Research highlights: speciation and transformations of silver released from Ag NPs in three species
Natalie V.
Hudson-Smith
*a,
Peter L.
Clement
a,
Richard P.
Brown
b,
Miriam O. P.
Krause
d,
Joel A.
Pedersen
c and
Christy L.
Haynes
a
aDepartment of Chemistry, University of Minnesota, Minneapolis, MN 55455, USA. E-mail: hudso283@umn.edu
bDepartment of Chemistry and Biochemistry, University of Maryland Baltimore County, Baltimore, MD 21250, USA
cDepartment of Soil Science, Environmental Chemistry and Technology Program, University of Wisconsin, 1525 Observatory Dr., Madison, WI 53706, USA
dCenter for Sustainable Nanotechnology, Department of Chemistry, University of Minnesota, Minneapolis, MN 55455, USA
First published on 21st November 2016
Abstract
Antimicrobial silver nanoparticles used in consumer products may be released during fabrication, during product use, or after disposal and may reach terrestrial and aquatic ecosystems, prompting concern about their potential to adversely impact the environment (Benn and Westerhoff, Environ. Sci. Technol., 2008, 42, 4133, DOI: 10.1021/es7032718). Although the toxicity of pristine silver nanoparticles is well studied and understood, silver nanoparticles can undergo transformation during release and in engineered and natural environments. The speciation of silver after release must therefore be explored to deepen understanding of the potential impact of these nanoparticles on the environment. Herein, we highlight three articles which use highly sensitive analytical techniques to define, and in some cases map, silver speciation in situ after exposure to organisms of varying size and complexity. First, we highlight research by Leonardo et al. which explores the transformations of silver acted upon by a microalgae species that is a candidate for heavy metal remediation in water. Next, we highlight research by Stegemeier et al. quantifying and mapping the speciation of silver in alfalfa after exposure to several silver sources, including two silver-based nanoparticles. Finally, we discuss work by Wang et al. on silver speciation in human monocyte cells as observed by synchrotron radiation techniques which leads to mechanistic insights on cytotoxicity.
Introduction
Silver nanoparticles (Ag NPs) are increasingly incorporated into commercial products due to their antibacterial properties. Prior research has focused on the mode of antibacterial activity and toxicity of pristine silver nanoparticles, including consideration of factors such as size, shape, surface character, and coatings (Reidy et al., Materials, 2013, 6, 2295, DOI: 10.3390/ma6062295). Ag+ is known to be bactericidal, and antibacterial activity of silver nanoparticles results from their oxidative dissolution (Maurer and Meyer, Environ. Sci.: Nano, 2016, 3, 311, DOI: 10.1039/C5EN00187K). While understanding the toxicity of pristine Ag NPs is important, the bioavailability and toxicity of Ag NP transformation products (dissolved and particulate) in municipal wastewaters and natural surface waters must also be considered to evaluate the potential risks posed by the release of these materials into the environment. Once Ag NPs or their transformation products have been taken up by organisms, understanding the speciation of the silver within the organism provides insight into the mechanism(s) of toxicity and is needed to predict any adverse biological effects. In the three highlighted articles, silver speciation is determined in situ, speciation is mapped in organisms, and toxic effects of silver released from different sources are explored.Uptake- and concentration-dependent silver speciation in green microalgae exposed to aqueous silver ion
Leonardo et al. (Environ. Sci. Technol., 2016, 50, 359, DOI: 10.1021/acs.est.5b03306) present evidence that aqueous Ag+, released either directly as dissolved silver ions or indirectly as a byproduct of released silver nanoparticles from consumer products, is taken up by microalgae and internally sequestered. The authors cultured the microalgae Coccomyxa actinabiotis in ultrapure water to maintain the state of Ag+ prior to uptake by the microalgae. Microalgae were exposed to four Ag+ concentrations prepared by dissolution of AgNO3: 10−6 M, 10−5 M, 10−4 M, and 10−3 M. Silver speciation inside harvested microalgae was investigated by X-ray absorption spectroscopy (XAS), X-ray diffraction (XRD), transmission electron microscopy (TEM), and inductively coupled plasma mass spectrometry (ICP-MS). Toxicity of the silver to the microalgae was quantified by studies of photosynthesis and cellular growth.The study found that speciation of silver within microalgae depended on the concentration of Ag+ exposure. The X-ray absorption near-edge structure (XANES) spectra from algae samples exposed at 10−6 M and 10−5 M Ag+ most resemble an Ag2S reference, while the exposures of 10−4 M and 10−3 M most resemble the Ag(0) reference (Fig. 1). Extended X-ray absorption fine structure (EXAFS) spectra corroborate the charge state of silver in microalgae exposed to low AgNO3 concentrations (Fig. 1A and B) and also gives evidence for Ag+ ions complexed with thiol species. Within microalgae, thiolation may reflect a response to render the silver non-toxic. From EXAFS spectra at higher concentrations, the authors show evidence for silver nanoclusters within the microalgae with an imperfect FCC structure. Complementary to EXAFS spectra, X-ray diffraction (XRD) of algae samples exposed to the highest concentration (10−2 M) of silver also indicated nanoparticles with an FCC crystal structure. Scherrer-type analysis determined a mean silver nanocrystallite size of 10 nm.
