Research highlights: engineering nanomaterial-based technologies for environmental applications
Stacey M.
Louie
* and
John M.
Pettibone
Materials Measurement Science Division, National Institute of Standards and Technology, Gaithersburg, MD 20899, USA. E-mail: stacey.louie@nist.gov
First published on 29th January 2016
Abstract
Nanomaterials are currently of interest for water treatment and remediation applications because they can exhibit high adsorption capacities and high reactivity to degrade or transform contaminants. Research is ongoing to further increase the adsorption capacity of the nanomaterials and to engineer nanomaterial-based treatment systems for contaminant removal. Here, we highlight three articles that advance this field by devising and testing approaches to improve the design of nanomaterials as well as their implementation in water treatment applications. One study demonstrates a method for non-covalent surface functionalization to produce silica and magnetite nanoparticles exhibiting thiol ligands for heavy metal removal. In another study, the surface coating chemistry of manganese oxide nanoparticles is optimized to enhance their uranyl sorption capacity. Finally, we highlight research that evaluates the overall implementation of magnetite nanoparticles for removal of hexavalent chromium (Cr(VI)) from water, including the production of the nanoparticles, their efficiency in removing Cr(VI) in a reactor, and the recovery of the used NPs in a magnetic separation system.
Nano impactResearch is needed to develop more effective nanomaterials for application in water treatment systems; we highlight recent articles that optimize the functionalization of nanomaterial surfaces for contaminant removal as well as the overall production, use, and post-use recovery of nanomaterials in a small-scale treatment system. |
Introduction
Prospective advancements in engineered systems for water treatment and environmental remediation will likely include nanomaterial-based solutions. Advantages of nanomaterials include their higher surface area and reactivity compared to bulk materials, which can result in lower material requirements and more effective removal of contaminants. Recent research has focused on modifying material properties to achieve higher contaminant treatment capacities. In addition, for nanomaterial-based technologies to be competitive with currently existing methods for water treatment, the nanomaterials must be cost-effective, which could be accomplished by lower costs in production and implementation, or increased recovery and reuse (Qu et al., Water Res., 2013, 47, 3931, DOI: 10.1016/j.watres.2012.09.058). We first highlight two articles that demonstrate the optimization of surface functionalization to maximize the contaminant sorption capacity of engineered nanoparticles (NPs). Nell et al. demonstrated a non-covalent approach for thiol functionalization of mesoporous silica and magnetite NPs for adsorption of heavy metal cations (Nell et al., Environ. Sci.: Nano, 2016, DOI: 10.1039/c5en00170f). In another approach, Lee et al. evaluated a library of surface coating chemistries on manganese oxide NPs for removal of uranium from water (Lee et al., Environ. Sci.: Nano, 2015, 2, 500, DOI: 10.1039/c5en00010f). We also highlight research by Simeonidis et al. that evaluated the implementation of magnetite NPs for removal of hexavalent chromium in a water treatment system, from synthesis and application of the NPs to recovery, reuse, and disposal of the used NPs (Simeonidis et al., Sci. Total Environ., 2015, 535, 61, DOI: 10.1016/j.scitotenv.2015.04.033).Heavy metal sorption methods using non-covalently functionalized nanomaterials
More effective functionalized NPs for environmental applications can be developed by tuning the surface coating to improve the coating's selectivity for the contaminant of interest, surface density on the NP, and stability against desorption during application. Nell et al. exploited mesoporous silica and magnetite (Fe3O4) NPs to obtain a high surface area for contaminant adsorption, and the surfaces of the nanomaterials were functionalized with thiol-bearing ligands to provide a high affinity to bind heavy metals (Nell et al., Environ. Sci.: Nano, 2016, DOI: 10.1039/c5en00170f). The authors focused on developing a non-covalent surface functionalization approach to produce coatings with a high thiol surface density on the NPs and low leaching of the coating into water.To functionalize the mesoporous silica sorbent, MCM-41, Nell et al. began by reacting the MCM-41 with trimethoxyphenyl silane to create a phenyl terminated self-assembled monolayer on mesoporous silica (Ph-SAMMS). This layer could then be non-covalently (NC) functionalized with a secondary layer of thiol-bearing aromatic ligands that have high affinity for heavy metal binding to the exposed thiol groups (Fig. 1a). To optimize NP performance, maximizing thiol ligand density on the surface and minimizing leaching of the secondary layer into water were balanced. This was accomplished by first adjusting the ratio of MCM-41 and trimethoxyphenyl silane for the Ph-SAMMS layer (Fig. 1b), and second, varying the chemistry and concentration of the aromatic thiols for the secondary layer (Fig. 1c). Through this systematic testing, 2-(mercaptomethyl)naphthalene was identified as the most promising ligand for the NC bound layer of the ligands tested (Fig. 1a), and the authors attained similar thiol ligand densities to a covalently bound thiol SAMMS (SH-SAMMS) layer.
