Research highlights: unveiling the mechanisms underlying nanoparticle-induced ROS generation and oxidative stress
Tian A.
Qiu
*a,
Miranda J.
Gallagher
b,
Natalie V.
Hudson-Smith
a,
Jiewei
Wu
c,
Miriam O. P.
Krause
d,
John D.
Fortner
c and
Christy L.
Haynes
a
aDepartment of Chemistry, University of Minnesota, Minneapolis, MN 55455, USA. E-mail: qiuxx152@umn.edu
bDepartment of Chemistry, Johns Hopkins University, Baltimore, MD 21218, USA
cDepartment of Energy, Environmental and Chemical Engineering, Washington University in St. Louis, St. Louis, MO 63130, USA
dCenter for Sustainable Nanotechnology, Department of Chemistry, University of Minnesota, Minneapolis, MN 55455, USA
First published on 11th August 2016
Abstract
The field of nanotoxicology has a long-tested hypothesis, supported by a significant body of evidence, that nanoparticle-induced reactive oxygen species (ROS) lead to oxidative stress in biological systems. Within this paradigm, it is critical to fundamentally understand the underpinning mechanisms of nanoparticle ROS production and the corresponding oxidative stress they induce. This Highlight is focused on four recent articles on this topic. The first highlighted work investigated ROS generation from various silica nanoparticle surfaces and demonstrated that porosity and surface functionalization are key factors influencing ROS generation and nanoparticle toxicity. The second article demonstrated plasmon-mediated ROS production via hot electron production on gold nanocage surfaces under near-infrared irradiation. The third highlighted work correlated electronic properties of metal oxide nanoparticles to ROS generation, and built a quantitative linear relationship between ROS generation and antibacterial activity. Finally, the fourth study provided insights regarding protein signatures and pathways sensitive to oxidative stress in macrophage cells using a redox proteomic approach. Together, these four reports reveal mechanisms underlying nanoparticle-induced ROS generation and the resulting cellular oxidative stress.
Introduction
Reactive oxygen species (ROS) are highly reactive oxygen-containing molecules, mostly radicals, including singlet oxygen (1O2), superoxide radical anion (O2˙−), hydrogen peroxide (H2O2), and hydroxyl radicals (˙OH), etc. ROS are natural byproducts of oxygen metabolism and are important in cell signaling and homeostasis. The over-production of ROS caused by nanoparticle exposure can perturb the cellular balance between ROS and antioxidants, resulting in oxidative stress, which can be detrimental. With regard to nanomaterial toxicity, excessive ROS have been shown to be a major mechanism of nanoparticle toxicity (von Moos and Slaveykova, Nanotoxicology, 2014, 8, 605–630, DOI: 10.3109/17435390.2013.809810; Manke et al., BioMed Res. Int., 2013, 942916, DOI: 10.1155/2013/942916; Sharifi et al., Chem. Soc. Rev., 2012, 41, 2323–2343, DOI: 10.1039/c1cs15188f). However, it is not enough to simply quantify material-induced ROS generation or the corresponding level of oxidative stress. Given the broader goal of nanoparticle safety through rational design, it is critical to understand the mechanisms behind ROS generation and related induced oxidative stress. In recent years, instead of one blanket optical measurement of ROS production using toolkits like fluorescent dyes, research trends have shifted to a more mechanistic understanding of ROS production and its biological effects. Such information has in turn been correlated to the toxicity of nanoparticles. Results also demonstrate that it is possible to tune nanoparticle toxicity through control of ROS production. In the four articles highlighted here, researchers investigated the chemical mechanisms behind ROS generation from various nanoparticles and correlated this ROS generation with toxic effects. Moreover, a proteomics approach was used to reveal a landscape of the redox-sensitive cellular pathways impacted by different levels of oxidative stress caused by nanoparticles.The role of surface properties in ROS production and toxicity of silica nanoparticles
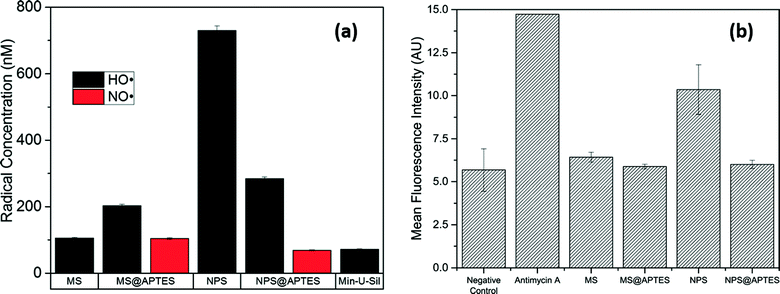
Lehman et al. (Lehman et al., Environ. Sci.: Nano, 2016, 3, 56, DOI: 10.1039/c5en00179j) investigated the relationship between the surface characteristics of wormhole-type mesoporous and Stöber-type nonporous silica nanoparticles and the generation of ROS. Each particle type was also functionalized with amine groups by reaction with aminopropyltriethoxysilane (APTES). The study of these four species – mesoporous silica (MS), nonporous silica (NPS), functionalized mesoporous silica (MS@APTES), and functionalized nonporous silica (NPS@APTES) particles – expands the understanding of particle surface properties and ROS generation as a predictor for cellular toxicity.Direct detection of transient ROS is not feasible due to their high reactivity and short lifetime. In this work, a spin-trap with electron paramagnetic resonance (EPR) spectroscopy was used to capture and quantify the transient ROS. As a spin-trap, 5,5-dimethyl-1-pyrroline N-oxide (DMPO) reacts with ˙OH and NO˙ radicals produced at the surface of the nanoparticles, and the product has a half-life long enough to be detected by EPR. The authors expressed surprise that the concentration of ˙OH was the highest on the nonporous silica surface, 7-fold higher than that produced by the MS nanoparticles (Fig. 1(a)), although MS particles had more surface area by a 20-fold difference. This resulted in much less radical production per surface area of MS nanoparticles compared to NPS nanoparticles. Particle functionalization of MS nanoparticles resulted in a decrease in surface area and pore volume and an increase in total radical production. Only functionalized particles produced both ˙OH and NO˙, which is not surprising due to the possible oxidation of amine groups.