To investigate the localization of the silver nanoparticles synthesized in vivo by C. actinabiotis, Leonardo et al. used TEM to image nanoparticles within cells (Fig. 2). The micrographs show nanoparticles ranging in size from 4–28 nm in diameter found mainly in the chloroplast and mitochondria of algae exposed to 10−4 M Ag+. At higher exposure concentrations, such as 10−2 M Ag+, these silver nanoparticles are found throughout the cell rather than being localized to the chloroplasts and mitochondria, although nanoparticle sizes remain comparable to those found with lower concentrations. The authors postulate that the initial localization of silver nanoparticles in the chloroplasts and mitochondria is due to the abundance of potential reducing agents, such as ferredoxin and NADH, within these organelles capable of reducing Ag+ to Ag(0).
Finally, toxicity of Ag+ to C. actinabiotis was assessed by monitoring growth and photosynthetic capacity of the microalgae. Quantification of photosynthetic capacity by measurement of the chlorophyll fluorescence yield is a particularly sensitive technique for assessing metal toxicity. Again, the microalgae demonstrated two different sets of behavior. At the concentration of 10−5 M Ag+, photosynthetic capacity dropped but the algae recovered after being transferred to a silver-free media. However, higher concentrations of Ag+ resulted in irreversible damage. Microalgae demonstrate two distinct pathways as a response to silver exposure. At low concentrations, internalized silver appears to be thiolated. At high concentrations, the microalgae synthesizes silver nanoparticles de novo within their cells. These mechanisms, resulting in modified silver speciation, suggest that this microalgae species may warrant consideration for recovering silver from waters that contain elevated levels of aqueous silver as a byproduct of silver nanoparticle release.
Uptake, distribution and speciation of silver in alfalfa exposed to silver and silver sulfide NPs
Next we highlight work by Stegemeier et al. (Environ. Sci. Technol., 2015, 49, 8451, DOI: 10.1021/acs.est.5b01147), which explored the differences in uptake, distribution and speciation of silver in hydroponically grown alfalfa plants exposed to AgNO3, Ag NPs (median size 6.3 nm by TEM) or Ag2S NPs (median size 7.8 nm by TEM). The authors began by analyzing silver uptake into the roots and the translocation of silver into the shoots of alfalfa plants exposed to either AgNO3, Ag NPs, or Ag2S NPs. For all silver sources, the authors found that 99% of alfalfa-associated silver remained in or on the root and only 1% or less of the alfalfa-associated silver was carried to the shoots. All three treatments led to statistically significant increases in the amount of silver in the roots and shoots compared to control plants not exposed to silver. Interestingly, despite the relatively low solubility of Ag2S NPs, uptake of silver from the three sources did not differ significantly. This suggests that dissolution of Ag2S NPs prior to interaction with the root is not a prerequisite for silver uptake resulting from exposure to Ag2S NPs. To explain these results, the authors suggest mechanisms of direct uptake of the Ag2S NPs and invoke a possible major role of root exudates in solubilizing Ag+ from Ag2S NPs.A major strength of this paper is the use of both high energy and low energy X-ray fluorescence (XRF) to produce elemental maps of alfalfa roots exposed to AgNO3, Ag NPs, or Ag2S NPs. This elemental mapping is helpful in understanding both the distribution and speciation of silver after adsorption or uptake. The low energy XRF (Fig. 3) provides high resolution images that indicate that Ag distribution differs depending on the Ag source. After exposure to Ag NPs, roots showed high Ag concentrations in the root cap and a fairly uniform, low concentration of Ag elsewhere in the root. In contrast, roots exposed to Ag2S NPs displayed little association of Ag with the root cap, instead showing highly concentrated areas of Ag on the exterior of the root and little Ag distributed elsewhere in the root. The preference of Ag from Ag2S NPs for the exterior of the root and the lack of Ag in the interior of the root points towards decreased uptake of Ag via dissolution of Ag2S NPs.
