Research highlights: examining the effect of shape on nanoparticle interactions with organisms
Joseph T.
Buchman
*a,
Miranda J.
Gallagher
b,
Chi-Ta
Yang
c,
Xi
Zhang
d,
Miriam O. P.
Krause
e,
Rigoberto
Hernandez
f and
Galya
Orr
g
aDepartment of Chemistry, University of Minnesota, Minneapolis, MN 55455, USA. E-mail: jbuchman@umn.edu
bDepartment of Chemistry, The Johns Hopkins University, Baltimore, MD 21218, USA
cDepartment of Chemistry, University of Iowa, Iowa City, IA 52242, USA
dDepartment of Chemistry, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA
eCenter for Sustainable Nanotechnology, Department of Chemistry, University of Minnesota, Minneapolis, MN 55455, USA
fSchool of Chemistry and Biochemistry, Georgia Institute of Technology, Atlanta, GA 30332, USA
gEnvironmental Molecular Sciences Laboratory, Pacific Northwest National Laboratory, Richland, WA 99352, USA
First published on 19th July 2016
Abstract
There are many variables that influence the toxicity of nanoparticles to organisms, such as nanoparticle size, shape, core composition, and ligand chemistry, composition, and coverage. Assessing the unique effects elicited by each of these parameters has been challenging as they impact each other. It is therefore difficult to change one parameter while keeping all other parameters constant. Here, we highlight three articles in which investigators carefully controlled as many confounding factors as possible while assessing the impacts of nanoparticle shape on their interactions with organisms. One study revealed shape-dependent effects of silver nanoparticles on the annual ryegrass, Lolium multiflorum. Another study identified shape-dependent effects of lanthanide-doped NaYF4 nanoparticles on nanoparticle association with a model cell membrane and on the cellular uptake and toxicity in selected cell lines. Finally, we highlight a study that used a coarse grain computational approach to effectively keep other parameters constant while determining the effect of shape on nanoparticle endocytosis.
Introduction
Due to their widespread incorporation in consumer goods, it is inevitable that nanoparticles will interact with organisms, whether intentionally in their use as drug delivery agents (Chen et al., Chem. Rev., 2016, 116, 2826–2885, DOI: 10.1021/acs.chemrev.5b00148) or unintentionally as they are released into the environment at any time during the production, use, or disposal of consumer products that contain them (Scheringer, Nat. Nanotechnol., 2008, 3, 322–323, DOI: 10.1038/nnano.2008.145; Klaine et al., Environ. Toxicol. Chem., 2008, 27, 1825–1851, DOI: 10.1897/08-090.1; Eduok et al., Ecotoxicol. Environ. Saf., 2013, 95, 1–9, DOI: 10.1016/j.ecoenv.2013.05.022; Benn and Westerhoff, Environ. Sci. Technol., 2008, 42, 4133–4139, DOI: 10.1021/es7032718). There is a need to understand how different nanoparticle characteristics will impact their interaction with organisms in order to guide the design of safer nanoparticles (Murphy et al., ACS Cent. Sci., 2015, 1, 117–123, DOI: 10.1021/acscentsci.5b00182). A recent workshop focusing on the environmental health and safety of nanoparticles identified several areas where more research is needed, including the impact of nanoparticle shape on its environmental transformation and interaction with organisms (Grassian et al., Environ. Sci. Nano, 2016, 3, 15–27, DOI: 10.1039/C5EN00112A). However, the effects of shape are difficult to assess, since changing nanoparticle shape often changes other features of the nanoparticle simultaneously. On the other hand, fundamental studies of nanoscale spheres and rods indicate that shape can indeed have an impact on their structure and motion in structured environments