An in vitro assay quantifying intracellular superoxide complemented the EPR data and found that among the four nanoparticles, only NPS particles induced more intracellular ROS compared to the negative control (Fig. 1(b)). Toxicity results also showed that only NPS nanoparticles elicited cytotoxicity in RAW 264.7 macrophage cells. Combined with the fact that NPS nanoparticles generated the most ROS detected by EPR, the data strongly suggested that ROS production, intracellular ROS levels, and cytotoxicity were correlated for these four silica nanoparticles. Functionalization of NPS particles reduced the number of free radicals formed at the surface, correlating with the non-cytotoxicity and unchanged intracellular ROS levels upon NPS@APTES exposure. The authors attributed this decrease in ROS to the curtailment of the surface silanol population by functionalization and steric blockage of approaching H2O2 to the particle surface where ROS production is catalyzed. On the contrary, amine functionalization of the MS nanoparticles resulted in an increased production of free radicals. The authors proposed that, due to surface properties of MS particles, there was a decreased effective diffusion of H2O2 to the surface. In addition, the functionalization of MS nanoparticles could disrupt the Stern layer that limits H2O2 diffusion. Functionalization therefore enhanced flux and resulted in increased cleavage of H2O2. The authors noted that, despite this proposal, the exact source of this phenomenon remains elusive.
Overall, increased porosity in MS silica nanoparticles and amine functionalization of NPS silica nanoparticles were found to decrease the ROS production at the solid–liquid surface, correlating with the decreased toxicity and intracellular ROS production. These results revealed the correlation between ROS production at the nanoparticle surface and the biological effects, and also showed possible strategies to make nanoparticles benign by design via altering surface properties and porosity to control ROS production.
Probing mechanisms of plasmon-mediated generation of ROS from gold nanocages under NIR irradiation
High levels of ROS can potentially cause oxidation of biomolecules, making them ideal for tumor photodynamic therapy (PDT). Thus, it is important to understand the mechanism of ROS generation to properly engineer the generation of ROS, eventually improving the efficiency of such therapeutic processes. The work by Gao et al. (Gao et al., ACS Nano, 2014, 8, 7260–7271, DOI: 10.1021/nn502325j) investigated the mechanisms of how gold nanocages (AuNCs) mediate the generation of ROS via hot electron generation under near-infrared (NIR) one/two-photon irradiation.In this work, AuNCs with an extinction peak at 810 nm were synthesized and characterized. Upon one-photon light irradiation at 808 nm, three types of ROS (singlet oxygen (1O2), superoxide radical anion (O2˙−), and hydroxyl radical (˙OH)) were detected by ESR using spin traps, called electron spin resonance (ESR) spectroscopy here (Fig. 2(a–c)). In the presence of corresponding ROS scavengers (e.g., histidine or mannitol), the signal for the ROS adduct disappeared, confirming the existence of these ROS on AuNC surfaces upon NIR irradiation. Meanwhile, hot electrons from irradiated AuNCs were detected using a three-electrode electrochemical workstation, with a working electrode of AuNC-modified indium tin oxide (ITO). Fig. 2(d) shows an obvious light switchable “on–off” pattern of photocurrent responses of a AuNC-modified electrode, and the intensity of photocurrent was proportional to the power density of illumination (Fig. 2(e)). Based on the explicit correlation between hot electron and ROS generation on AuNCs, the authors proposed a plasmon-mediated ROS generation mechanism via hot electrons (Fig. 2(f)). In brief, NIR illumination excites the surface plasmons of AuNCs, which then decay into hot electrons with an energy distribution. As the nanoparticle suspension contains oxygen, high-energy electrons then transfer to the 2π* antibonding (AB) orbital in the oxygen molecule to create O2˙−, and O2˙− further reacts to produce 1O2 and ˙OH.
