stability over their bare PES counterparts. Table 3 summarizes some of the most recent development of organic membranes.
| Membrane material | Performance (L (m2 h bar)−1) | Key features |
|---|---|---|
| Microfiltration | ||
| Polyvinyl alcohol (PVA)/polypropylene (PP) | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 346 L m−2 h−1 at 0.24 bar 346 L m−2 h−1 at 0.24 bar |
Combined solution and melt electrospinning methods to achieve smaller avg. pore size than nonwoven membranes192 |
| Poly(vinylidene fluoride) (PVDF)/hydroxyethylmethacrylate (HEMA) | Electrospun nanofibrous membrane coated with a surface-charged chitosan polymer, enhanced hydrophilicity and improved flux193 | |
| Ultrafiltration | ||
| Cellulose acetate (CA) nanofiber | 3540 | 10× higher flux than commercial membranes118 |
| Polyimide (PI)/polyethersulfone (PES) | 3565–1780 | Controllable coating of PI on PES via ALD, enhanced thermal resistance and mechanical strength194 |
| Polyethylene terephthalate (PET) | 0.1–0.21 mL cm−2 s−1 at pH 4–8 | Reversible pH-responsive permeation167 |
| Polysulfone (PSU)/poly[2,2′-(m-phenylene)-5,5′-dibenzimidazole] (PBI) | 355 L m−2 h−1 | Enhanced porosity, hydrophilicity, and thermal stability195 |
| Polyvinyl chloride (PVC) and polyvinyl formal (PVF) | 52–323 L m−2 h−1 at 0.1 MPa | Enhanced antifouling property131 |
| Cellulose acetate (CA)/polyethylene glycol (PEG) | Max 360 L m−2 h−1 at 0.05 MPa | CA and PEG concentration effect thickness and cross sectional structure of the membranes173 |
| Hydrophilic polyurethane additive (L2MM)/Polyvinylidene fluoride (PVDF) flat sheet | Max 130 L m−2 h−1 | A new type of hydrophilic additives (L2MM(PEG-600) and L2MM(PEG-200)) result in ~6 times higher fluxes than the pure PVDF membranes134 |
| Cellulose acetate derivatives | 316–406 L m−2 h−1 atm | Hollow fiber, cellulose acetate (CA), cellulose acetate butyrate (CAB), and cellulose acetate propionate (CAP) hydrophilicities CA > CAB > CAP CA with the highest antifouling properties for humic acid and BSA117 |
| Polysulfone (PSU) | 227.8 L m−2 h−1 | Polyethylene glycol methyl ether (PEGME) as an additive, decreased contact angle from 71° to 47° (ref. 132) |
| Nano-chitin whisker (NCW)/poly(vinylidene fluoride) (PVDF) | 392 L m−2 h−1 | Improvement of mechanical properties, permeability and antifouling property via non-solvent induced phase separation method196 |
| Polysulfone (PSU) | 7.5–8.9 L m−2 h−1 | Gravity-driven membrane filtration, improved permeate quality in the presence of biofilm on membrane surface but over long-term, accumulation of organic matter resulting in deteriorating the permeate quality197 |
| Poly(acrylic acid-co-polyethylene glycol methyl ether methacrylate) | 0.794 L m−2 h−1 kPa | Hydrophilic, pH responsive 90% flux recovery ratio after bovine serumal bumin separation as compared to 37% flux recovery ratio of unmodified membrane165 |
| Cellulose acetate (CA) nanofiber | 3540 | Free-standing CA nanofibrous layer on a CA microfiltration membrane, uniform porous structure with porosity of up to 71%, ultrahigh water permeability (10 times greater than that of most commercial membranes)118 |
| Nanofiltration | ||
| Poly(ethylene glycol) diglycidyl ether (PEGDE)/polyamide | 50–92 L m−2 h−1 | Improved fouling resistance with small changes in the surface properties, after small amount of grafting material (PEGDE), additional PEGDE has much less impact on membrane performance152 |
| Polysulfone (PSU) | Max 70 L m−2 h−1 | Improved hydrophilicity and cadmium removal (up to 98%) by addition of an amphiphilic additive, IGEPAL surfactant and by using the lowest level of coagulation bath temperature119 |
4.3. Inorganic–organic hybrid membrane materials
The latest development in membrane material design is the use of hybrid (inorganic–organic) materials, with much of the drive originating in overcoming limitations associated with polymeric membrane systems. Inorganic materials that have been explored are metal oxides (e.g., Al2O3, TiO2, SiO2, ZnO, Fe2O3), metals (e.g., Cu, Ag) and carbon-based materials (e.g., graphene and carbon nanotubes). Introducing inorganic moieties into a polymeric matrix system can offer multi-functionality beyond separation alone and can enhance hydrophilicity, mechanical strength, water permeability, rejection rate, and antifouling properties. This is, in part, because such additives can modify the kinetics and thermodynamics of the formation process of the polymeric membrane such that the membrane surface and pore structure can be altered.Various fabrication methods have been developed to incorporate these nanomaterials in a polymer matrix as shown in Fig. 14. These include blending, phase inversion methods (resulting in well-mixed nanomaterials in the matrix), interfacial polymerization (resulting in a thin layer of nanocomposite on the surface of the membrane or a thin layer with nanocomposite membrane substrates), self-assembly of nanoparticles, surface coatings, layer-by-layer processing, and surface grafting.198 Wang et al. recently reviewed the behavior of various nanomaterials in polymeric matrices during the phase-inversion process and their structural performance during filtration;199 membranes consisting of nanomaterials in a polymer matrix for water treatment were recently reviewed by Goh et al.200 and Yin et al.198 Recent developments in thin film composite membranes201 have received increasing attention as these systems exhibit superior performance compared with asymmetric membranes for desalination and have been reviewed by Lau et al.202,203
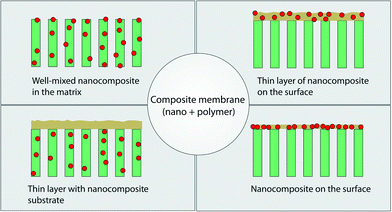 | ||
| Fig. 14 Various types of composite membranes containing nanomaterials and polymer. Adapted from ref. 198 Copyright (2015), with permission from Elsevier. | ||
Modifying the membranes by blending organic and inorganic materials (especially nanoparticles) may offer advantages such as excellent filtration performance, thermal and chemical stability, as well as membrane forming ability. Recently, NPs such as oxide species (e.g., SiO2, TiO2, Al2O3, ZnO) and metal particles such as Ag have been used to modify organic membranes. Widely used polymeric membranes such as PES and PVDF with improved antifouling property were realized by incorporating various NPs.
One of the earlier reports on hydrophilic modification of PES membranes with NPs was carried out by Luo et al.204 TiO2 NPs were assembled on the surface of PES by coordination and hydrogen-bond interactions between the hydroxyl group of TiO2 and the sulfone. The composite UF membrane showed good separation performance. TiO2/PES composite membranes revealed that incorporation of TiO2 (0.5 wt%) into PES did not affect the structure of the membrane, and performance metrics such as hydrophilicity, thermal stability, mechanical strength, and anti-fouling ability were enhanced. If, however, more than 0.5 wt% of TiO2 was used, a defective pore structure formed and the performance declined.205 More recent reports about TiO2 incorporation include PES–TiO2 NP composite membranes synthesized from casting a solution consisting of polar solvents (DMF and EtOH) and TiO2 additive showing concentration-(TiO2 and EtOH) dependent membrane performance (permeation and rejection rates, pore size, and porosity),206 UF composite membranes containing reduced graphene oxide/TiO2 in PVDF matrix showing greater hydrophilicity and higher water flux (54.9% increase) than bare PVDF,207 and improved performance and hydrophilic properties of PVDF–TiO2 membranes by various methods (sol–gel and blending methods,208 non-solvent induced phase separation,209 and phase-inversion method123). It should be noted that determination of optimal concentration of TiO2 plays a key role in enhancement of membrane properties (the concentration ranges vary from 2–25% among the studies mentioned above).
Balta et al. used zinc oxide as an alternative to TiO2 as it offers lower material cost.210 They reported the synthesis of ZnO/PES membranes and investigated the performance of the composite membranes by varying ZnO nanoparticle content (0.035–4 wt%). The results showed improved permeability, dye rejection, and fouling resistance by adding ZnO NPs. Methylene blue was used for rejection tests, and the rejection rate was increased from 47.5% for neat membranes to 82.3% for composite membranes even at 0.035 wt% of ZnO nanoparticles. Another example illustrates that the incorporation of ZnO NPs can tune pore diameters (ranging from sub-20 nm up to 100 nm) of membranes as shown in Fig. 15.211 ZnO NPs/glycerol were used as pore templates in a sense that removal of ZnO NPs/glycerol from the PES matrix resulted in pores that were controlled by the ratio of ZnO NPs/glycerol.
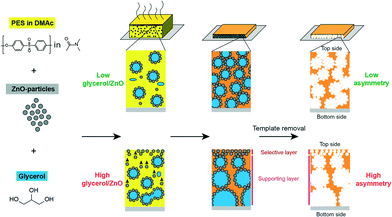 | ||
| Fig. 15 Schematic illustration of fabricating asymmetric membrane. From left to right: poly(ether sulfone)/dimethylacetamide (PES/DMAc) solution is mixed with ZnO NPs as pore templates and with glycerol. Composite film is cast on a glass substrate. After solvent evaporation and polymer coagulation the pore-templating NPs are dissolved with diluted HCl. High glycerol/ZnO ratios result in highly asymmetrical membranes. Reprinted with permission from ref. 211 Copyright (2015) American Chemical Society. | ||
Halloysite (formula: Al2Si2O5 (OH)4·2H2O) nanotubes (HNTs) have recently been used as catalyst supports, nanoreactors, and filler for polymer to improve the mechanical and thermal properties of the composites. Studies showed that HNTs are easy to blend with a polymer matrix due to their well-crystallized structure, low density of hydroxyl functional groups, and tubular shape.212 Examples of HNT composite membranes include dextran-grafted HNT as a hydrophilic filler in PES membranes showing higher flux and good antifouling properties (the content of dextran-grafted HNT in the hybrid membranes was an important factor affecting the morphology and separation properties of the membranes)213 and 2-methacryloyloxyethyl phosphorylcholine (MPC)-grafted HNT/PES composite membrane also addressing antifouling properties (MPC-based materials are known to resist protein adsorption, and the results indicated that the hybrid membranes possessed higher water flux, good antifouling performance, and stability).214
Another NP additive is Al2O3, which is stable, inexpensive, and non-toxic. Many studies demonstrated that the addition of Al2O3 results in enhanced mechanical strength, hydrophilicity and, consequently, antifouling properties.215,216 Recently, the influence of combined additives (inorganic Al2O3 and organic polyethyleneglycol (PEG)) via phase inversion method in various polymers such as PSU, PES, and PEI was studied (PEG has been extensively used as an organic additive to improve membrane selectivity and hydrophilicity as well as a pore forming agent)217 to determine the material with the best antifouling properties.218 Combining Al2O3 with PES resulted in superior antifouling properties caused by reduced hydrophobic interaction between foulants and the membrane surface. Among these composites, PES/PEG/Al2O3 membranes displayed the best antifouling properties.