but they lacked the specificity of interactions at the nano–bio interface (Tucker and Hernandez, J. Phys. Chem. B, 2012, 116, 1328–1334, DOI: 10.1021/jp207346j; Tucker and Hernandez, J. Phys. Chem. B, 2011, 115, 4412–4418, DOI: 10.1021/jp201867f). While many studies attempting to identify the effect of nanoparticle shape on organisms have been published, only a few focused carefully on keeping all parameters consistent while changing only shape. Here we highlight three recent articles in which the authors were careful about keeping as many nanoparticle properties consistent as possible to monitor the effect of nanoparticle shape on its interaction with organisms. By understanding the impact of nanoparticle shape on the interaction with organisms, we should be able to better engineer nanoparticles to elicit the desired effects depending on their intended applications.Shape-dependent toxicity of silver nanoparticles to a panel of organisms
While it is often difficult to control all the characteristics of nanoparticles to assess the effect of only one specific property, Gorka et al. conducted a thorough study in which they carefully attempted to keep other factors constant to investigate the effects of silver nanoparticle shape on toxicity across a panel of different organisms (Gorka et al., Environ. Sci. Technol., 2015, 49, 10093–10098, DOI: 10.1021/acs.est.5b01711). Silver nanoparticles were constructed in the shapes of nanospheres (diameter: 44.1 ± 7.0 nm), nanocubes (edge length: 36.6 ± 5.7 nm), and nanowires (length: 82.2 ± 17.3 nm, width: 6775 ± 3441 nm). The concentration of each nanoparticle type was chosen to achieve similar total surface areas, with the nanospheres and nanocubes having almost identical surface areas (13.0 ± 2.44 cm2 mL−1 and 15.6 ± 2.88 cm2 mL−1, respectively). The surface chemistries of each nanoparticle were manufactured to be the same as they were all coated with polyvinylpyrrolidone.Since nanoparticles interact with many different organisms after they are released into the environment, Gorka et al. utilized a terrestrial plant, a Gram-positive bacterium, two Gram-negative bacteria, zebrafish, and a terrestrial nematode to represent multiple environmental facets. Except in the model plant, Lolium multiflorum, they found that the shape of the silver nanoparticles did not affect their toxicity toward the organisms. For Lolium multiflorum, the seeds were exposed to nanoparticles during a 5 day germination period, after which the lengths of the roots and shoots were measured with digital calipers and the number of root hairs was assessed with light microscopy. Silver nanospheres were toxic to both the roots and shoots whereas nanocubes and nanowires were nontoxic to both (Fig. 1a). Very few root hairs were present after exposure to nanospheres (12 ± 4 root hairs per cm), compared with exposures to nanocubes (29 ± 6 root hairs per cm), nanowires (33 ± 10 root hairs per cm), or the control (34 ± 10 root hairs per cm) (Fig. 1b).
Based on their findings, the authors argue that silver nanocubes and nanowires are not toxic to their model plant and that nanospheres are the most toxic shape studied. They indicate that even though root hairs help facilitate the uptake of water and nutrients for the plant, they could not determine whether the decreased root hair abundance seen in the nanosphere exposure was the cause of the observed reduced root and shoot growth, a question that needs to be investigated further. The authors concluded that deliberate engineering of nanoparticle shapes could reduce overall environmental toxicity, while recognizing the complexity of multiple factors that influence a nanoparticle's environmental impact.