To compare the ROS generation under one/two photon irradiation, the singlet oxygen (1O2) and superoxide radical anion (O2˙−) were further quantified using fluorescent dyes, and the quantum yield of 1O2 was estimated by comparing the photosensitizing reaction rate of AuNCs to that of indocyanine green as a reference material. A surprising 6-fold increase in the quantum yield of 1O2 was observed in two-photon irradiation compared to that of one-photon irradiation, and this enhancement was attributed to the different heating mechanisms between the different irradiation modes. Thus two-photon irradiation was suggested as the preferred irradiation mode for a higher ROS generation efficiency. Modifying the AuNC surface with PEG ligands did not compromise the ROS generation efficiency of AuNCs. Subsequent assays on PEG-AuNC-treated HeLa cells showed an increased level of intracellular ROS, ROS-induced mitochondrial depolarization, and a decrease in caspase protein expression, leading to cell apoptosis.
In general, this work clearly illustrated the ROS generation mechanism mediated with AuNC nanoparticles under one/two photon NIR irradiation. ROS generation efficiency was not impacted upon ligand functionalization of AuNCs with PEG units, and the pathways of cell death were examined. Although this work was aimed to engineer ROS generation at a high level in order to kill cancer cells, the mechanism it explored could also be informative for researchers aiming to make nanoparticles with limited ROS generation capability.
Nanoparticle metal oxide electronic structure predicts ROS generation and subsequent E. coli inactivation
Li et al. (Li et al., ACS Nano, 2012, 6, 5164–5173, DOI: 10.1021/nn300934k) provided a unique perspective on predicting the formation of ROS by focusing on the band gap of a material as being the key indicator of UV-induced ROS generation (Fig. 3(a)). The generation of three types of ROS (1O2, O2˙−, and ˙OH) on seven bulk and nano-scale metal oxides (TiO2, CeO2, SiO2, Al2O3, ZnO, CuO, Fe2O3) upon 365 nm illumination were quantified using the standard methods of furfuryl alcohol degradation, XTT salt reduction and pCBA reduction, respectively. Taking account of the electronic structures, particle sizes, and accompanying dissolution data, the mechanisms of ROS production were discussed in detail. The measured ROS generation of the three radicals was summed to determine the total ROS generation. Using this total ROS generation data, a linear correlation was found between the bacterial toxicity and the total ROS generation among six out of seven metal oxides (Fig. 3(b)).When a metal oxide is exposed to the correct energy of light, electrons are promoted from the valance band (Ev) to the conduction band (Ec). The resultant electron–hole pair can react with electron acceptors (e.g. molecular oxygen) and donors (e.g. water) to form ROS. The Ec and Ev values of all seven metal oxides at pH 5.6 were plotted together in comparison to the redox potentials needed to generate different ROS (Fig. 3(a)). For O2˙− generation, nano-TiO2 and nano-CeO2 were expected to generate O2˙− as their Ec values are smaller than the redox potential of O2/O2˙− (−0.2 eV) as it was measured. The authors attributed the unexpected generation of O2˙− in nano-ZnO and nano-Fe2O3 to a possible upward-bending conduction band due to an accumulation of positive charge near the nanoparticle surface in water. With Ev values higher than that needed to form ˙OH (H2O/˙OH, 2.2 eV), the holes in nano-TiO2, nano-ZnO, nano-CuO, and nano-Fe2O3 were expected to react with water to form ˙OH, and ˙OH generation was observed in all these nanoparticles except nano-CuO. This exception is probably due to the small difference between the Ev of nano-CuO (2.39 eV) and ˙OH generation. The generation of 1O2 was explained in a similar way. It is worth noting that nano-Al2O3 and nano-SiO2, while not supposed to be excited due to their band gaps being larger than the incident light (3.4 eV), still generated 1O2, and this phenomenon cannot be explained simply by band gap theory.
Quantitative correlation between the total ROS generation and antibacterial activity on E. coli was explored using linear regression (Fig. 3(b)). After excluding data from nano-CuO due to the known antibacterial effects of Cu2+, the model suggested a statistically significant linear relationship that correlated the increase of antibacterial activity to a greater amount of total ROS generation (p < 0.05, ANOVA), and the slope was determined.