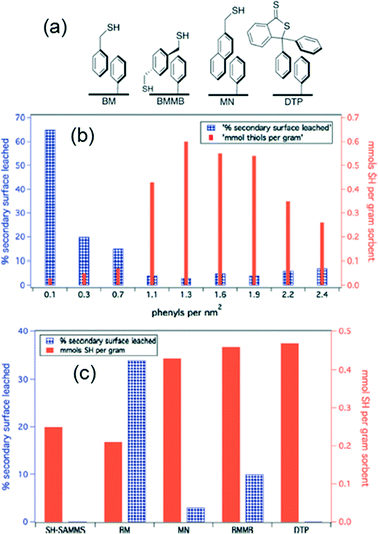 | ||
| Fig. 1 Thiol-bearing aromatic ligands were non-covalently adsorbed onto a Ph-SAM base layer on mesoporous silica and Fe3O4 NPs (a), and the Ph-SAMMS coverage (b) and type of secondary ligand (c) were optimized to obtain high thiol density and low leaching of the secondary surface layer from the mesoporous silica. Ligands tested were benzyl mercaptan (BM), 1,4-bis(mercaptomethyl)benzene (BMMB), 2-(mercaptomethyl)naphthalene (MN), and 3,3-diphenylbenzo(c)thiophene-1(3H)-thione (DTP), with results shown for MN in (b). Reprinted with permission from Nell et al., Environ. Sci.: Nano, DOI: 10.1039/c5en00170f. Copyright 2016 Royal Society of Chemistry. | ||
The NC functionalized materials were then tested for removal of Cd(II), Hg(II), Pb(II), and Ag(I) from water. The authors demonstrated that the optimized NC material removed nearly 100% of heavy metals from river water samples. The distribution coefficients of the four metals to the NC material (i.e., mass of metal adsorbed per mass of sorbent) were significantly higher than those of the control (non-functionalized or only Ph-SAMMS functionalized) MCM-41 sorbent and a commercial thiol-functionalized resin, resulting from the high binding affinity of the thiol ligands and the high surface area to mass ratio of the NPs, respectively. The NC material also provided similar distribution coefficients to the covalently bound SH-SAMMS, indicating the stability and surface density of the NC layer was similar to the covalently bound counterparts.
The authors also demonstrated the NC approach to functionalize Fe3O4 NPs with thiol ligands. The Fe3O4 NPs were first prepared with lauric acid shells, which were exchanged for benzoic acid to form the phenyl surface layer for NC functionalization with the aromatic thiol ligands. Again, the NC functionalized NPs had distribution coefficients for Cd(II) and Hg(II) that were higher than the control (benzoic acid) NPs and thiol resin and comparable to Fe3O4 NPs that were covalently functionalized with mercaptopropionic acid, indicating high stability and surface density of the NC layer on the NPs, which demonstrates the general applicability of the approach.
In summary, Nell et al. demonstrated that NC functionalization is a viable approach to form stable, versatile and high affinity surface coatings on NPs for contaminant removal applications. Furthermore, the authors note that the NC approach could enable regeneration and reuse of the materials if the secondary layer (comprising the thiol groups with irreversibly bound heavy metals) can be removed after the adsorption capacity is reached. The authors also suggest that this approach could be applied for the facile production of material arrays with a variety of functionalities in order to optimize the surface chemistry of the NPs. In particular, the authors propose that the NC approach can be advantageous to a purely covalent approach when the ligand for contaminant binding is incompatible with the anchor group used for attachment to the NP (e.g., hydroxypyridinone (HOPO) ligands and siloxane anchors) (Fryxell et al., J. Mater. Chem., 2007, 17, 2863, DOI: 10.1039/b702422c); in these cases, the non-covalent approach would eliminate the need to protect the ligand (e.g., with a benzyl group) during the SAMMS deposition and to subsequently remove the protecting group by chemical reactions that may damage the functionalized NPs.