Stegemeier et al. used high energy XRF to map the ratio of Ag to S (Fig. 3) in alfalfa roots, which enabled the authors to study Ag distribution as well as speciation. After treatment with Ag NPs, high energy XRF confirms accumulation of Ag in the root cap and shows regions of high Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S on the exterior of the root cap. Aside from the enhanced accumulation in the root cap, the maps of Ag in AgNO3- and Ag NP-treated roots show similar distribution throughout the root. These observations indicate Ag NP association with the root cap prior to uptake and then partial dissolution of Ag NPs allowing for distribution throughout the root. This is supported by TEM images showing both direct uptake and partial dissolution of Ag NPs before uptake.
S on the exterior of the root cap. Aside from the enhanced accumulation in the root cap, the maps of Ag in AgNO3- and Ag NP-treated roots show similar distribution throughout the root. These observations indicate Ag NP association with the root cap prior to uptake and then partial dissolution of Ag NPs allowing for distribution throughout the root. This is supported by TEM images showing both direct uptake and partial dissolution of Ag NPs before uptake.
Roots of plants treated with Ag2S NPs showed no enhanced accumulation in the root cap, instead showing elevated levels of Ag in the root elongation zone. This points toward uptake occurring during periods of growth. Compared to the roots exposed to AgNO3 or Ag NPs, which showed distribution of silver throughout the root, the roots exposed to Ag2S NPs displayed lower levels of Ag throughout the root. Additionally, the ratio of Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S remained fairly low for roots exposed to Ag2S NPs, suggesting that little free Ag exists. Instead, Ag appears to either remain in the nanoparticles or be bound by thiol groups within the root.
S remained fairly low for roots exposed to Ag2S NPs, suggesting that little free Ag exists. Instead, Ag appears to either remain in the nanoparticles or be bound by thiol groups within the root.
In this article, Stegemeier et al. demonstrated that Ag from AgNO3, Ag NPs and Ag2S NPs is internalized by alfalfa via differing mechanisms depending on the source of silver exposure. Transformation of Ag NPs to Ag2S NPs reduces solubility of silver and has been shown to negate toxicity towards some organisms (Levard et al., Environ. Sci. Technol., 2013, 47, 13440, DOI: 10.1021/es403527n), and in this study, the plants treated with Ag2S NPs qualitatively appeared healthier than those treated with Ag NPs or AgNO3. However, this work shows that despite a decrease in solubility, Ag2S NPs are still internalized in alfalfa through direct uptake or after partial dissolution, likely facilitated by rhizosphere exudates. More work is clearly needed to fully understand the impact of Ag2S NPs and other environmentally relevant Ag NP transformation products on other organisms.
In situ detection of Ag NP speciation to determine mechanism of toxicity in human monocytes
Much work has gone into examining the toxicity of various nanoparticles in terms of cell death or survival, giving information about the endpoint without describing the process. To advance the goal of making safer nanomaterials, researchers must understand the underlying mechanism of toxicity, including a nanoparticle's transformation from an intact particle to all of its downstream chemical identities. Towards this objective, Wang et al. (ACS Nano, 2015, 9, 6532, DOI: 10.1021/acsnano.5b02483) provide a detailed view of the speciation and fate of silver nanoparticles in human monocyte cells. Their use of synchrotron radiation transmission X-ray microscopy (SR-TXM) and SR-X-ray absorption near edge structure (SR-XANES) allowed for detailed determination of the localization of silver nanoparticles (Ag NPs) upon uptake into human monocytes (THP-1) and of chemical transformation of the Ag NPs from Ag(0) to oxidized and thiolated chemistries (Ag+, Ag–O– and Ag–S–). Correlation of localization and speciation to markers for cell stress or death gives an in situ picture of nanoparticle toxicity and provides insight into ways that could make these materials safer.First, Wang et al. characterized the dose- and time-dependent cytotoxicity of the Ag NPs and Ag ions using conventional methods. They used water soluble Ag NPs (core particle diameter (TEM): ∼20 nm; hydrodynamic diameter (dynamic light scattering): ∼38 nm) coated in Tween-20 with a near-neutral zeta potential of −4 mV. Both Ag NPs and Ag ions showed similar trends in cell toxicity. Further experiments used 24 h exposures at 10 μg mL−1 for Ag NPs and 5 μg mL−1 for Ag ions to investigate underlying mechanisms.