Arsuaga et al. reported the effects of the type, size, and spatial distribution of metal oxide NPs (TiO2 (485 ± 148 nm), Al2O3 (438 ± 131 nm) and ZrO2 (398 ± 122 nm)) on the properties of composite PES UF membranes (Fig. 16).219 They observed a correlation between physico-chemical properties (porosity, hydrophilicity, and permeability of composite membranes) and the spatial particle distribution in the membrane structure. General improvement of water flux and rejection upon incorporation of the particles was seen. Also, the study showed that metal oxide NP-doped PES membranes exhibited reduced fouling due to increased hydrophilicity of the membrane surface. NPs distribution is a key parameter for membrane fouling reduction, but there is no effect on the rejection potential of the composite membranes.
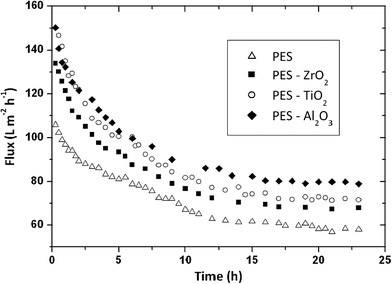 | ||
| Fig. 16 Flux comparison of prepared membranes (PES as a control, PES/ZrO2, PES/TiO2, PES/Al2O3) during 100 mg L−1 BSA aqueous filtration. Membrane performance improves in the order Al2O3 > TiO2 > ZrO2. Spatial NP distribution on PES membrane is a key parameter for membrane fouling reduction. Reprinted from ref. 219 Copyright (2013), with permission from Elsevier. | ||
There are several reports on the use of silver nanoparticles to address bio-fouling issues in polymeric membranes (Ag NPs are a common biocide material). An example includes incorporation of Ag NPs onto PAA brush-grafted PVDF membranes via a physisorbed free-radical-grafting technique. Silver ions from silver nitrate solution were bound to PAA-grafted PVDF membranes via coordination bond formation between carboxyl groups of PAA and silver ions; further reduction of silver ions resulted in the formation of Ag NPs on the grafted membranes. The resultant composite membranes showed enhanced surface hydrophilicity and antifouling performance.220 Another report addressing the bio-fouling issue was carried out by modifying PSU membranes with Ag NPs synthesized using various ionic surfactants (silver sulfadiazine, dodecyltrimethylammonium bromide, benzalkonium chloride, or sodium dodecylbenzene sulfonate).221 All membranes displayed better hydrophilicity than neat PSU. E. coli and S. aureus were used to test the sensitivity of the antimicrobial effect, and the lowest adhesion of E. coli was found with 6% and 10% Ag NP additions. These studies also found that the Ag particle size played an important role for the antimicrobial effect. Loss of antimicrobial compounds by the membranes during operation is an ongoing challenge in this field. Prince et al. showed functional modification of PES hollow fiber membranes using PEG and Ag NPs through thermal grafting (poly(acrylonitrile-co-maleic acid) (PANCMA) was used as a linker to chemically attach PEG and Ag NPs to the PES membrane).222 The modified UF membrane exhibited an increase of hydophilicity and antibacterial activity.
GO nanosheet additives offer good chemical stability and high surface area. GO-doped PSU polymer matrix exhibits enhanced hydrophilicity, water flux, and salt rejection.223 There are also reports in which GO nanosheets act as a hydrophilic modifier for polymeric membranes such as PVDF,224 PSF,223 GO/PVP on PVDF,225 as well as enhancing antifouling226,227 and mechanical strength of MF membranes (e.g., GO/PVDF with 55.11% and 67.14% increase in the tensile strength and Young's modulus, respectively).228 Cross-linked GO nanosheets on a polydopamine-modified PES support displayed 4–10 times higher flux (80 and 276 Lmh MPa−1) than that of most commercial nanofiltration membranes.115Fig. 17 shows a schematic illustration of a GO membrane fabrication procedure and reaction mechanisms. Although GO membranes are still in the development stage, they exhibited relatively high flux range (80 to 276 Lmh MPa−1) as compared to commercially available NF membranes. Low to moderate rejection of salts and organic dyes was reported, and these unwanted solute rejections are believed to be the result of size exclusion and surface-charge effects.
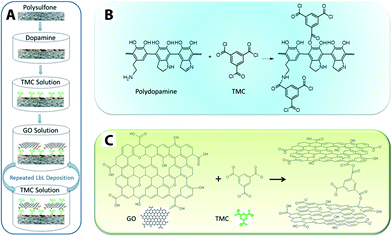 | ||
| Fig. 17 Schematic illustration of (A) GO membrane fabrication procedure, (B) the mechanism of reactions between polydopamine and TMC (cross-linker: 1,3,5-benzenetricarbonyl trichloride), and (C) reaction mechanism between GO and TMC. Reprinted with permission from ref. 115 Copyright (2013) American Chemical Society. | ||
Similar to GO, CNTs offer thermal and mechanical stability and possess high surface area and selective adsorption properties via surface functionalization of the CNTs. An earlier report by Choi et al. showed an increase in water flux with the introduction of carboxylated multi-walled CNTs into PSU membranes. This enhanced flux is related to improved hydrophilicity arising from the carboxylic acid functional groups.229 PES is one of the most common polymeric materials used in UF applications as mentioned in an earlier section. Despite its excellent properties (e.g., permeability, selectivity, mechanical stability, and chemical resistance), PES is inherently hydrophobic resulting in low membrane flux and poor antifouling properties. In addressing antifouling properties, various modification techniques (e.g., the use of additives, ultraviolet irradiation, chemical treatments, grafting, and surface coatings) have been reported.120,230–232 Recent reports on CNT incorporation include the fabrication of multi-walled CNT-blended PES composite membrane via the phase-inversion method,233 and amine functionalized multi-walled CNTs/PES composite membranes, which showed improved antifouling properties and hydrophilicity.234,235
Table 4 summarizes the recent development of hybrid membrane and their performance and features.
| Membrane material | Performance (L m−2 h−1 at 1 bar) | Key features |
|---|---|---|
| Microfiltration | ||
| Polyisoprene (PI), polystyrene (PS), poly(4-vinylpyridine) (P4VP)/TiO2 NPs | 3200 ± 500 | Structural asymmetry with a thin nanoporous surface layer236 |
| AgNP/polysulfone (PSU) | 0.181–0.086 kg m2 h1 Pa1 at 1000 kPa (UF + MF) | Ag NP loading via cold spray jet, anti-microbial capabilities (lysing bacteria via 15 wt% Ag loading)92 |
| Ultrafiltration | ||
| CNTs/polyethersulfone (PES) | Max 10–90 L m−2 h−1 at 10 to 60 psi | More hydrophilic, higher flux and slower fouling rate than neat PES membranes (0.5% C/P blend optimal)233 |
| Poly(ether sulfone)/dimethylacetamide (PES/DMAc) solution, glycerol, and ZnO NPs | 5600 L m−2 h−1 | Solvent-evaporation-based process 97% retention 45 nm silica beads gradient pore sizes by using ZnO NPs as a template211 |
| Ag–SiO2/polyethersulfone (PES) | Max 140 L m−2 h−1 | Anti-bacterial and antifouling performance, introduction of silver in the membrane by Ag NP deposition on the silica sphere surface237 |
| Amine functionalized Multi-walled carbon nanotubes (MWCNTs)/polyethersulfone (PES) | Max 180 L m−2 h−1 | Increased hydrophilicity and improved BSA rejection and antifouling properties of PES membrane234 |
| Al2O3/polycarbonate | Max 11 | Al2O3 deposition and pore size control via ALD, improved hydrophilicity and resistance to acids and organic solvents238 |
| TiO2/Polyvinylidene fluoride (PVDF) or polypropylene (PP) | 190, 300–420 | Uniform and conformal TiO2 deposition via ALD, improved water flux and retention through increased hydrophilicity and reduced pore sizes189,239 |
| Anodic alumina membranes | ~2–7 g h−1 at 1–4.5 bar | Demonstration of water slippage on hydrophilic surfaces, flow enhancement increases with decreasing diameter and are a function of the channel length240 |
| Polyvinylidene fluoride (PVDF) and graphene oxide (GO) | 271.29–346.14 L m−2 h−1 | Improved hydrophilic, mechanical strength, permeation properties (96.4% increase)224 |
| Polyethersulfone (PES)/TiO2 | 365–596 L m−2 h−1 | Addition of TiO2, no effect on membrane structure, enhanced hydrophilicity, thermal stability, mechanical strength and anti-fouling ability205 |
| Modified TiO2/Polyvinylideneflouride (PVDF) | 82.5 L m−2 h−1 | LiCl·H2O and TiO2 nanoparticles as an additive, lower TiO2 nanoparticle loading, the higher hydrophilicity, small pore size, and high porosity123 |
| AgNPs/polysulfone (PSU) | 6–12 ml min−1 | 6% and 10% of newly synthesized Ag NP exhibited the best anti-biofouling characteristics, improved hydrophilicity, antibacterial effect221 |
| AgNPs/Polyethyleneimine (PEI) and poly(sodium styrenesulfonate) (PSS) | 9.5 m3 (m2 day atm)−1 | Layer-by-layer deposition by using electrostatic interaction of polyelectrolyte-stabilized Ag NPs95 |
| Dextran-grafted halloysite nanotube (HNT)/polyethersulfone (PES) | 100–220 L m−2 h−1 as a function of HNTs contents | Blending with HNTs-Dextran composites via phase inversion method, improved antifouling property, hydrophilicity, pure water flux and mean pore size of the membranes213 |
| Nanofiltration | ||
| (NH2-MWCNTs)/polyethersulfone (PES) | 23.7 L m−2 h−1 | Enhanced hydrophilicity, pure water flux with increase of NH2-MWCNTs amount, improved fouling recovery ratio, more negatively charged surface for BSA filtration, salt retention Na2SO4 (65%) > MgSO4 (45%) > NaCl (20%)235 |
| Polyethersulfone (PES)/O-carboxymethyl chitosan + Fe3O4 NPs | 20–40 kg m−2 h−1 at 4 bar | Improved hydrophilicity, pure water flux recovery ratio (91.7%), higher rejection and good fouling resistance, undesired agglomeration when high content of CC-Fe3O4 NPs (>0.5%) are used, lowest irreversible fouling resistance value 8.33%241 |
| ZnO/PES | Max 60 | ZnO as an alternative NP additive, improved permeability, dye rejection and fouling resistance242 |
5. Analytical methods
Analytical methods such as physical/chemical characterization of membranes, modeling filtration processes, and understanding different operation modes are a crucial part of design, development, and application of membrane technology. In the following sections, we survey each of these topics, drawing special attention to their relevance to materials selection.5.1. Membrane operation processes
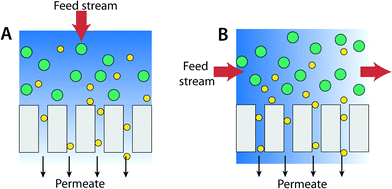
Membrane filtration-based water purification processes such as RO, NF, UF, and MF are most commonly pressure-driven (other methods include dialysis, distillation, and electro-potential driven processes). The primary membrane system architectures are (A) dead-end filter and (B) cross-flow operations (Fig. 18).243Dead-end filtration mode is the most common process for water treatment in the research lab. In this mode, the flow of water to be filtered is directed perpendicular to the membrane surface such that water is pushed through the membrane by the applied pressure. This technique is useful if the concentration of particles or targeted pollutant is low. It is typically used in home water filtrations and also to concentrate compounds in contrast to industrial applications where amount of materials to be filtered can be as high as 30%. If the concentration of targeted species is high, the filtered materials can accumulate as a layer on the surface of the membrane. This layer formation results in a pressure drop across the membrane leading to increased resistance and reduced permeate flux.