Improved uranyl sorption through NP surface chemistry
In addition to tuning NP surface coatings for increased packing density, the chemistry and structure of their surface coatings can be modified to improve the colloidal stability (thus increasing surface availability for sorption) as well as to attach functional groups with high sorption affinity for contaminants. Lee et al. demonstrated a systematic study to compare different surface coatings on manganese oxide NPs for removal of uranium (Lee et al., Environ. Sci.: Nano, 2015, 2, 500, DOI: 10.1039/c5en00010f). Manganese oxide NPs were targeted because of their potential reactivity to reduce uranium(VI) to uranium(IV), which is less soluble in water. The authors synthesized libraries of manganese oxide NPs with surface coatings of various structures and chemistries, including organic acid bilayers and polyethylene glycol (PEG) coatings, and tested these materials to determine the optimal surface coating for uranyl sorption.The manganese oxide NPs were synthesized from manganese oleate in oleic acid and 1-octadecene, resulting in MnO@Mn3O4 core–shell NPs with an oleic acid base coating. After purification and redispersion in hexane, the NPs were transferred to water using two coating strategies. In the bilayer strategy, a surface stabilizer (oleyl phosphate (OP), oleic acid (OA), octadecylphosphonic acid (ODP), or stearic acid (SA)) was assembled onto the oleic acid base layer to present outer-facing phosphonate and carboxylate groups. In the single layer strategy, PEG (200 Da, 1 kDa, or 10 kDa) was added to displace the oleic acid base layer. The bilayer and 200 Da PEG produced thin, compact coatings, whereas thicker coatings based on hydrodynamic size were obtained with 1 kDa and 10 kDa PEG (Fig. 2a). The zeta potentials for the coated NPs were the most negative for the phosphonate-functionalized (OP and ODP) layers, followed by the carboxylate-functionalized (OA and SA) layers and the PEG layers.
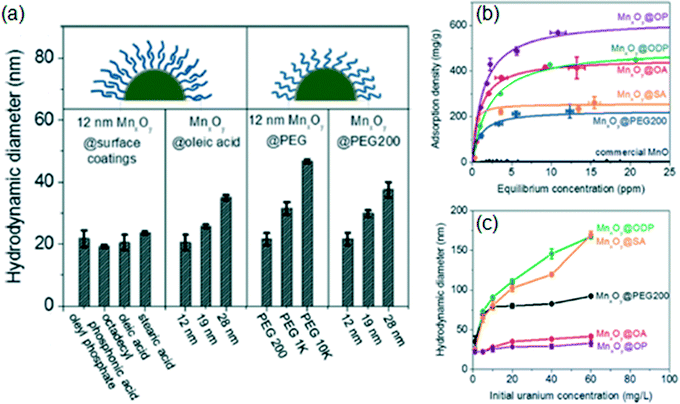 | ||
| Fig. 2 (a) Bilayers formed from OP, ODP, OA, and SA (on an oleic acid base coating) and a PEG (200 Da) single layer formed compact coatings on MnxOy NPs, whereas thicker coatings were formed from 1 kDa and 10 kDa PEG layers. The maximum adsorption capacity of uranium onto the NPs (b) was highest for the phosphonate-bearing layers (OP and ODP). Bilayers formed from the unsaturated molecules (OP and OA) showed higher sorption capacities than the counterpart saturated molecules (ODP and SA, respectively), which was attributed to the better colloidal stability of the OP- and OA-coated NPs against aggregation (c). Reprinted with permission from Lee et al., Environ. Sci.: Nano, DOI: 10.1039/c5en00010f. Copyright 2015 Royal Society of Chemistry. | ||
The library of coated MnxOy NPs was then tested for uranium sorption, with sorption data fitted to a Langmuir isotherm. All coated NPs showed maximum uranium sorption capacities (i.e., maximum adsorbed mass of uranium per mass of manganese) that were orders of magnitude higher than that of commercial MnO (Fig. 2b), which was attributed to the higher NP stability and surface area as well as the favorable surface chemistries of the coated NPs. Comparing across coating types, the highest maximum sorption capacity was observed for OP, followed by ODP, OA, SA, and PEG (200 Da) coatings (Fig. 2b). The authors attribute this trend to two effects. First, the uranium was bound to the phosphonate and carboxylate groups on the OP, ODP, OA, and SA coatings, as demonstrated in attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectra. Second, the higher adsorption capacity for OP (compared to ODP) and OA (compared to SA) was attributed to their increased colloidal stability in the presence of elevated uranyl concentrations (as well as in NaCl and CaCl2 solutions), resulting in a higher effective surface area for sorption (Fig. 2c).