Uptake of silver by THP-1 cells after 24 h exposure to Ag NPs was determined by ICP-MS. Exocytosis of the Ag NPs was measured by exchanging the cell media with nanoparticle-free media and measuring silver concentration by ICP-MS. After 24 and 48 h, 71% and 82% of Ag had been eliminated from the cells, respectively.
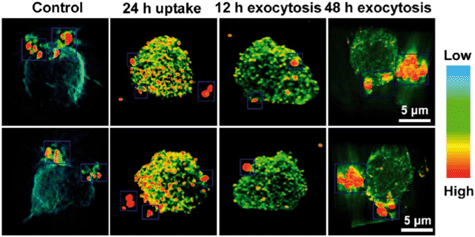
Silver localization was visualized in 3D using SR-TXM (Fig. 4) which relies on heavy elements (such as silver) absorbing X-rays much more strongly than light elements such as carbon, sulfur, and oxygen, to provide strong contrast between metallic nanoparticles and organic components of cells. Images of sequential optical slices were reconstructed to reveal high resolution 3D distribution of Ag NPs in a single THP-1 cell after 24 h of exposure to Ag NPs as well as following exocytosis of Ag NPs over time. Wang et al. provide video footage showing this process in the article's supporting information. The 3D localization provides a better understanding of the path that particles take into and out of the cell compared to 2D imaging techniques.
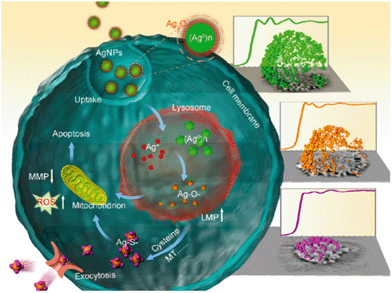
Perhaps the most significant contribution of their study comes from the use of SR-XANES to determine Ag NP speciation in situ (Fig. 5). Once Ag NPs enter the cell, they can end up in lysosomes where they are transformed to Ag+ in the acidic environment. This ionic form of silver does not persist in the biological environment, as Cl−, thiols, peptides and other ligands encounter and bind it. Measuring SR-XANES spectra of dried cell pellets, they followed the time-correlated transformation of Ag in THP-1 cells. Formation of Ag–S species can be attributed to cysteine-rich metal transport proteins associated with uptake and exclusion of Ag in and out of cells.
The XANES results were correlated temporally to various biological markers for Ag exposure linked to toxicity. These included fluorometric assays for reactive oxygen species production and real-time polymerase chain reaction for monitoring mRNA levels of metal chelation and exporter genes. In addition, they used circular dichroism spectroscopy to show structural changes to metallothioneins upon binding of Ag species and other assays to monitor lysosomal leakage.
By integrating well-known methods of characterization with more detailed analysis offered by synchrotron radiation X-ray experiments, the authors proposed a detailed model for the effects of Ag NP exposure in human cells (Fig. 6). This level of investigation may open the door for innovative solutions and strategies for mitigating some of the harmful effects of metallic nanoparticles as well as finding new ways to exploit their beneficial properties.
Acknowledgements
This highlight was initiated from the literature discussion of a biweekly student group meeting in the Center for Sustainable Nanotechnology. The Center for Sustainable Nanotechnology is supported by a grant awarded to Prof. Robert J. Hamers by the National Science Foundation under grant number CHE-1503408.| This journal is © The Royal Society of Chemistry 2016 |