In the case of a cross-flow (or tangential flow) operation process, the feed stream is parallel to the membrane surface such that the feed water flow is perpendicular to the filtration flow as shown in Fig. 18B. Continuous turbulent flow along the membrane surface (cross-flow velocity is typically 0.5 to 1 m s−1, four to five orders of magnitude greater than the superficial water velocity toward the membrane) creates a shear force that reduces the accumulation of species.244 As such, the cross-flow operation mode is particularly useful for filtering high concentrations of materials or macromolecules such as cells and proteins. For municipal water treatment applications, input (surface) water typically has dilute contamination (concentration of solids is about 0.01%). Therefore, the advantage of cross-flow operation is less significant. Also, costs for the membrane system and operation costs associated with the cross-flow system are higher than those for dead-end operation. (Some cross-flow operation systems can also operate in a dead-end mode.)
5.2. Membrane characterization
Structural, physical, and chemical properties of membranes – especially surface properties of the membranes – must be well understood in order to develop successful membrane technologies. Understanding the surface properties of the membranes, which depend on the membrane materials, membrane type, and interactions between membrane and solute, is not only of scientific importance but also technological importance in the water treatment industry and is critical to membrane performance metrics including permeation, flux, rejection, lifetime, and fouling.There are various qualitative and quantitative analytical tools for characterizing membranes. These include scanning electron microscopy (SEM), transmission electron microscopy (TEM), scanning tunneling microscopy (STM), secondary ion mass spectrometry (SIMS), contact angle measurements, zeta potential, X-ray photoelectron spectroscopy (XPS), laser scanning confocal microscopy (LSCS), electron spin resonance (ESR), neutron reflectivity (NR), thermogravimetric analysis (TGA), Fourier transform infrared (FTIR) spectroscopy with attenuated total reflection (FTIR-ATR), Raman spectroscopy, atomic force microscopy (AFM), and X-ray diffraction (XRD). These tools elucidate structural information, elemental composition, surface morphology, and fouling phenomena. In this section, brief descriptions of several of the most commonly used tools are provided. Readers who seek additional details on specific tools should refer to recent reviews by Agboola et al. and Lau et al.201,245
The most widely used technique for structural and chemical composition characterization of membranes is SEM. An SEM image is formed by scanning a focused electron beam across the sample and recording the intensity of scattered or secondary electrons. In addition to electrons, X-rays are ejected from the sample, and these can be detected using energy dispersive X-ray spectroscopy (EDX), or wavelength dispersive X-ray spectroscopy (WDX). The SEM electron beam can also generate photons in the UV-vis-IR range and these can be recorded using cathodoluminescence. When high-resolution images of membrane structures are necessary, TEM can be used. Many of these microscopes also offer qualitative and quantitative evaluation of different types of foulants and chemical composition of membranes. Various signals obtained from a collection of detectors contain information about the surface topology, pore size, pore size distribution, pore shape, chemical composition, and thickness of the membrane. If the sample is cross-sectioned (or is viewed tilted), the cross-sectional images can provide thickness and structural information (e.g., membrane support, pre-layer, and skin layer).246 Sample preparation and imaging parameters greatly affect the resultant images. Recently, Abdullah et al. pointed out the importance of noting and reporting imaging parameters and membrane sample preparation for SEM characterization.247
Another imaging technique often used to elucidate the surface roughness, pore size and its distribution, nodule size, and aggregate size at the surface of the membrane is AFM.248 AFM uses mechanical interactions between the sample and a probe tip mounted at the end of a cantilever, which is scanned across the sample surface. As the tip nears the sample surface, deflection of the cantilever is measured by a laser beam reflected from the cantilever onto a photodiode. AFM studies have been applied to various membrane materials (both organic and inorganic)249–251 and processes (from MF to RO).249,252–256 Determination of the surface roughness by AFM has become a routine analytical method as it relates to membrane fouling.257,258
In the case of the surface charge properties of the membranes, zeta potential measurements are commonly used.259 Zeta (surface) potentials are determined from electro-kinetic measurements; changes in membrane surface potential can be used to study cake deposition and fouling behavior during filtration, and the measurements are also useful for NF/RO applications in which electrolyte solutions (e.g., KCl) containing different pH values are used.260–262 Membrane charge electrostatically interacts with ions and results in a change in charge density near the surface of the membrane. The separation efficiency of ions is governed by the relative sign of charge on the membrane's surface, ions, colloids, or molecules (e.g., attractive forces through opposite charge interactions result in fouling).
IR and Raman spectroscopy measure the vibrations of molecules and are used to identify or study structural/chemical composition of samples (Raman-active transitions require a change in the polarizability of the molecule, whereas, IR-active transitions require a change in the dipole moment). These techniques can be used to characterize polymeric membranes for example, to monitor surface modification and bio-fouling of the membrane.263,264 Recently, surface-enhanced Raman spectroscopy has also been utilized to examine fouling of organic species on membrane surfaces.265,266
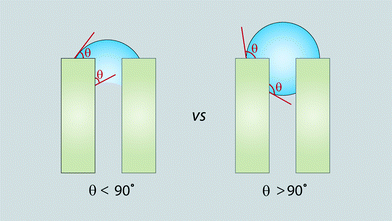
As stated earlier, it is generally accepted that fouling increases for membranes with more hydrophobic, less negatively charged, and rougher surfaces. Hydrophilic surfaces hydrate the membrane surface by water molecules, which makes it less susceptible to initial organic fouling than hydrophobic surfaces.124 Analytical tools used for membrane protein-fouling characterization include SEM, TEM, radiolabelling, XPS, microspectrophotometry, EPRS (quantitative analysis of protein fouling/denaturation by chemical attachment of spin labels to protein), SANS (in situ measurement for quantification and location of protein fouling, thickness, and structural characterization), NMR, and SIMS (differentiating adsorbed proteins and determining orientation/conformation of adsorbed proteins).267
Water contact angle is a measure of wettability of the membrane. This measurement is the most commonly used technique to evaluate hydrophobicity/hydrophilicity, where smaller contact angles correspond to more hydrophilic surfaces. Contact angle measurement is based on three-phase equilibrium, which occurs at solid/liquid/vapor or solid/liquid/liquid interfaces (e.g., membrane/water/air). Typically, a drop of water (~ a few μL) is placed on a membrane surface, and the contact angle θ between the water droplet and the membrane surface is measured using a goniometer. In principle, if the measured contact angle is less than 90°, spontaneous water intrusion to the pore can occur without additional pressure whereas if the contact angle is higher than 90°, extra pressure is needed for permeation to occur as shown in Fig. 19. However, even if the contact angle is less than 90°, it is possible to take some time to wet the membrane due to some degree of surface roughness. Also, even if the contact angle is higher than 90°, pores of the membrane may eventually be wet due to surface defects, condensation of water vapor or other processes. Interestingly, despite the widespread understanding in the field that hydrophobicity and fouling are intimately related, a recent article by Rana and Matsuura identifies only a few studies in which membrane fouling is directly correlated to the hydrophilicity/hydrophobicity of the membrane surface. Further, except for the membrane surface charge, these parameters are based on correlation of data, which are, at best, valid within a small range of interfacial property values.129 As such, one should be cautious when extrapolating correlation data, especially for extreme contact angle values.
5.3. Membrane modeling and simulation
Ideally, a modeling system will not only predict membrane performance but also help to optimize the separation process. Accurate modeling will shed light on separation mechanisms during filtration that can produce substantial opportunities for productivity improvements and cost savings. As such, modeling and simulation are an integral part of membrane research and have become an increasingly necessary tool for industry as well.In general, modeling and simulation for filtration processes can be divided into three levels – molecular, mesoscale, and macroscale modeling. Macroscale modeling, which is beyond the focus of this review, deals with design and optimization of processing parameters of membrane-based filter modules for application in, for example, wastewater treatment plants.
Mesoscale modeling (such as at the single-filter module level) generally deals with flow, rejection, flux, and fluidic transport. As discussed in section 4, membranes with various structures can be fabricated using different synthetic methods and materials. In the case of UF and MF processes, when a membrane is porous and water flow is laminar (flow layers travel a regular path or travel smoothly over one another cf. turbulent flow, in which the flow pattern involves irregular fluctuations and is time-dependent) a simple hydrodynamic theory can be applied and modelled using empirical equations: Darcy's Law, Hagen–Poiseuille equation, and Carman–Kozeny equation.268,269 Application of a specific equation depends on the pore structure factors such as pore size, shape, porosity, average capillary length, pore-size distribution, surface area, and tortuosity. In this section, we shall discuss the practical use of these equations. More detailed mathematical derivations and mechanisms of membrane transport can be found in ref. 268.
The law governing the flow of fluid established using porous plugs of sand between two fluid reservoirs was developed by Darcy. He observed that the flow rate is directly proportional to the hydrostatic pressure along the length of a membrane consisting of sand, 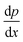 . Darcy's law expresses that the average velocity, ū, through the porous membrane has the following relation:
. Darcy's law expresses that the average velocity, ū, through the porous membrane has the following relation:
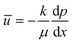 | (5.1) |
In the case of a cylindrical pore structure (Fig. 20), the Hagen–Poiseuille equation describing laminar flow in a pipe is used to characterize the flow rate:
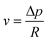 | (5.2) |
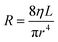 | (5.3) |
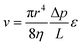 | (5.4) |
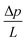 is taken as a pressure gradient across the capillary as seen in Darcy's Law,
is taken as a pressure gradient across the capillary as seen in Darcy's Law, 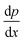 . It should be noted that the flow rate is sensitive to the radius of the capillary (v ∝ r4). For example, a typical pore diameter of a MF membrane ranges from 0.1–5 μm, which is approximately 100-fold larger than the average pore diameter of an UF membrane as shown in Fig. 3. This means the permeance (flux per unit pressure difference) in MF is significantly higher than that in UF, as such, different operating pressures are required.
. It should be noted that the flow rate is sensitive to the radius of the capillary (v ∝ r4). For example, a typical pore diameter of a MF membrane ranges from 0.1–5 μm, which is approximately 100-fold larger than the average pore diameter of an UF membrane as shown in Fig. 3. This means the permeance (flux per unit pressure difference) in MF is significantly higher than that in UF, as such, different operating pressures are required.