Overall, Lee et al. demonstrated a systematic approach to test the effects of surface chemistry and structure (as well as NP size and pH, described in the highlighted article) to maximize the uranium sorption capacity of manganese oxide NPs. Future application of this approach is needed for other NPs and contaminants of interest to identify the fundamental surface properties that control contaminant sorption and thereby design more effective materials for environmental applications.
Holistic evaluation of magnetite NP-based technologies for Cr(VI) treatment
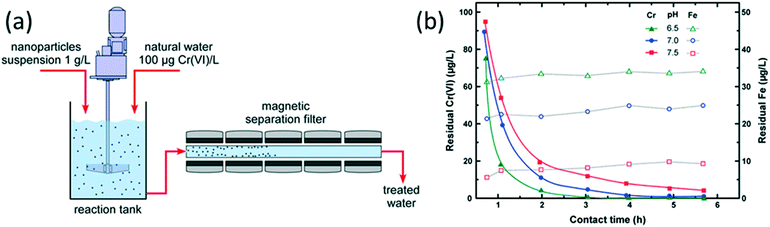
For nanomaterial-based technologies to be competitive with existing water treatment technologies, the nanomaterials must be able to be produced inexpensively in large volumes, and feasible implementation of the nanomaterials in water treatment operations must be demonstrated. In the current research, Simeonidis et al. evaluated a broad parameter space for the development of candidate magnetite (Fe3O4) NPs that efficiently removed hexavalent chromium (Cr(VI)) from water and were cost-efficient options in applied systems (Simeonidis et al., Sci. Total Environ., 2015, 535, 61, DOI: 10.1016/j.scitotenv.2015.04.033).Simeonidis et al. first evaluated various inexpensive iron precursor materials for the Fe3O4 NP synthesis, as well as the effects of water chemistry on the Cr(VI) removal efficiency in batch studies. Iron oxide NPs were synthesized at the kilogram scale by aqueous co-precipitation of Fe2+/Fe3+ salts in three combinations: FeCl2 with FeCl3; FeSO4 with Fe2(SO4)3; and Mohr's salt ((NH4)2Fe(SO4)2) with Fe2(SO4)3. The NPs produced from the iron chloride precursors were smaller (≈10 nm diameter) and more easily oxidized to Fe2O3 (i.e., the Fe2+/Fe3+ ratio in the NPs was lower than expected for Fe3O4), resulting in lower Cr(VI) reduction and removal capacity as well as lower magnetization. Comparing the NPs produced from the two iron sulfate syntheses, similar Fe2+/Fe3+ ratios, magnetizations, and Cr(VI) removal capacities were obtained. FeSO4 was chosen as the optimal Fe2+ precursor for further testing because of its lower cost and lack of NH4+ waste production compared to Mohr's salt. Partial to nearly complete reduction of the Cr(VI) to Cr(III) precipitates on the Fe3O4 NPs was observed by X-ray photoelectron spectroscopy. The authors identified the need to account for the effects of water chemistry and pH on the removal efficiency of the NPs, with lower efficiencies obtained at higher pH and in waters containing interfering ions.
The Fe3O4 NPs were then applied in a system that comprised a 2 L reactor and 50 cm long magnetic separator (Fig. 3a), varying the contact time in the reactor and the magnetic field and flow rate through the separator. The authors demonstrated that nearly 100% removal of the NPs could be achieved in the separator at flow rates up to 80 mL min−1. To achieve Cr(VI) removal from a 100 μg L−1 starting concentration to a residual 10 μg L−1 concentration in the treated water, contact times of (2 to 3) h in the reactor were required at pH 7, with lower contact times possible at pH 6.5 to achieve similar residual concentrations (Fig. 3b). The authors also determined that the collected NPs could be reused eight times without significant loss of their Cr(VI) removal efficiency. Furthermore, the spent NPs could be safely disposed of based on results from leaching of Cr(VI) using U.S. Toxicity Characteristic Leaching Procedure (TCLP) tests. Finally, the authors estimated low production and treatment costs for the application of the Fe3O4 NPs for Cr(VI) removal.
Overall, Simeonidis et al. provided a thorough and holistic evaluation of Fe3O4 NPs for Cr(VI) treatment. Moving forward, additional studies are needed that provide similar evaluations of the feasibility and cost effectiveness of other nanomaterial-based technologies for water treatment. These studies should contribute to the development of viable and sustainable nanotechnologies for environmental applications.
| This journal is © The Royal Society of Chemistry 2016 |