Understanding shape-dependent particle–membrane association of lanthanide-doped NaYF4 nanoparticles
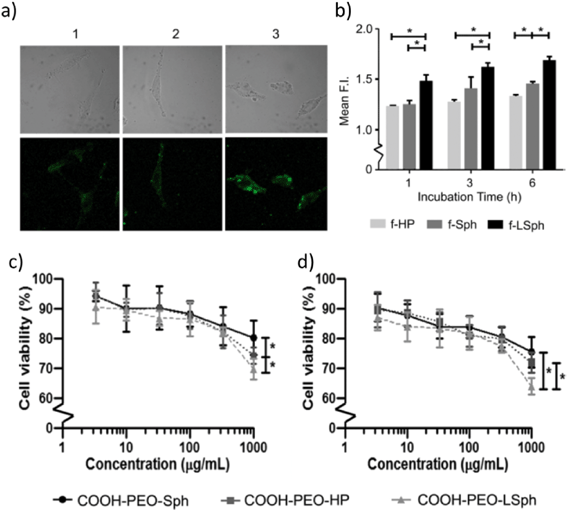
To study the effects of differently shaped NaYF4 nanoparticles doped with 30 mol% Yb3+ and 0.5 mol% Tm3+, Tree-Udom et al. utilized nanospheres, elongated nanospheres, and nanohexagonal prisms (Tree-Udom et al., ACS Appl. Mater. Interfaces, 2015, 7, 23993–24000, DOI: 10.1021/acsami.5b06781). They ensured that the differently shaped nanoparticles were consistent in other ways, demonstrating that they had the same crystal structure, surface coating (polyethylene oxide), zeta potential, and fluorescence emission upon irradiation at 980 nm. The diameter of the nanospheres (27 ± 1 nm) was similar to that of the elongated nanospheres (diameter: 25 ± 1 nm, length: 38 ± 1 nm). The nanohexagonal prisms were slightly larger, with an edge length of 48 ± 3 nm and height of 64 ± 2 nm. The authors covalently linked 6-aminofluorescein to the nanoparticles to allow for investigations using confocal laser microscopy and flow cytometry.To determine interactions of nanoparticles with a model membrane, the authors exposed the nanoparticles to liposomes composed of dioleoyl L-α phosphatidylcholine and monitored their association using confocal laser microscopy. The fluorescence intensity was highest for the elongated nanospheres, indicating the greatest membrane association, followed by the nanospheres and nanohexagonal prisms. The authors explain that nanospheres and elongated nanospheres, which have soft edges, have more favorable curvature free energies for association than the nanohexagonal prisms, which have sharp edges that cause extreme curving in the membrane. Nanohexagonal prisms only have a favorable curvature free energy for association when a flat face of the nanoparticle is parallel with the flat face of the membrane.
Confocal laser fluorescence microscopy (Fig. 2a) and flow cytometry (Fig. 2b) were also used to quantify and compare cellular uptake of the differently shaped nanoparticles by human melanoma (A-375) and human liver carcinoma (HepG2) cell lines. The observed cellular uptake correlated well with the liposome association results, indicating that the elongated nanospheres were taken up into cells more efficiently than nanospheres and nanohexagonal prisms. Also, the cytotoxicity of the nanoparticles was tested on A-375 cells (Fig. 2c), a normal skin cell line (WI-38) (Fig. 2d), and HepG2 cells using the MTT assay after a 24 hour exposure. All three shaped particles induced low cytotoxicity to the cell lines, but a significantly higher cytotoxicity by the elongated nanospheres was observed, which could be a result of the higher cellular uptake of these particles. Interestingly, the in vitro cytotoxicity of nanohexagonal prisms was higher than that of nanospheres at the highest concentration used for exposures of WI-38 and A-375 cells. The authors speculated that this observation could be a result of the different intracellular responses of the cells toward the two shaped particles.
The authors concluded that nanoparticle shape matters when considering the binding of nanoparticles to model membranes. The degree of nanoparticle association with the membrane can be correlated with the degree of cellular uptake in the cell lines. To some extent, cytotoxicity is also correlated with association and cellular uptake of nanoparticles. The MTT assay showed that elongated nanospheres were the most toxic to mammalian cells. Nanospheres were the least toxic to the mammalian cell lines in this study, which contradicts the results seen by Gorka et al., which showed that nanospheres were the most toxic to a model plant (Gorka et al., Environ. Sci. Technol., 2015, 49, 10093–10098, DOI: 10.1021/acs.est.5b01711). This demonstrates the complexity inherent in understanding the effect that nanoparticle shape can have on organisms. Toxicity of nanoparticles with different shapes may vary with the nanoparticle material and may not be generalizable across all biological systems (e.g. plant cells versus mammalian cells).