For porous media with noncircular cross section, Kozeny developed a hydrodynamic equation based on the assumption that the flow path is random and tortuous.268 Using the concept of the hydraulic radius, the Carman–Kozeny equation is
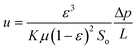 | (5.5) |
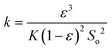 | (5.6) |
For NF processes, transport through the membrane is broken down into two separate components: convection and diffusion. Electrostatic interactions are an important factor when addressing charged membrane surfaces and charged molecules such as salts and ions. Modeling water flux for NF is related primarily to the pressure (Δp), which is analogous to MF and UF described earlier using the Hagen–Poiseuille equation (osmotic pressure needs to be taken into account when water contains high salt concentrations).270 In the case of convection, water flux is dependent on the applied pressure (water flux and convective flux of species in water are high at high pressure). In contrast, the diffusion process (solute flux) is independent of pressure, as such, diffusion processes permeate the species through the membrane regardless of the pressure but rather related to the solute concentration gradient across the membrane. Commonly adopted NF models are those based on the Extended Nernst–Planck equation with the Donnan steric equilibrium at the membrane and solution interfaces.271,272 The Extended Nernst–Planck equation describing the solute flux (Ji) is:
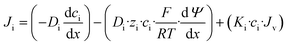 | (5.7) |
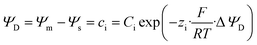 | (5.8) |
 | ||
| Fig. 21 Schematic illustration of porous membranes with different tortuosity (τ). Fig. adapted from ref. 10. | ||
In order to have better understanding of filtration mechanisms, numerical simulations on a smaller scale are often carried out. Molecular dynamics (MD) is one such tool where the physical motion of atoms and molecules is simulated. Newtonian equations of motion are solved numerically for a system of interacting particles, and the forces between particles are defined by inter atomic potentials. Through MD simulations, static and dynamic properties (e.g., membrane material property characterization or solute movement through and on the membrane including phase-space trajectory followed by individual atoms) of a membrane system may be investigated on a molecular level.276 Graphene-derived functional membranes (especially GO) have emerged as excellent candidates for filtration process as described in an earlier section.277,278 Ning Wei et al. have numerically studied the water permeation mechanism in graphene oxide membranes by performing atomistic simulations and continuum mechanics-based analysis.279 By considering flow through the interlayers of GO, expanded channels such as wrinkles of inter-edge spaces and pores within the sheet, the study concluded that the porous microstructure is the origin of fast flow of water and suggests a hydrogen-bond-mediated side-pinning effect by water confined between oxidized and pristine regions in GO membranes (Fig. 22). The previously proposed mechanism of ultrafast flow between pristine GO membranes invoked an atomically smooth graphitic surface leading to ultralow friction.100,277
 | ||
| Fig. 22 Schematic illustration of (A) microstructures of graphene-derived membranes. The percolated water transport channel is composed of interlayer, inter-edge spaces, wrinkles, and pores. The pristine and oxidized patterns on GO (bottom left) are modeled in a quasi-2D molecular model (bottom center) with oxygen-containing functionalization groups on both sides (bottom right). (B) Schematic models for water flow between graphene or GO membranes. Water flow between graphene sheets experiencing significant boundary slip as such velocity profile is almost flat. (C) Reduction of flow between GO sheets with a much shorter slip length. (D) Flow within pristine and oxidized graphene regions with widths wG and wO, respectively. The edge-pinning effect breaks down the ultrafast flow within the pristine channel. Reprinted from ref. 279 Copyright (2014) American Chemical Society. | ||
Numerical simulations can also provide insight into membrane fouling.280–282 It is found that the adhesion force between the foulant and membrane originates from (i) hydrogen bonding (ii) van der Waals interaction and (iii) ionic-bridge binding. MD studies of CNT-based membranes also have been carried out. However, the majority of MD studies including CNTs focus on desalination and RO processes (beyond the scope of this review) dealing with aqueous salt solutions of different concentrations, ionic properties, and water properties (flow, diffusion, solvation energy, etc.).283–287 Along with a brief background about classical MD, Ebro et al. recently summarized MD simulations used in these membrane-based water treatment processes.276
6. Outlook and conclusions
Energy and water represent perhaps the two most pressing challenges we face as a society, and they are intimately interrelated. Sustainable, low-cost supplies of clean water and energy are crucial for global prosperity, health, and security. Membrane technology, in particular, can be anticipated to maintain a dominant role in water purification because it is both effective and energy-efficient.Membranes effectively remove molecular and mesoscopic pollutants, including microorganisms and colloids, though challenges remain in their large-scale utilization. Regardless of membrane type, common goals are: 1) high flux, permeation, and rejection, 2) mechanical, chemical, thermal, and temporal stability, 3) system design including processibility into large scale, 4) cost-effectiveness, and 5) anti-fouling.
In practice, these demands placed on membrane technology for water purification are aggressive. Remarkable progress has been made in establishing new fabrication methods for tailoring membrane pore structures, surface properties, and morphology. Despite recent advances in synthesis of novel membrane materials, surface modification/functionalization methods, and optimization of operating design and conditions there remains an urgent need to produce reliable membranes with designed characteristics especially toward addressing membrane fouling (biofouling, scaling, organic, and colloidal fouling) issues.129,130,288,289 The prevention of fouling remains an unsolved problem in water treatment leading to high operational costs and low product efficiency. To enable the next generation of progress in membrane technology, innovative surface engineering and fabrication methods to develop multi-functional membranes with exceptional antifouling, antimicrobial, and photocatalytic properties may be needed. In this regard, composite membrane materials are promising candidates, especially those incorporating functional nanomaterials in a “smart” polymer matrix. Indeed, GO-, CNT-, and inorganic NP-based membranes (and hybrid membranes) represent a true next generation of materials. In depth understanding of their physicochemical properties and development of controlled interactions between nanomaterials and hosts need to be established in order to increase reproducibility of production and performance. Thorough understanding of transport mechanisms of water and solute in the membrane, and the role of microscopic membrane properties in macroscopic performance still remains elusive. In addition, common frameworks for risk research/assessment/management need to be established. Environmental impact and toxicology of these nanomaterials in long-term use must be evaluated and mitigated.
Clean water scarcity is a massive and burgeoning challenge worldwide; advanced water purification technologies will be an indispensable pillar required to meet our future needs. Through innovative fabrication, processing methods, materials selection, and systematic studies for identifying key parameters (effect/influence of membrane structures, pores, surface roughness and charge, and understanding/predicting interactions between solutes and membrane), research and development of membrane technology promises to play a key role in addressing this global water crisis.
Acknowledgements
Use of the Center for Nanoscale Materials was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. The authors gratefully acknowledge support from a University of Chicago-Argonne National Laboratory Water Initiative grant.Notes and references
- http://www.un.org/waterforlifedecade/background.shtml .
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Marinas and A. M. Mayes, Nature, 2008, 452, 301–310 CrossRef CAS PubMed.
- J. Eliasson, Nature, 2015, 517, 6 CrossRef CAS PubMed.
- M. Elimelech and W. A. Phillip, Science, 2011, 333, 712–717 CrossRef CAS PubMed.
- L. F. Greenlee, D. F. Lawler, B. D. Freeman, B. Marrot and P. Moulin, Water Res., 2009, 43, 2317–2348 CrossRef CAS PubMed.
- K. W. Lawson and D. R. Lloyd, J. Membr. Sci., 1997, 124, 1–25 CrossRef CAS.
- M. F. A. Goosen, S. S. Sablani, H. Ai-Hinai, S. Ai-Obeidani, R. Al-Belushi and D. Jackson, Sep. Sci. Technol., 2004, 39, 2261–2297 CrossRef CAS.
- R. Semiat, Environ. Sci. Technol., 2008, 42, 8193–8201 CrossRef CAS PubMed.
- N. Ghaffour, J. Bundschuh, H. Mahmoudi and M. F. A. Goosen, Desalination, 2015, 356, 94–114 CrossRef CAS.
- R. W. Baker, Membrane technology and applications, John Wiley & Sons, Ltd., Chichester, 2nd edn, 2004 Search PubMed.
- B. S. Lalia, V. Kochkodan, R. Hashaikeh and N. Hilal, Desalination, 2013, 326, 77–95 CrossRef CAS.
- P. Apel, Radiat. Meas., 2001, 34, 559–566 CrossRef CAS.
- Y.-H. Zhao, Y.-L. Qian, B.-K. Zhu and Y.-Y. Xu, J. Membr. Sci., 2008, 310, 567–576 CrossRef CAS.
- S. H. Tabatabaei, P. J. Carreau and A. Ajji, J. Membr. Sci., 2008, 325, 772–782 CrossRef CAS.
- S. Loeb and S. Sourirajan, in Advances in Chemistry Series Number 28, American Chemical Society, Washington, DC, 1963, pp. 117–132 Search PubMed.
- M. Ulbricht, Polymer, 2006, 47, 2217–2262 CrossRef CAS.
- M.-L. Pellegrin, J. Aguinaldo, S. Arabi, M. E. Sadler, K. Min, M. Liu, C. Salamon, A. D. Greiner, J. Diamond, R. McCandless, C. Owerdieck, J. Wert and L. P. Padhye, Water Environ. Res., 2013, 85, 1092–1175 CrossRef.
- G. Jonsson, Desalination, 1985, 53, 3–10 CrossRef CAS.
- E. Arkhangelsky, A. Duek and V. Gitis, J. Membr. Sci., 2012, 394, 89–97 CrossRef.
- H. Bechhold, Z. Phys. Chem., 1907, 60, 257 Search PubMed.
- N. Hilal, H. Al-Zoubi, N. A. Darwish, A. W. Mohamma and M. Abu Arabi, Desalination, 2004, 170, 281–308 CrossRef CAS.
- K. P. Lee, T. C. Arnot and D. Mattia, J. Membr. Sci., 2011, 370, 1–22 CrossRef CAS.
- B. Van der Bruggen and C. Vandecasteele, Desalination, 2002, 143, 207–218 CrossRef CAS.
- T. Humplik, J. Lee, S. C. O'Hern, B. A. Fellman, M. A. Baig, S. F. Hassan, M. A. Atieh, F. Rahman, T. Laoui, R. Karnik and E. N. Wang, Nanotechnology, 2011, 22, 292001 CrossRef CAS PubMed.
- T. Y. Cath, A. E. Childress and M. Elimelech, J. Membr. Sci., 2006, 281, 70–87 CrossRef CAS.
- A. ElMekawy, H. M. Hegab and D. Pant, Energy Environ. Sci., 2014, 7, 3921–3933 CAS.
- M. C. Porter, Handbook of industrial membrane technology, 1989 Search PubMed.
- W. S. Winston Ho and K. K. Sirkar, Membrane Handbook, Springer Science & Business Media, New York, 1992 Search PubMed.
- Comprehensive Membrane Science and Engineering, ed. E. Drioli and L. Giorno, Elsevier B.V., 2010 Search PubMed.
- K. A. DeFriend, M. R. Wiesner and A. R. Barron, J. Membr. Sci., 2003, 224, 11–28 CrossRef CAS.