Overall, this work investigated the ROS generation kinetics of seven metal oxide nanoparticles and their bulk counterparts. With a few exceptions, the authors elucidated the ROS production mechanism by interpreting electronic structures and built a quantitative relationship between total ROS and antibacterial activity of the nano-metal oxides. The matrix conditions used in this study were far from environmentally relevant at pH 5.6 and 22 °C; with ROS generation and ion dissolution from metal oxides being pH-dependent (He et al., Biomaterials, 2012, 33, 7547–7555, DOI: 10.1016/j.biomaterials.2012.06.076; Pourbaix, Atlas of electrochemical equilibria in aqueous solutions, 2nd edn, National Association of Corrosion Engineers, 1974), it would be interesting to see if the same ROS trend still applies at a different pH, temperature and accounting for competing ions. In general, the straightforward explanation of how the band gap determines the production of 1O2, O2˙−, and ˙OH radicals, and the construction of a quantitative relationship between total ROS generation and bacterial toxicity are two significant contributions of this paper to the field. Follow-up studies further built the quantitative structure–activity relationships among band gap, hydration energies, and nanoparticle toxicity (Kaweeteerawat et al., Environ. Sci. Technol., 2015, 49, 1105–1112, DOI: 10.1021/es504259s), contributing to the construction of benign-by-design guidelines for nanoparticle synthesizers as well as size, shape and ligand composition.
Looking deeper into the mechanisms underlying cellular oxidative stress
While cellular oxidative stress is generally measured using fluorescent dyes or total amount of glutathione, these measurements fail to provide a mechanistic understanding of how cells respond to oxidative stress on a molecular level. In their recent work, Duan et al. (Duan et al., ACS Nano, 2016, 10, 524–538, DOI: 10.1021/acsnano.5b05524) demonstrated how to use a quantitative redox proteomics approach to profile the whole collection of protein S-glutathionylation (SSG), a specific post-translational modification of protein by reactive oxygen and nitrogen species (ROS and RNS), in macrophages. This -omics approach allowed them to capture a landscape of cell responses to different levels of oxidative stress with a great amount of molecular details.The redox proteomic strategy was designed to detect site-specific protein S-glutathionylation which occurs on the cysteine residues of proteins (Fig. 4(a)). After nanoparticle treatment, cells were lysed, and N-ethylmaleimide (NEM) was used to block the free thiols on the cysteine residues that were not glutathionylated. A selective reduction was performed to reduce only the SSG sites to free thiols, and the reduced proteins were captured on a resin column with thiol-affinity. The enriched proteins were then labeled, eluted, and quantified using LC-MS/MS. In this approach, only the proteins with SSG modification were collected and characterized; to confirm that the changes in SSG abundance were not due to the changes in total protein abundance, total proteins were also collected and characterized for the same treatment (Fig. 4(a)).
Three metal oxide nanoparticles, SiO2, Fe3O4 and CoO, were used to represent low, moderate and high level of oxidative stress in exposed macrophages, respectively. Results showed that the overall patterns of SSG modifications (Fig. 4(b)) matched with the oxidative stress measurement results using general indicators, such as total glutathione. The most and highest SSG modification was induced by CoO NPs, which represented the highest level of oxidative stress. Fe3O4 NPs also induced robust oxidative stress, though less than that by CoO. SiO2 NPs barely induced any increase in SSG modification. It is interesting to note that in the work by Lehman et al., nonporous SiO2 NPs induced cytotoxicity and intracellular ROS generation; this discrepancy might be due to the different sources of SiO2 NPs used in these two studies. Functional analysis revealed pathway-specific differences in SSG modifications in responding to moderate (Fe3O4) and high (CoO) levels of oxidative stress (Fig. 4(c)). When the oxidative stress was moderate, pathways regulating protein synthesis, protein stability and phagocytosis were significantly enriched with SSG-modified proteins. A higher level of oxidative stress, however, reduced the specificity in the patterns of SSG modifications, and a broader set of pathways involving classical stress responses were triggered (Fig. 4(c)). Findings from the SSG profiling results also led to subsequent experiments to confirm the role of SSG in endoplasmic reticulum (ER) stress response, a list of selected SSG sites as potential signatures to indicate different levels of oxidative stress induced by nanoparticles, and a proposed scheme plotting the potential pathways involved in nanoparticle-induced oxidative stress.
Overall, the study revealed proteome-wide scale molecular details in how nanoparticle-induced oxidative stress affected cellular functions and pathways by profiling protein S-glutathionylation, a redox-sensitive post-translational modification. There is no doubt that such an -omics approach will lead to a deeper understanding of nanoparticle-induced biological effects at a molecular level and stimulate new hypotheses regarding nano–bio interactions in various research areas.
Acknowledgements
This highlight was initiated from the literature discussion of a biweekly student group meeting in the Center for Sustainable Nanotechnology. The Center for Sustainable Nanotechnology is supported by a grant awarded to Prof. Bob Hamers by the National Science Foundation under grant number CHE-1503408.| This journal is © The Royal Society of Chemistry 2016 |