Modeling endocytosis with differently shaped PEGylated nanoparticles
In the interest of using nanoparticles for targeted drug delivery, Li et al. recognized that there are four important factors potentially determining the cellular uptake of nanoparticles: size, shape, surface properties, and material composition (Li et al., Nanoscale, 2015, 7, 16631–16646, DOI: 10.1039/C5NR02970H). Controlling for three out of the four, the authors study shape effects using a coarse grain model of dissipative particle dynamics simulations along with self-consistent field theory and Flory theory. Since nanoparticles used as drug delivery agents are often coated with polyethylene glycol (PEG) to improve biocompatibility and circulation time (Vllasaliu et al., Expert Opin. Drug Delivery, 2014, 11, 139–154, DOI: 10.1517/17425247.2014.866651), the authors built the PEGylated nanospheres, nanorods, nanocubes, and nanodisks with the same surface area of 201 nm2 to ensure an equal number of PEG molecules on the surface regardless of shape (Li et al., Nanoscale, 2015, 7, 16631–16646, DOI: 10.1039/C5NR02970H). The need for control of surface area is also in keeping with the work by Gorka et al. as noted above.To simulate the interaction of PEGylated nanoparticles with cell membranes, Li et al. investigated the process of endocytosis with each nanoparticle shape. Their simulation shows that at high grafting density (1.6 chains per nm2), all four nanoparticle shapes were internalized by the cell in similar amounts of time (2.24–2.77 μs). At low grafting density (0.2 chains per nm2), the internalization was incomplete for all nanoparticle shapes. Interestingly, at intermediate grafting density (0.6 chains per nm2), the endocytic kinetics start to reveal the shape effect with nanospheres possessing the fastest internalization rate followed by nanocubes, nanorods, and then nanodisks (Fig. 3a). Hence, the shape of nanoparticles plays an important role in particle–cell interaction and endocytosis.
The time it takes each of the differentially shaped nanoparticles to be wrapped by the membrane as part of the internalization process is analyzed to quantify the effect of grafting density and shape. This analysis also indicates that nanospheres have the highest cellular uptake efficiency among the shapes studied, which agrees with gold nanosphere experimental results also reported in the paper (Cho et al., Small, 2010, 6, 517–522, DOI: 10.1002/smll.200901622). There are three major free energy (F) level changes that occur during the receptor-mediated endocytosis of PEGylated nanoparticles, namely, ligand–receptor interactions (ΔFlig), membrane bending energy (ΔFmemb), and conformational free energy loss of PEG polymer (ΔFpoly). In analyzing the importance of ΔFpoly, the authors observed that the two parts of conformational free energy loss (elastic energy change and interaction energy change) are impartial to the shape of the nanoparticles, which indicates the minor role of ΔFpoly in the shape effect. The membrane bending energy was determined for each nanoparticle shape by Helfrich–Canham–Evans free energy (Fig. 3b).
The authors conclude that of the three free energy level variables for the differently shaped nanoparticles, differing ΔFmemb is the major contributor to their differential endocytosis behavior. The membrane bending energies correlate well with the internalization rates seen for the nanoparticles, suggesting that low aspect ratio nanoparticles such as spheres have the best internalization rates. The shape effect is most prominent when PEG is at an intermediate grafting density, because at high density, the core shape is masked by the shape of the dense ligand coverage, while at low grafting density, nanoparticles are only slightly wrapped by the membrane due to weak ligand–receptor interactions. Lastly, when entry angle is considered, the anisotropic rod and disk nanoparticles have further reduced cellular uptake efficiencies, further supporting the idea that spherical nanoparticles would undergo endocytosis most efficiently.
In summary, despite the difficulties in attributing the specific contribution of nanoparticle characteristics to their toxicity, the three articles highlighted here suggest that particle shape plays a significant role and should be considered as a design control parameter as researchers move towards the grand challenge of creating useful and sustainable new nanoparticles (Murphy et al., ACS Cent. Sci., 2015, 1, 117–123, DOI: 10.1021/acscentsci.5b00182).
Acknowledgements
This highlight was initiated from the literature discussion of a biweekly student group meeting in the Center for Sustainable Nanotechnology. The Center for Sustainable Nanotechnology is supported by a grant awarded to Prof. Bob Hamers by the National Science Foundation under grant number CHE-1503408.| This journal is © The Royal Society of Chemistry 2016 |