- I. Mohmood, C. B. Lopes, I. Lopes, I. Ahmad, A. C. Duarte and E. Pereira, Environ. Sci. Pollut. Res., 2013, 20, 1239–1260 CrossRef CAS PubMed.
- S. Kumar, W. Ahlawat, G. Bhanjana, S. Heydarifard, M. M. Nazhad and N. Dilbaghi, J. Nanosci. Nanotechnol., 2014, 14, 1838–1858 CrossRef CAS PubMed.
- X. W. Zhang, D. K. Wang and J. C. D. da Costa, Catal. Today, 2014, 230, 47–54 CrossRef CAS.
- Z. Liu, Y. He, F. Li and Y. Liu, Environ. Sci. Pollut. Res., 2006, 13, 328–332 CrossRef CAS PubMed.
- D. Kanakaraju, B. D. Glass and M. Oelgemoller, Environ. Chem. Lett., 2014, 12, 27–47 CrossRef CAS.
- J. H. Pan, X. Zhang, A. J. Du, D. D. Sun and J. O. Leckie, J. Am. Chem. Soc., 2008, 130, 11256–11257 CrossRef CAS PubMed.
- M. N. Chong, B. Jin, C. W. K. Chow and C. Saint, Water Res., 2010, 44, 2997–3027 CrossRef CAS PubMed.
- E. M. Espeland and R. G. Wetzel, Microb. Ecol., 2001, 42, 572–585 CrossRef CAS PubMed.
- M. R. Hoffmann, S. T. Martin, W. Y. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- I. H. Cho, J. H. Park and Y. G. Kim, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2005, 40, 1033–1044 CrossRef CAS.
- N. H. H. Hairom, A. W. Mohammad and A. A. H. Kadhum, Sep. Purif. Technol., 2014, 137, 74–81 CrossRef CAS.
- K. Hashimoto, H. Irie and A. Fujishima, Jpn. J. Appl. Phys., Part 1, 2005, 44, 8269–8285 CrossRef CAS.
- A. Fujishima, X. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63, 515–582 CrossRef CAS.
- U. I. Gaya and A. H. Abdullah, J. Photochem. Photobiol., C, 2008, 9, 1–12 CrossRef CAS.
- J. Schneider, M. Matsuoka, M. Takeuchi, J. L. Zhang, Y. Horiuchi, M. Anpo and D. W. Bahnemann, Chem. Rev., 2014, 114, 9919–9986 CrossRef CAS PubMed.
- H. Choi, A. C. Sofranko and D. D. Dionysiou, Adv. Funct. Mater., 2006, 16, 1067–1074 CrossRef CAS.
- H. Choi, E. Stathatos and D. D. Dionysiou, Desalination, 2007, 202, 199–206 CrossRef CAS.
- S. W. Leong, A. Razmjou, K. Wang, K. Hapgood, X. W. Zhang and H. T. Wang, J. Membr. Sci., 2014, 472, 167–184 CrossRef CAS.
- T. Hasegawa, A. B. Béléké and M. Mizuhata, J. Power Sources, 2013, 233, 148–156 CrossRef CAS.
- X. Zhang, T. Zhang, J. Ng and D. D. Sun, Adv. Funct. Mater., 2009, 19, 3731–3736 CrossRef CAS.
- A. Hu, X. Zhang, K. D. Oakes, P. Peng, Y. N. Zhou and M. R. Servos, J. Hazard. Mater., 2011, 189, 278–285 CrossRef CAS PubMed.
- S. P. Albu, A. Ghicov, J. M. Macak, R. Hahn and P. Schmuki, Nano Lett., 2007, 7, 1286–1289 CrossRef CAS PubMed.
- C. P. Athanasekou, G. E. Romanos, F. K. Katsaros, K. Kordatos, V. Likodimos and P. Falaras, J. Membr. Sci., 2012, 392, 192–203 CrossRef.
- Y. F. Gu and S. T. Oyama, J. Membr. Sci., 2009, 345, 267–275 CrossRef CAS.
- H. Zhang, H. Zhao, P. Liu, S. Zhang and G. Li, J. Membr. Sci., 2009, 343, 212–218 CrossRef CAS.
- L. Liu, Z. Y. Liu, H. W. Bai and D. D. Sun, Water Res., 2012, 46, 1101–1112 CrossRef CAS PubMed.
- X. W. Zhang, A. J. Du, P. Lee, D. D. Sun and J. O. Leckie, Appl. Catal., B, 2008, 84, 262–267 CrossRef CAS.
- A. J. Karabelas, K. V. Plakas, V. C. Sarasidis and S. I. Patsios, Global NEST J., 2014, 16, 516–524 CAS.
- N. Ma, Y. Zhang, X. Quan, X. Fan and H. Zhao, Water Res., 2010, 44, 6104–6114 CrossRef CAS PubMed.
- M. A. Lazar, S. Varghese and S. S. Nair, Catalysts, 2012, 2, 572–601 CrossRef CAS.
- J. Romao, D. Barata, P. Habibovic, G. Mul and J. Baltrusaitis, Anal. Chem., 2014, 86, 7612–7617 CrossRef CAS PubMed.
- Q. Wen, J. Di, Y. Zhao, Y. Wang, L. Jiang and J. Yu, Chem. Sci., 2013, 4, 4378–4382 RSC.
- V. Tajer-Kajinebaf, H. Sarpoolaky and T. Mohammadi, Ceram. Int., 2014, 40, 1747–1757 CrossRef CAS.
- M. Pelaez, N. T. Nolan, S. C. Pillai, M. K. Seery, P. Falaras, A. G. Kontos, P. S. M. Dunlop, J. W. J. Hamilton, J. A. Byrne, K. O'Shea, M. H. Entezari and D. D. Dionysiou, Appl. Catal., B, 2012, 125, 331–349 CrossRef CAS.
- R. Asahi, T. Morikawa, H. Irie and T. Ohwaki, Chem. Rev., 2014, 114, 9824–9852 CrossRef CAS PubMed.
- J. Blanco-Galvez, P. Fernández-Ibáñez and S. Malato-Rodríguez, J. Sol. Energy Eng., 2006, 129, 4–15 CrossRef.
- S. Rehman, R. Ullah, A. M. Butt and N. D. Gohar, J. Hazard. Mater., 2009, 170, 560–569 CrossRef CAS PubMed.
- D. A. Keane, K. G. McGuigan, P. F. Ibanez, M. I. Polo-Lopez, J. A. Byrne, P. S. M. Dunlop, K. O'Shea, D. D. Dionysiou and S. C. Pillai, Catal. Sci. Technol., 2014, 4, 1211–1226 CAS.
- S. Banerjee, S. C. Pillai, P. Falaras, K. E. O'Shea, J. A. Byrne and D. D. Dionysiou, J. Phys. Chem. Lett., 2014, 5, 2543–2554 CrossRef CAS PubMed.
- N. G. Moustakas, F. K. Katsaros, A. G. Kontos, G. E. Romanos, D. D. Dionysiou and P. Falaras, Catal. Today, 2014, 224, 56–69 CrossRef CAS.
- X. P. Cao, D. Li, W. H. Jing, W. H. Xing and Y. Q. Fan, J. Mater. Chem., 2012, 22, 15309–15315 RSC.
- A. K. Geim, Science, 2009, 324, 1530–1534 CrossRef CAS PubMed.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- S. Morales-Torres, L. Pastrana-Martínez, J. Figueiredo, J. Faria and A. T. Silva, Environ. Sci. Pollut. Res., 2012, 19, 3676–3687 CrossRef CAS PubMed.
- C. P. Athanasekou, S. Morales-Torres, V. Likodimos, G. E. Romanos, L. M. Pastrana-Martinez, P. Falaras, D. D. Dionysiou, J. L. Faria, J. L. Figueiredo and A. M. T. Silva, Appl. Catal., B, 2014, 158, 361–372 CrossRef.
- S. Ciston, R. M. Lueptow and K. A. Gray, J. Membr. Sci., 2009, 342, 263–268 CrossRef CAS.
- S. Ciston, R. M. Lueptow and K. A. Gray, J. Membr. Sci., 2008, 320, 101–107 CrossRef CAS.
- M. Facciotti, V. Boffa, G. Magnacca, L. B. Jorgensen, P. K. Kristensen, A. Farsi, K. Konig, M. L. Christensen and Y. Yue, Ceram. Int., 2014, 40, 3277–3285 CrossRef CAS.
- K. Konig, V. Boffa, B. Buchbjerg, A. Farsi, M. L. Christensen, G. Magnacca and Y. Yue, J. Membr. Sci., 2014, 472, 232–240 CrossRef CAS.
- M. M. Pendergast and E. M. V. Hoek, Energy Environ. Sci., 2011, 4, 1946–1971 CAS.
- T. Pradeep and Anshup, Thin Solid Films, 2009, 517, 6441–6478 CrossRef CAS.
- J. G. Li, T. T. Zhao, T. K. Chen, Y. B. Liu, C. N. Ong and J. P. Xie, Nanoscale, 2015, 7, 7502–7519 RSC.
- S. Thatai, P. Khurana, J. Boken, S. Prasad and D. Kumar, Microchem. J., 2014, 116, 62–76 CrossRef CAS.
- M. T. Amin and A. A. Alazba, Membrane Water Treatment, 2014, 5, 123–146 CrossRef.
- P. K. Stoimenov, R. L. Klinger, G. L. Marchin and K. J. Klabunde, Langmuir, 2002, 18, 6679–6686 CrossRef CAS.
- G. Ghasemzadeh, M. Momenpour, F. Omidi, M. R. Hosseini, M. Ahani and A. Barzegari, Front. Environ. Sci. Eng., 2014, 8, 471–482 CrossRef CAS.
- Y. C. Sharma, V. Srivastava, S. N. Upadhyay and C. H. Weng, Ind. Eng. Chem. Res., 2008, 47, 8095–8100 CrossRef CAS.
- S. M. Ponder, J. G. Darab and T. E. Mallouk, Environ. Sci. Technol., 2000, 34, 2564–2569 CrossRef CAS.
- B. J. Allred and B. C. Tost, Water Environ. Res., 2014, 86, 2221–2232 CrossRef CAS PubMed.
- T. Ahmed, S. Imdad, K. Yaldram, N. M. Butt and A. Pervez, Desalin. Water Treat., 2014, 52, 4089–4101 CrossRef CAS.
- J. Wehling, J. Koser, P. Lindner, C. Luder, S. Beutel, S. Kroll and K. Rezwan, Mater. Sci. Eng., C, 2015, 48, 179–187 CrossRef CAS PubMed.
- L. F. Dumee, L. He, P. C. King, M. Le Moing, I. Gueller, M. Duke, P. D. Hodgson, S. Gray, A. J. Poole and L. Kong, J. Membr. Sci., 2015, 475, 552–561 CrossRef CAS.
- M. Cecilia Cruz, G. Ruano, M. Wolf, D. Hecker, E. Castro Vidaurre, R. Schmittgens and V. Beatriz Rajal, Chem. Eng. Res. Des., 2015, 94, 524–537 CrossRef PubMed.
- M. S. Mauter, Y. Wang, K. C. Okemgbo, C. O. Osuji, E. P. Giannelis and M. Elimelech, ACS Appl. Mater. Interfaces, 2011, 3, 2861–2868 CAS.
- S. Kawada, D. Saeki and H. Matsuyama, Colloids Surf., A, 2014, 451, 33–37 CrossRef CAS.
- H. Liu, H. Wang and X. Zhang, Adv. Mater., 2015, 27, 249–254 CrossRef CAS PubMed.
- H. Huang, Y. Ying and X. Peng, J. Mater. Chem. A, 2014, 2, 13772–13782 CAS.
- Y. Han, Z. Xu and C. Gao, Adv. Funct. Mater., 2013, 23, 3693–3700 CrossRef CAS.
- D. Cohen-Tanugi and J. C. Grossman, Nano Lett., 2012, 12, 3602–3608 CrossRef CAS PubMed.
- B. Mi, Science, 2014, 343, 740–742 CrossRef CAS PubMed.
- R. K. Joshi, P. Carbone, F. C. Wang, V. G. Kravets, Y. Su, I. V. Grigorieva, H. A. Wu, A. K. Geim and R. R. Nair, Science, 2014, 343, 752–754 CrossRef CAS PubMed.
- Y. Y. Lou, G. P. Liu, S. N. Liu, J. Shen and W. Q. Jin, Appl. Surf. Sci., 2014, 307, 631–637 CrossRef CAS.
- P. Sun, F. Zheng, K. Wang, M. Zhong, D. Wu and H. Zhu, Sci. Rep., 2014, 4, 6798 CrossRef CAS PubMed.
- A. S. Brady-Estevez, M. H. Schnoor, S. Kang and M. Elimelech, Langmuir, 2010, 26, 19153–19158 CrossRef CAS PubMed.
- M. S. Rahaman, C. D. Vecitis and M. Elimelech, Environ. Sci. Technol., 2012, 46, 1556–1564 CrossRef CAS PubMed.
- A. Srivastava, O. N. Srivastava, S. Talapatra, R. Vajtai and P. M. Ajayan, Nat. Mater., 2004, 3, 610–614 CrossRef CAS PubMed.
- Y. Baek, C. Kim, D. K. Seo, T. Kim, J. S. Lee, Y. H. Kim, K. H. Ahn, S. S. Bae, S. C. Lee, J. Lim, K. Lee and J. Yoon, J. Membr. Sci., 2014, 460, 171–177 CrossRef CAS.
- R. Das, M. E. Ali, S. B. Abd Hamid, S. Ramakrishna and Z. Z. Chowdhury, Desalination, 2014, 336, 97–109 CrossRef CAS.
- H. Ebro, Y. M. Kim and J. H. Kim, J. Membr. Sci., 2013, 438, 112–125 CrossRef CAS.
- H. M. Hegab and L. Zou, J. Membr. Sci., 2015, 484, 95–106 CrossRef CAS.
- Y. Cao and X. Li, Adsorption, 2014, 20, 713–727 CrossRef CAS.
- F. B. Li, Y. Yang, Y. Q. Fan, W. H. Xing and Y. Wang, J. Membr. Sci., 2012, 397, 17–23 CrossRef.
- V. Tajer-Kajinebaf, H. Sarpoolaky and T. Mohammadi, Ceram. Int., 2014, 40, 1747–1757 CrossRef CAS.
- X. W. Zhang, D. K. Wang, D. R. S. Lopez and J. C. D. da Costa, Chem. Eng. J., 2014, 236, 314–322 CrossRef CAS.
- M. Hu and B. Mi, Environ. Sci. Technol., 2013, 47, 3715–3723 CrossRef CAS PubMed.
- J. J. Qin, Y. Li, L. S. Lee and H. Lee, J. Membr. Sci., 2003, 218, 173–183 CrossRef CAS.
- T. Shibutani, T. Kitaura, Y. Ohmukai, T. Maruyama, S. Nakatsuka, T. Watabe and H. Matsuyama, J. Membr. Sci., 2011, 376, 102–109 CrossRef CAS.
- F. Soyekwo, Q. G. Zhang, C. Deng, Y. Gong, A. M. Zhu and Q. L. Liu, J. Membr. Sci., 2014, 454, 339–345 CrossRef CAS.
- E. Saljoughi and S. M. Mousavi, Sep. Purif. Technol., 2012, 90, 22–30 CrossRef CAS.
- A. L. Ahmad, A. A. Abdulkarim, B. S. Ooi and S. Ismail, Chem. Eng. J., 2013, 223, 246–267 CrossRef CAS.
- H. R. Lohokare, M. R. Muthu, G. P. Agarwal and U. K. Kharul, J. Membr. Sci., 2008, 320, 159–166 CrossRef CAS.
- I. C. Kim, H. G. Yun and K. H. Lee, J. Membr. Sci., 2002, 199, 75–84 CrossRef CAS.
- E. Yuliwati and A. F. Ismail, Desalination, 2011, 273, 226–234 CrossRef CAS.
- F. Liu, N. A. Hashim, Y. Liu, M. R. M. Abed and K. Li, J. Membr. Sci., 2011, 375, 1–27 CrossRef CAS.
- C. Zhao, J. Xue, F. Ran and S. Sun, Prog. Mater. Sci., 2013, 58, 76–150 CrossRef CAS.
- N. Nady, M. C. R. Franssen, H. Zuilhof, M. S. M. Eldin, R. Boom and K. Schroen, Desalination, 2011, 275, 1–9 CrossRef CAS.
- B. Van der Bruggen, J. Appl. Polym. Sci., 2009, 114, 630–642 CrossRef CAS.
- G.-D. Kang and Y.-M. Cao, J. Membr. Sci., 2014, 463, 145–165 CrossRef CAS.
- D. Rana and T. Matsuura, Chem. Rev., 2010, 110, 2448–2471 CrossRef CAS PubMed.
- V. Kochkodan, D. J. Johnson and N. Hilal, Adv. Colloid Interface Sci., 2014, 206, 116–140 CrossRef CAS PubMed.
- X. Fan, Y. Su, X. Zhao, Y. Li, R. Zhang, J. Zhao, Z. Jiang, J. Zhu, Y. Ma and Y. Liu, J. Membr. Sci., 2014, 464, 100–109 CrossRef CAS.
- M. K. Sinha and M. K. Purkait, J. Membr. Sci., 2013, 437, 7–16 CrossRef CAS.
- W. R. Bowen, T. A. Doneva and H. B. Yin, J. Membr. Sci., 2001, 181, 253–263 CrossRef.
- N. Pezeshk, D. Rana, R. M. Narbaitz and T. Matsuura, J. Membr. Sci., 2012, 389, 280–286 CrossRef CAS.
- E.-S. Kim, Q. Yu and B. Deng, Appl. Surf. Sci., 2011, 257, 9863–9871 CrossRef CAS.
- M. Ulbricht and G. Belfort, J. Appl. Polym. Sci., 1995, 56, 325–343 CrossRef CAS.
- M. Ulbricht and G. Belfort, J. Membr. Sci., 1996, 111, 193–215 CrossRef CAS.
- H. Chen and G. Belfort, J. Appl. Polym. Sci., 1999, 72, 1699–1711 CrossRef CAS.
- I. Yared, S.-L. Wang and M.-J. Wang, IEEE Trans. Plasma Sci., 2014, 42, 3847–3857 CrossRef CAS.
- X. Li, Y. Cao, G. Kang, H. Yu, X. Jie and Q. Yuan, J. Appl. Polym. Sci., 2014, 131, 41144 Search PubMed.
- H. Meng, Q. Cheng, H. Wang and C. Li, J. Chem., 2014, 304972 Search PubMed.
- M. Tao, F. Liu and L. Xue, J. Membr. Sci., 2015, 474, 224–232 CrossRef CAS.
- B. P. Tripathi, N. C. Dubey and M. Stamm, J. Membr. Sci., 2014, 453, 263–274 CrossRef CAS.
- Z. Wang, H. Ma, B. S. Hsiao and B. Chu, Polymer, 2014, 55, 366–372 CrossRef CAS.
- J.-S. Gu, H.-Y. Yu, L. Huang, Z.-Q. Tang, W. Li, J. Zhou, M.-G. Yan and X.-W. Wei, J. Membr. Sci., 2009, 326, 145–152 CrossRef CAS.
- M. I. Vázquez, R. de Lara, P. Galán and J. Benavente, Colloids Surf., A, 2005, 270–271, 245–251 CrossRef.
- K. J. Moses and Y. Cohen, J. Colloid Interface Sci., 2014, 436, 286–295 CrossRef CAS PubMed.
- C. Yang, X. Ding, R. J. Ono, H. Lee, L. Y. Hsu, Y. W. Tong, J. Hedrick and Y. Y. Yang, Adv. Mater., 2014, 26, 7346–7351 CrossRef CAS PubMed.
- R. Zhou, P.-F. Ren, H.-C. Yang and Z.-K. Xu, J. Membr. Sci., 2014, 466, 18–25 CrossRef CAS.
- D. J. Miller, D. R. Paul and B. D. Freeman, Polymer, 2014, 55, 1375–1383 CrossRef CAS.
- D. J. Miller, S. Kasemset, L. Wang, D. R. Paul and B. D. Freeman, J. Membr. Sci., 2014, 452, 171–183 CrossRef CAS.
- E. M. Van Wagner, A. C. Sagle, M. M. Sharma, Y.-H. La and B. D. Freeman, J. Membr. Sci., 2011, 367, 273–287 CrossRef CAS.
- W. Zhao, Y. Su, C. Li, Q. Shi, X. Ning and Z. Jiang, J. Membr. Sci., 2008, 318, 405–412 CrossRef CAS.
- J.-W. Lee, J. Jung, Y. H. Cho, S. K. Yadav, K. Y. Baek, H. B. Park, S. M. Hong and C. M. Koo, ACS Appl. Mater. Interfaces, 2014, 6, 14600–14607 CAS.
- D. Rana, R. M. Narbaitz, A.-M. Garand-Sheridan, A. Westgate, T. Matsuura, S. Tabe and S. Y. Jasim, J. Mater. Chem. A, 2014, 2, 10059–10072 CAS.
- P. Kanagaraj, S. Neelakandan and A. Nagendran, J. Appl. Polym. Sci., 2014, 131, 40320 CrossRef.
- S. H. Maruf, A. R. Greenberg, J. Pellegrino and Y. Ding, J. Membr. Sci., 2014, 452, 11–19 CrossRef CAS.
- J. R. Du, S. Peldszus, P. M. Huck and X. S. Feng, J. Membr. Sci., 2015, 475, 488–495 CrossRef CAS.
- A. M. Mika, R. F. Childs, J. M. Dickson, B. E. McCarry and D. R. Gagnon, J. Membr. Sci., 1995, 108, 37–56 CrossRef CAS.
- K. Hu and J. M. Dickson, J. Membr. Sci., 2007, 301, 19–28 CrossRef CAS.
- J. Hendri, A. Hiroki, Y. Maekawa, M. Yoshida and R. Katakai, Radiat. Phys. Chem., 2001, 60, 617–624 CrossRef CAS.
- Q. Wei, J. Li, B. Qian, B. Fang and C. Zhao, J. Membr. Sci., 2009, 337, 266–273 CrossRef CAS.
- J. Alam, L. A. Dass, M. S. Alhoshan and A. W. Mohammad, Adv. Polym. Technol., 2013, 32, E189–E197 CrossRef CAS.
- X. Chen, Y. He, C. C. Shi, W. G. Fu, S. Y. Bi, Z. Y. Wang and L. Chen, J. Membr. Sci., 2014, 469, 447–457 CrossRef CAS.
- M. K. Sinha and M. K. Purkait, J. Membr. Sci., 2014, 464, 20–32 CrossRef CAS.
- Z. Y. Han, C. Cheng, L. S. Zhang, C. D. Luo, C. X. Nie, J. Deng, T. Xiang and C. S. Zhao, Desalination, 2014, 349, 80–93 CrossRef CAS.
- K. Pan, R. Ren, B. Liang, L. Li, H. Li and B. Cao, J. Appl. Polym. Sci., 2014, 131, 40912 Search PubMed.
- H. R. Lohokare, S. C. Kumbharkar, Y. S. Bhole and U. K. Kharul, J. Appl. Polym. Sci., 2006, 101, 4378–4385 CrossRef CAS.
- D. Menne, F. Pitsch, J. E. Wong, A. Pich and M. Wessling, Angew. Chem., Int. Ed., 2014, 53, 5706–5710 CrossRef CAS PubMed.
- Y. H. La, B. D. McCloskey, R. Sooriyakumaran, A. Vora, B. Freeman, M. Nassar, J. Hedrick, A. Nelson and R. Allen, J. Membr. Sci., 2011, 372, 285–291 CrossRef CAS.
- S. Loeb and S. Sourirajan, in Saline Water Conversion—II, American Chemical Society, 1963, ch. 9, vol. 38, pp. 117–132 Search PubMed.
- D. Klemm, B. Heublein, H.-P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3393 CrossRef PubMed.
- T. Mohammadi and E. Saljoughi, Desalination, 2009, 243, 1–7 CrossRef CAS.
- C. Mu, Y. Su, M. Sun, W. Chen and Z. Jiang, J. Membr. Sci., 2010, 350, 293–300 CrossRef CAS.
- M. Hossein Razzaghi, A. Safekordi, M. Tavakolmoghadam, F. Rekabdar and M. Hemmati, J. Membr. Sci., 2014, 470, 547–557 CrossRef CAS.
- R. Mahendran, R. Malaisamy, G. Arthanareeswaran and D. Mohan, J. Appl. Polym. Sci., 2004, 92, 3665 CrossRef.
- S. J. Eichhorn, A. Dufresne, M. Aranguren, N. E. Marcovich, J. R. Capadona, S. J. Rowan, C. Weder, W. Thielemans, M. Roman, S. Renneckar, W. Gindl, S. Veigel, J. Keckes, H. Yano, K. Abe, M. Nogi, A. N. Nakagaito, A. Mangalam, J. Simonsen, A. S. Benight, A. Bismarck, L. A. Berglund and T. Peijs, J. Mater. Sci., 2010, 45, 1–33 CrossRef CAS.
- H. Ma, C. Burger, B. S. Hsiao and B. Chu, J. Membr. Sci., 2014, 454, 272–282 CrossRef CAS.
- R. A. Mulvenna, J. L. Weidman, B. Jing, J. A. Pople, Y. Zhu, B. W. Boudouris and W. A. Phillip, J. Membr. Sci., 2014, 470, 246–256 CrossRef CAS.
- W. A. Phillip, R. M. Dorin, J. Werner, E. M. V. Hoek, U. Wiesner and M. Elimelech, Nano Lett., 2011, 11, 2892–2900 CrossRef CAS PubMed.
- R. M. Dorin, W. A. Phillip, H. Sai, J. Werner, M. Elimelech and U. Wiesner, Polymer, 2014, 55, 347–353 CrossRef CAS.
- M. M. Pendergast, R. Mika Dorin, W. A. Phillip, U. Wiesner and E. M. V. Hoek, J. Membr. Sci., 2013, 444, 461–468 CrossRef CAS.
- S. P. Nunes and A. Car, Ind. Eng. Chem. Res., 2013, 52, 993–1003 CrossRef CAS.
- S. Y. Yang, J. Park, J. Yoon, M. Ree, S. K. Jang and J. K. Kim, Adv. Funct. Mater., 2008, 18, 1371–1377 CrossRef CAS.
- E. A. Jackson and M. A. Hillmyer, ACS Nano, 2010, 4, 3548–3553 CrossRef CAS PubMed.
- W. A. Phillip, M. Amendt, B. O'Neill, L. Chen, M. A. Hillmyer and E. L. Cussler, ACS Appl. Mater. Interfaces, 2009, 1, 472–480 CAS.
- S. Y. Yang, I. Ryu, H. Y. Kim, J. K. Kim, S. K. Jang and T. P. Russell, Adv. Mater., 2006, 18, 709–712 CrossRef CAS.
- W. A. Phillip, B. O'Neill, M. Rodwogin, M. A. Hillmyer and E. L. Cussler, ACS Appl. Mater. Interfaces, 2010, 2, 847–853 CAS.
- Q. Q. Wang, X. T. Wang, Z. H. Wang, J. Huang and Y. Wang, J. Membr. Sci., 2013, 442, 57–64 CrossRef CAS.
- S. M. George, Chem. Rev., 2010, 110, 111–131 CrossRef CAS PubMed.
- T. Sheng, H. Chen, S. Xiong, X. Chen and Y. Wang, AIChE J., 2014, 60, 3622 CrossRef.
- X. H. Li, W. M. Yang, H. Y. Li, Y. Wang, M. M. Bubakir, Y. M. Ding and Y. C. Zhang, J. Appl. Polym. Sci., 2015, 132, 41601 Search PubMed.
- S. A. A. N. Nasreen, S. Sundarrajan, S. A. S. Nizar, R. Balamurugan and S. Ramakrishna, Polym. J., 2014, 46, 167–174 CrossRef CAS.
- T. Sheng, H. Chen, S. Xiong, X. Q. Chen and Y. Wang, AIChE J., 2014, 60, 3614–3622 CrossRef CAS.
- E. Eren, A. Sarihan, B. Eren, H. Gumus and F. O. Kocak, J. Membr. Sci., 2015, 475, 1–8 CrossRef CAS.
- A. Qin, X. Li, X. Zhao, D. Liu and C. He, J. Membr. Sci., 2015, 480, 1–10 CrossRef CAS.
- N. Derlon, J. Mimoso, T. Klein, S. Koetzsch and E. Morgenroth, Water Res., 2014, 60, 164–173 CrossRef CAS PubMed.
- J. Yin and B. Deng, J. Membr. Sci., 2015, 479, 256–275 CrossRef CAS.
- P. Wang, J. Ma, F. Shi, Y. Ma, Z. Wang and X. Zhao, Ind. Eng. Chem. Res., 2013, 52, 10355–10363 CrossRef CAS.
- P. S. Goh, B. C. Ng, W. J. Lau and A. F. Ismail, Sep. Purif. Rev., 2015, 44, 216–249 CrossRef CAS.
- W. J. Lau, A. F. Ismail, P. S. Goh, N. Hilal and B. S. Ooi, Sep. Purif. Rev., 2015, 44, 135–156 CrossRef CAS.
- W. J. Lau, A. F. Ismail, N. Misdan and M. A. Kassim, Desalination, 2012, 287, 190–199 CrossRef CAS.
- A. F. Ismail, M. Padaki, N. Hilal, T. Matsuura and W. J. Lau, Desalination, 2015, 356, 140–148 CrossRef CAS.
- M. L. Luo, J. Q. Zhao, W. Tang and C. S. Pu, Appl. Surf. Sci., 2005, 249, 76–84 CrossRef CAS.
- G. P. Wu, S. Y. Gan, L. Z. Cui and Y. Y. Xu, Appl. Surf. Sci., 2008, 254, 7080–7086 CrossRef CAS.
- A. Sotto, A. Boromand, R. Zhang, P. Luis, J. M. Arsuaga, J. Kim and B. Van der Bruggen, J. Colloid Interface Sci., 2011, 363, 540–550 CrossRef CAS PubMed.
- M. Safarpour, A. Khataee and V. Vatanpour, Ind. Eng. Chem. Res., 2014, 53, 13370–13382 CAS.
- L.-Y. Yu, H.-M. Shen and Z.-L. Xu, J. Appl. Polym. Sci., 2009, 113, 1772 Search PubMed.
- J. P. Mericq, J. Mendret, S. Brosillon and C. Faur, Chem. Eng. Sci., 2015, 123, 283–291 CrossRef CAS.
- S. Balta, A. Sotto, P. Luis, L. Benea, B. Van der Bruggen and J. Kim, J. Membr. Sci., 2012, 389, 155–161 CrossRef CAS.
- S. C. Hess, A. X. Kohll, R. A. Raso, C. M. Schumacher, R. N. Grass and W. J. Stark, ACS Appl. Mater. Interfaces, 2015, 7, 611–617 CAS.
- M. Liu, Z. Jia, D. Jia and C. Zhou, Prog. Polym. Sci., 2014, 39, 1498–1525 CrossRef CAS.
- H. Yu, Y. Zhang, X. Sun, J. Liu and H. Zhang, Chem. Eng. J., 2014, 237, 322–328 CrossRef CAS.
- Z. Wang, H. Wang, J. Liu and Y. Zhang, Desalination, 2014, 344, 313–320 CrossRef CAS.
- L. Yan, Y. S. Li, C. B. Xiang and S. Xianda, J. Membr. Sci., 2006, 276, 162–167 CrossRef CAS.
- F. Liu, M. R. M. Abed and K. Li, J. Membr. Sci., 2011, 366, 97–103 CrossRef CAS.
- Y. Liu, G. H. Koops and H. Strathmann, J. Membr. Sci., 2003, 223, 187–199 CrossRef CAS.
- J. Garcia-Ivars, M. I. Alcaina-Miranda, M. I. Iborra-Clar, J. A. Mendoza-Roca and L. Pastor-Alcaniz, Sep. Purif. Technol., 2014, 128, 45–57 CrossRef CAS.
- J. M. Arsuaga, A. Sotto, G. del Rosario, A. Martinez, S. Molina, S. B. Teli and J. de Abajo, J. Membr. Sci., 2013, 428, 131–141 CrossRef.
- J. H. Li, X. S. Shao, Q. Zhou, M. Z. Li and Q. Q. Zhang, Appl. Surf. Sci., 2013, 265, 663–670 CrossRef CAS.
- P. C. Bernardes, N. J. de Andrade, L. H. M. da Silva, A. F. de Carvalho, P. E. Fernandes, E. A. Araujo, C. A. Lelis, P. C. G. Mol and J. P. N. de Sa, J. Nanosci. Nanotechnol., 2014, 14, 6355–6367 CrossRef CAS PubMed.
- J. A. Prince, S. Bhuvana, K. V. K. Boodhoo, V. Anbharasi and G. Singh, J. Membr. Sci., 2014, 454, 538–548 CrossRef CAS.
- B. M. Ganesh, A. M. Isloor and A. F. Ismail, Desalination, 2013, 313, 199–207 CrossRef CAS.
- Z. H. Wang, H. R. Yu, J. F. Xia, F. F. Zhang, F. Li, Y. Z. Xia and Y. H. Li, Desalination, 2012, 299, 50–54 CrossRef CAS.
- X. Chang, Z. Wang, S. Quan, Y. Xu, Z. Jiang and L. Shao, Appl. Surf. Sci., 2014, 316, 537–548 CrossRef CAS.
- C. Xu, Y. Xu and J. Zhu, ACS Appl. Mater. Interfaces, 2014, 6, 16117–16123 CAS.
- S. Zinadini, A. A. Zinatizadeh, M. Rahimi, V. Vatanpour and H. Zangeneh, J. Membr. Sci., 2014, 453, 292–301 CrossRef CAS.
- C. Zhao, X. Xu, J. Chen and F. Yang, Desalination, 2014, 334, 17–22 CrossRef CAS.
- J. H. Choi, J. Jegal and W. N. Kim, J. Membr. Sci., 2006, 284, 406–415 CrossRef CAS.
- G. Wu, S. Gan, L. Cui and Y. Xu, Appl. Surf. Sci., 2008, 254, 7080–7086 CrossRef CAS.
- F. G. Wilhelm, I. G. M. Pünt, N. F. A. van der Vegt, H. Strathmann and M. Wessling, J. Membr. Sci., 2002, 199, 167–176 CrossRef CAS.
- M. L. Steen, A. C. Jordan and E. R. Fisher, J. Membr. Sci., 2002, 204, 341–357 CrossRef CAS.
- E. Celik, H. Park, H. Choi and H. Choi, Water Res., 2011, 45, 274–282 CrossRef CAS PubMed.
- A. Rahimpour, M. Jahanshahi, S. Khalili, A. Mollahosseini, A. Zirepour and B. Rajaeian, Desalination, 2012, 286, 99–107 CrossRef CAS.
- V. Vatanpour, M. Esmaeili and M. H. D. A. Farahani, J. Membr. Sci., 2014, 466, 70–81 CrossRef CAS.
- Y. Gu, R. M. Dorin and U. Wiesner, Nano Lett., 2013, 13, 5323–5328 CrossRef CAS PubMed.
- J. Huang, H. Wang and K. Zhang, Desalination, 2014, 336, 8–17 CrossRef CAS.
- F. Li, L. Li, X. Liao and Y. Wang, J. Membr. Sci., 2011, 385, 1–9 CrossRef.
- Q. Xu, J. Yang, J. W. Dai, Y. Yang, X. Q. Chen and Y. Wang, J. Membr. Sci., 2013, 448, 215–222 CrossRef CAS.
- K. P. Lee, H. Leese and D. Mattia, Nanoscale, 2012, 4, 2621–2627 RSC.
- S. Zinadini, A. A. Zinatizadeh, M. Rahimi, V. Vatanpour, H. Zangeneh and M. Beygzadeh, Desalination, 2014, 349, 145–154 CrossRef CAS.
- S. Balta, A. Sotto, P. Luis, L. Benea, B. Van der Bruggen and J. Kim, J. Membr. Sci., 2012, 389, 155–161 CrossRef CAS.
- L. K. Wang, J. P. Chen, Y.-T. Hung and N. K. Shammas, Membrane and Desalination Technologies, Springer, 2011 Search PubMed.
- K. J. Howe, D. W. Hand, J. C. Crittenden, R. R. Trussell and G. Tchobanoglous, Principles of Water Treatment, John Wiley & Sons, 2012 Search PubMed.
- O. Agboola, J. Maree and R. Mbaya, Environ. Chem. Lett., 2014, 12, 241–255 CrossRef CAS.
- Y. Wyart, G. Georges, C. Demie, C. Amra and P. Moulin, J. Membr. Sci., 2008, 315, 82–92 CrossRef CAS.
- S. Z. Abdullah, P. R. Berube and D. J. Horne, J. Membr. Sci., 2014, 463, 113–125 CrossRef CAS.
- D. Johnson and N. Hilal, Desalination, 2015, 356, 149–164 CrossRef CAS.
- Y. Wyart, R. Tamime, L. Siozade, I. Baudin, K. Glucina, C. Deumie and P. Moulin, J. Membr. Sci., 2014, 472, 241–250 CrossRef CAS.
- D. Y. Khanukaeva, A. N. Filippov and A. V. Bildyukevich, Pet. Chem., 2014, 54, 498–506 CrossRef CAS.
- K. Singh, S. Devi, H. C. Bajaj, P. Ingole, J. Choudhari and H. Bhrambhatt, Sep. Sci. Technol., 2014, 49, 2630–2641 CrossRef CAS.
- W. R. Bowen and T. A. Doneva, Surf. Interface Anal., 2000, 29, 544–547 CrossRef CAS.
- A. Rahimpour, S. S. Madaeni and Y. Mansourpanah, J. Membr. Sci., 2010, 364, 380–388 CrossRef CAS.
- S. Waheed, A. Ahmad, S. M. Khan, G. Sabad e, T. Jamil, A. Islam and T. Hussain, Desalination, 2014, 351, 59–69 CrossRef CAS.
- A. M. ElHadidy, S. Peldszus and M. I. Van Dyke, J. Membr. Sci., 2013, 429, 373–383 CrossRef CAS.
- K. Boussu, B. Van der Bruggen, A. Volodin, J. Snauwaert, C. Van Haesendonck and C. Vandecasteele, J. Colloid Interface Sci., 2005, 286, 632–638 CrossRef CAS PubMed.
- A. M. Zaky, I. C. Escobar and C. L. Gruden, Environ. Prog. Sustainable Energy, 2013, 32, 449–457 CrossRef CAS.
- R. Heffernan, O. Habimana, A. J. C. Semião, H. Cao, A. Safari and E. Casey, Water Res., 2014, 67, 118–128 CrossRef CAS PubMed.
- D. B. Burns and A. L. Zydney, J. Membr. Sci., 2000, 172, 39–48 CrossRef CAS.
- D. Nanda, K.-L. Tung, Y.-L. Li, N.-J. Lin and C.-J. Chuang, J. Membr. Sci., 2010, 349, 411–420 CrossRef CAS.
- B. B. Vyas and P. Ray, Desalination, 2015, 362, 104–116 CrossRef CAS.
- G. Hagmeyer and R. Gimbel, Sep. Purif. Technol., 1999, 15, 19–30 CrossRef CAS.
- K. C. Khulbe, B. Kruczek, G. Chowdhury, S. Gagne, T. Matsuura and S. P. Verma, J. Membr. Sci., 1996, 111, 57–70 CrossRef CAS.
- K. C. Khulbe, T. Matsuura and H. J. Kim, J. Appl. Polym. Sci., 2000, 77, 2558–2560 CrossRef CAS.
- R. Lamsal, S. G. Harroun, C. L. Brosseau and G. A. Gagnon, Sep. Purif. Technol., 2012, 96, 7–11 CrossRef CAS.
- P. Chen, L. Cui and K. Zhang, J. Membr. Sci., 2015, 473, 36–44 CrossRef CAS.
- R. Chan and V. Chen, J. Membr. Sci., 2004, 242, 169–188 CrossRef CAS.
- R. B. Bird, W. E. Stewart and E. N. Lightfoot, Transport Phenomena, Revised, 2nd edn, John Wiley & Sons, USA, 2007 Search PubMed.
- S. S. H. R. A. K. Pabby and A. M. Sastre Requena, Handbook of Membrane Separations:Chemical, Pharmaceutical, Food, and Biotechnological Applications, Tayer&Francis Group, LLC, 2009 Search PubMed.
- S. Bandini and D. Vezzani, Chem. Eng. Sci., 2003, 58, 3303–3326 CrossRef CAS.
- T. Tsuru, S. Nakao and S. Kimura, J. Chem. Eng. Jpn., 1991, 24, 511–517 CrossRef CAS.
- W. R. Bowen, A. W. Mohammad and N. Hilal, J. Membr. Sci., 1997, 126, 91–105 CrossRef CAS.
- G. M. Geise, H.-S. Lee, D. J. Miller, B. D. Freeman, J. E. McGrath and D. R. Paul, J. Polym. Sci., Part B: Polym. Phys., 2010, 48, 1685–1718 CrossRef CAS.
- S. I. Patsios and A. J. Karabelas, Desalin. Water Treat., 2010, 21, 189–201 CrossRef CAS.
- M. F. R. Zuthi, H. H. Ngo and W. S. Guo, Bioresour. Technol., 2012, 122, 119–129 CrossRef CAS PubMed.
- H. Ebro, Y. M. Kim and J. H. Kim, J. Membr. Sci., 2013, 438, 112–125 CrossRef CAS.
- R. R. Nair, H. A. Wu, P. N. Jayaram, I. V. Grigorieva and A. K. Geim, Science, 2012, 335, 442–444 CrossRef CAS PubMed.
- H. Huang, Z. Song, N. Wei, L. Shi, Y. Mao, Y. Ying, L. Sun, Z. Xu and X. Peng, Nat. Commun., 2013, 4, 2979 Search PubMed.
- N. Wei, X. Peng and Z. Xu, ACS Appl. Mater. Interfaces, 2014, 6, 5877–5883 CAS.
- Y. Xiang, Y. Liu, B. Mi and Y. Leng, Langmuir, 2014, 30, 9098–9106 CrossRef CAS PubMed.
- A. Ronen, S. Lerman, G. Z. Ramon and C. G. Dosoretz, J. Membr. Sci., 2015, 475, 320–329 CrossRef CAS.
- Z. E. Hughes and J. D. Gale, J. Mater. Chem., 2012, 22, 175–184 RSC.
- G. Hummer, J. C. Rasaiah and J. P. Noworyta, Nature, 2001, 414, 188–190 CrossRef CAS PubMed.
- L. Wang, R. S. Dumont and J. M. Dickson, J. Chem. Phys., 2013, 138, 124701 CrossRef PubMed.
- L. Vukovic, E. Vokac and P. Kral, J. Phys. Chem. Lett., 2014, 5, 2131–2137 CrossRef CAS PubMed.
- A. Kalra, S. Garde and G. Hummer, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 10175–10180 CrossRef CAS PubMed.
- A. Striolo, Nano Lett., 2006, 6, 633–639 CrossRef CAS PubMed.
- G. Mustafa, K. Wyns, P. Vandezande, A. Buekenhoudt and V. Meynen, J. Membr. Sci., 2014, 470, 369–377 CrossRef CAS.
- I. Armentano, C. R. Arciola, E. Fortunati, D. Ferrari, S. Mattioli, C. F. Amoroso, J. Rizzo, J. M. Kenny, M. Imbriani and L. Visai, Sci. World J., 2014, 410423 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |