Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Multimedia environmental fate and speciation of engineered nanoparticles: a probabilistic modeling approach†
J. A. J.
Meesters
*a,
J. T. K.
Quik
b,
A. A.
Koelmans
cd,
A. J.
Hendriks
a and
D.
van de Meent
ab
aInstitute for Water and Wetland Research, Department of Environmental Science, Radboud University Nijmegen, P.O. Box 9010, NL-6500 GL Nijmegen, The Netherlands. E-mail: joris.meesters@rivm.nl
bNational Institute of Public Health and the Environment (RIVM), P.O. Box 1, 3720 BA, Bilthoven, The Netherlands
cAquatic Ecology and Water Quality Management Group, Department of Environmental Sciences, Wageningen University, P.O. Box 47, 67e00 AA Wageningen, The Netherlands
dIMARES − Institute for Marine Resources & Ecosystem Studies, Wageningen UR, P.O. Box 68, 1970 AB IJmuiden, The Netherlands
First published on 24th May 2016
Abstract
The robustness of novel multimedia fate models in environmental exposure estimation of engineered nanoparticles (ENPs) remains unclear, because of uncertainties in the emission, physicochemical properties and natural variability in environmental systems. Here, we evaluate the uncertainty in predicted environmental concentrations (PECs) by using the SimpleBox4nano (SB4N) model. Monte Carlo (MC) simulations were performed on the environmental fate, concentrations and speciation of nano-CeO2, -TiO2 and -ZnO. Realistic distributions of uncertainty and variability were applied for all of SB4N's input and model parameter values. Environmental distribution over air, water, soil and sediment as well as nanomaterial speciation across natural colloid and coarse particles appeared to be similar for nano-CeO2, -TiO2 and -ZnO. ENPs in the atmosphere were effectively removed by deposition. ENPs in the water column were removed through hetero-aggregation–sedimentation with natural particles. ENPs accumulated in soil by attachment to grains. The sources of uncertainty and variability driving variation in PECs, which was identified in Spearman rank analysis, were related to production, emission, compartment volumes, and removal by dissolution or advection and appeared to be similar for the three ENPs. The variation in speciation within environmental compartments was influenced most by the physicochemical properties of the ENP and by model parameters that relate to the compartment of interest.
Nano impactIt is not yet clear whether novel multimedia fate models for environmental exposure estimation of engineered nanoparticles (ENPs) are sufficiently robust for environmental risk assessment. Analyzing model sensitivity to uncertainties in emission estimations, physiochemical properties of nano-CeO2, -TiO2 and -ZnO and natural variability of the environmental system demonstrates significant trends in environmental fate that appear to be general for ENPs. Hence, the identified variation in predicted environmental concentrations can be applied to nanoparticles with chemical compositions other than nano-CeO2, -TiO2 and -ZnO but with comparable physicochemical properties. Screening level exposure estimation becomes more feasible, since the variation in model outcomes due to uncertainty and variability has been made explicit. |
Introduction
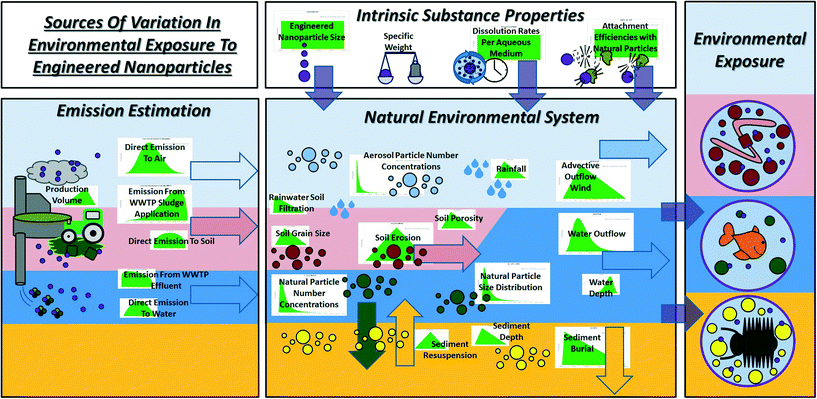
The nanotechnology industry is rapidly developing engineered nanoparticles (ENPs) that are applied in a great variety of consumer and industrial products.1 Release of ENPs to the environment is anticipated during production, use and disposal.2 However, ENP environmental fate, bioavailability, bioaccumulation, and toxicity and thus the biological risks require more knowledge in order to be fully understood3 and in order to prevent unforeseen environmental and toxicological impacts.4Chemical safety assessment frameworks, such as the European Commission (EC)'s legislation program REACH (Registration, Evaluation, Assessment and Restriction of Chemicals) and the Toxic Substances Control Act of the U.S. Environmental Protection Agency (USEPA), have been founded to protect human health and the environment from potential toxic effects of chemicals.5,6 Their guidance is not yet able to cope with chemicals occurring as nanomaterials that possess physicochemical properties that diverge from those of the chemical's equivalent bulk material or dissolved state.7,8 A major challenge in environmental risk assessment of ENPs is to turn the models that are currently used to describe and predict the fate of ‘conventional chemical substances’ into models that are fit for use with nanosubstances.7,9,10 Probabilistic mass flow analysis has been suggested for this purpose.11–14 However, such models require input data that are not readily available for implementation in chemical safety assessment frameworks.14–16 Therefore, new tools are being developed to estimate environmental exposure to ENPs from their emission and physicochemical properties alone15–19 by simulating the key mechanisms in the environmental fate of ENPs, which are attachment to natural particles, settling and removal by dissolution.7–10,20 However, emission of ENPs to the environment is uncertain, because data on production volumes13 and release during the different life cycles of nanomaterial products,2 generally, are inaccessible. Physicochemical properties of ENPs that drive their environmental fate are also considered to be uncertain.9,10 Therefore, some important interactions between the ENP and the environmental matrix are hard to predict, e.g. dissolution rates of ENPs in different aqueous media and their attachment to natural particles.21 Such uncertainty in determining the input parameters of new models obviously leads to variation in their predicted environmental concentrations (PECs). At the same time, limitations in techniques measuring ENPs in environmental samples hamper model calibration and validation.14,16,22,23 Consequently, the robustness of the latest multimedia fate models remains unclear. We argue that this robustness can be evaluated by making the uncertainties in input parameters and the natural variability in the environmental system explicit. Here, we present such an evaluation for SimpleBox4nano, a multimedia environmental fate model that predicts concentrations, distributions and speciation of nanomaterials in the environment.15 This evaluation is done to meet three main objectives: (i) to identify commonalities and differences among the environmental fates of nanomaterials with different chemical compositions, (ii) to derive confidence intervals for PECs calculated by mechanistic multimedia fate models such as SB4N, and (iii) to rank the most important sources of uncertainty and variability in environmental exposure estimation of ENPs.
Our study approach is as follows. SB4N is an adaptation of the SimpleBox model,24 which serves as a regional distribution module in the European Union System for Evaluation of Substances (EUSES) model, used by REACH as guidance in the environmental exposure estimation of chemicals.25 In an earlier proof-of-concept study,15 we have evaluated the model concept of SB4N with a scenario of nano-TiO2 emission in Switzerland.11 However, this evaluation was based on a model concept with single model and input parameter values only and model outcomes apply as point estimates of parameters from this source.15 In reality, the model parameters are subject to natural variability and the input parameters are subject to uncertainty.10 To take this into account, data were collected for all of SB4N's input and model parameters reflecting realistic distributions of variability and uncertainty including emission estimation, natural variability in the environmental system, and uncertainty in physicochemical properties of ENPs (Fig. 1). These probability distributions were used for Monte Carlo (MC) simulations of the environmental fate of the three mostly used metal oxide ENPs in Europe: nano-TiO2, nano-ZnO and nano-CeO2.13,26 The MC simulations yielded distributions of outputs (medians and 95% confidence intervals) for the PECs of three physical species of ENPs in the environment: (i) free ‘primary’ or ‘pristine’ ENPs, (ii) ENPs attached to natural colloids, and (iii) ENPs attached to coarse particulate matter.
Methods
Monte Carlo simulations with SimpleBox4nano
The software package Crystal Ball27 was used to perform Monte Carlo (MC) simulation of the SBN4 output; median values and 95% confidence intervals for PECs were calculated from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 iterations. Output distributions of PEC were simulated for (i) freely dispersed particles, (ii) hetero-aggregated particles and (iii) particles attached to suspended particulate matter, prone to gravitational forces in aqueous media. The model solves mass balance equations for a steady-state situation in all compartments and species through matrix algebra:15,28
000 iterations. Output distributions of PEC were simulated for (i) freely dispersed particles, (ii) hetero-aggregated particles and (iii) particles attached to suspended particulate matter, prone to gravitational forces in aqueous media. The model solves mass balance equations for a steady-state situation in all compartments and species through matrix algebra:15,28| m = −A−1e |
Here, e (kg s−1) is the vector of emission rates of ENPs into the environment. The system matrix A (s−1) represents first-order rate constants for (i) transport between compartments and to media outside the system, (ii) the rates at which ENPs attach to natural colloids or coarse particles, and (iii) the rates at which ENPs are subjected to removal processes such as degradation and dissolution (ESI,† Chapter A, S4–S6).15 These rate constants were calculated using equations that express the interactions between ENPs and the environmental system.15 For these calculations, the MC simulations require probability distributions of the natural variability in model parameters that define the environmental system and of the uncertainty of the physicochemical properties and emission of the ENPs. The collected data (ESI,† Chapters B–E, S7–S29) did not always provide the shapes of the uncertainty and variability distributions (e.g. normal, lognormal, Pareto). Uniform distributions were assumed in those cases that data only provided minima and maxima, whereas triangular distributions were assumed when data provided a minimum, a most-likely value and a maximum. For a detailed account of the probability distributions used, see Chapters B to E, ESI.† Environmental exposure estimated with the model is expressed as PECs (gENP mmedium−3; gENP kgdryweight−1 for porous media). PECs are expressed for (i) the free pristine particles, because these are most compatible with toxicity testing protocols for risk assessment,16 (ii) the sum of free ENPs and ENPs hetero-aggregated, because in a regulatory context the fraction of metal (oxides) able to pass through a filter <450 nm is considered bioavailable,25 and (iii) total concentrations for each environmental compartment.
Multimedia fate is evaluated with environmental distributions (EDs) (% gENP in compartment i gENP in env. system−1) and speciation distribution patterns (SDPs) within each compartment (% gENP species i in compartment j gENP in compartment j−1).
Emission rates
The vector of emission was calculated by multiplying estimated production volumes13,26 with estimated fractions that are released to air, water or soil during stages of the life cycle of the nanomaterial product (ESI,† Chapter B, S7–S11).2 Direct emission patterns to soil and air are considered during the use and production stage of the nanomaterials, whereas direct emission to surface water is only expected during the use stage. The fractions that are released during use are calculated as the product of the use patterns of the nanomaterials, e.g. cosmetics, electronics, coatings and paints, and the estimated fractions of release for these use patterns (ESI,† Chapter B, Tables S1 and S2). Indirect emission is calculated for the disposal stage through waste incineration and wastewater treatment containing the nanomaterial products (ESI,† Chapter B, Fig. S1 and Table S1). Here, it was assumed that all nano-CeO2 and -TiO2 that flow from wastewater treatment effluents to surface waters2 are emitted as ENPs that are attached to natural colloid particles.29–33 Nano-ZnO is assumed to be absent in these effluents, because it has been dissolved completely.34 All nano-CeO2, -TiO2 and -ZnO that are emitted to agricultural soil by application of sewage sludge13 are assumed to be attached to natural coarse particles prone to sedimentation during the wastewater treatment process.35 Prior to emission, nano-ZnO in the sludge is assumed to be reduced to 0–1% of its initial amount.34 The amount of sewage sludge that is applied to agricultural soils is assumed to range between 0 and 55%.13 ENPs released during production, use and waste incineration are all assumed to be in their primary state as free ENPs, because release of free ENPs causes the most environmental concern.36Uncertainties in physicochemical properties of ENPs
The physicochemical properties of ENPs that are used as input parameters in the SB4N model are size, specific weight, attachment efficiencies with natural particles, and dissolution rates in different aqueous media.15 A uniform distribution was applied to express the variability in ENP diameter with 1 and 100 nm as the minimum and maximum, respectively, because more detailed data on the actual sizes of ENPs produced in the EU is not available.4,37 The specific weights of the nanomaterials are equal to the densities of the solid states of the pure chemical of which the ENP consists. Possible influences of coatings on the ENPs' weight were assumed negligible. Hence, there were no distributions inserted in the MC simulations related to the mass density of ENPs. Dissolution is interpreted here as the transformation of ENPs into ionic or molecular forms7 either spontaneously or as a result of chemical reactions with the environmental media such as (re-)oxidation and sulfidication.38 Dissolution rates for nano-ZnO in storm (rain)water “within weeks”, in freshwater “within days”, and in groundwater “within weeks” have been derived from the rough estimations of residence times published by Garner and Keller (ESI,† Chapter C, Table S1).21 Dissolution rates in sediment are assumed equal to those in freshwater. Nano-CeO2 and -TiO2 are not expected to dissolve to any significant extent, even over long periods of time regardless of water type,21 so that their dissolution rate has been set to zero.The data for the attachment efficiencies were collected from experimental studies, if not available from the Derjaguin and Landau,39 Verwey and Overbeek40 (DLVO) theory (ESI,† Chapter D, S13–S14). Experimentally obtained attachment efficiencies are often presented as a set of values reflecting a range of environmental conditions. A uniform distribution was applied with the minimal and maximal efficiencies observed within the boundaries of relevant environmental conditions (ESI,† Chapter D, Table S4).
The parameters applied in the DLVO theory are accompanied by ranges of variability and uncertainty in environmental conditions and ENP properties. These ranges were included in separate MC simulations that were performed on the DLVO expression (ESI,† Chapter D, S13–S17). However, it still remains uncertain to what extent experimentally derived attachment efficiencies are suitable for integration in an environmental fate model.41 Therefore, the acquired uncertainty distributions were not directly included in the evaluation of the SB4N model. Instead, the fundamental uncertainty of the applied methods to derive attachment efficiencies was also evaluated. In each iteration of the MC simulations, a value for each attachment efficiency was generated randomly from the distributions obtained from the DLVO theory or experimental work (ESI,† Chapter D, Table S4). Then, this value was used as the mode in a triangular distribution, in which the values of 0 and 1 have been selected as minimum and maximum, so that the fundamental uncertainty of these two methods was included.
Natural variabilities of environmental system parameters
For the purpose of this study, we used SB4N with one spatial scale resembling a regional environmental system (ESI,† Chapter E, Table S1) as defined in REACH.25 According to the REACH guidelines in environmental exposure estimation, 10% of the total production volume in the EU is located at this regional scale.25 The data were collected to reflect natural variability in model parameters of the environmental system from (i) earlier parameter uncertainty analyses on SimpleBox, (ii) other model studies on the environmental fate of nanoparticles and (iii) experimental, model and literature review studies on the natural variability in environmental materials, conditions and flows (ESI,† Chapter E, Table S2).Spearman rank analyses
Contributions of model input variances to model-predicted fate were analyzed by means of Spearman rank correlation analysis. Derived rank correlation coefficients express to what extent SB4N's model outcomes statistically depend on the uncertainties and variabilities of its input parameters. The Spearman rank analysis assigns the highest coefficients to the parameters that are most important to variation in PECs, environmental fate and speciation of the ENPs, thus revealing the relative importance of the different sources of uncertainty and variability.Three sets of Spearman rank analyses were performed to identify which input and model parameters are the major sources of uncertainty and variability in the PECs and SDPs of nano-CeO2, -TiO2, and -ZnO. The analyses were performed on the extract data of the MC simulations containing the selected model and parameter values and calculated output values for each iteration. The Spearman's rank correlation is calculated as the Pearson's correlation coefficient on the ranks of these data.
A first set of Spearman rank correlation coefficients was derived for the correlation between the PECs and all input and model parameters with a defined probability distribution. The uncertainties in the estimated production volumes are generally so large13,26 that influences of other input variances on model-calculated PECs could easily become obscured. Since the relation between emission rates and PECs is known to be linear in mass balance models,42 it was decided to run a separate second set of analyses in which uncertainty in production volumes was excluded by setting them at a constant value of 1 ton per year. It should be noted that this is a common procedure in life cycle impact assessment of toxic chemicals, where such ratios of steady-state masses and emission rates are named ‘environmental fate factor’.43 These fate factors are also applicable to nanomaterials.44 The PECs1t/y illustrate how ENPs spread through the environment independent of the total volume that is produced, reflecting the accuracy of the SB4N model.
Finally, a third set of Spearman rank analyses was performed to determine the most important parameters that influence the extent to which the ENPs occur as free pristine species or attached to natural colloids or coarse particles. This analysis was performed on the extract data of derived species distribution patterns (SDPs) within each environmental compartment (gspecies of ENP in compartment gtotal ENP in compartment−1).
Results and discussion
Environmental fate and exposure of nano-CeO2, -TiO2, and -ZnO
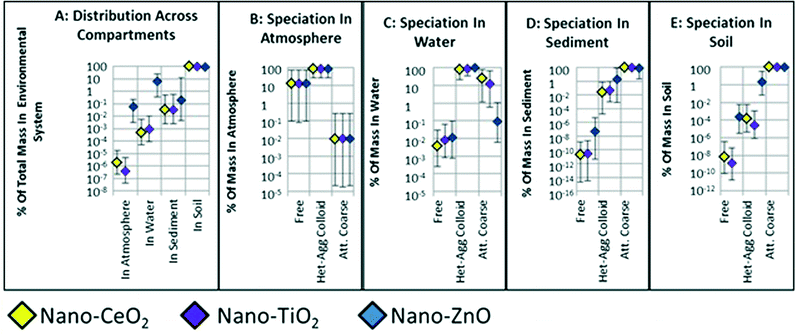
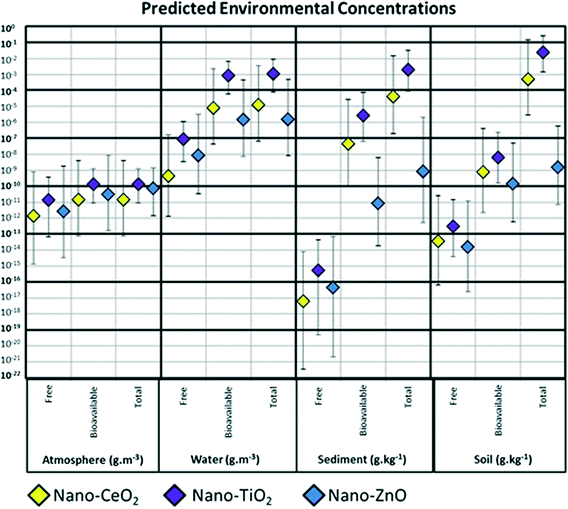
The environmental exposure to nano-CeO2, -TiO2, and -ZnO simulated with SB4N is expressed with median values for PECs and their 95% CIs calculated for the free, bioavailable (the sum of free ENPs and ENPs hetero-aggregated with natural colloids), and total concentrations in the atmosphere, water, sediment and soil compartments (Fig. 3). Speciation of the ENPs as free, hetero-aggregated with natural colloid particles or attached to natural coarse particles is expressed with median values and 95% CIs for the SDPs (Fig. 2). Differences in PECs and SDPs between nano-CeO2, -TiO2, and -ZnO are considered meaningful if 95% CIs do not overlap.The environmental fate across the compartments air, soil, water and sediment appear to be quite similar for nano-CeO2, -TiO2 and -ZnO, because most of their respective 95% CIs calculated by SB4N overlap (Fig. 2). The relative amounts of nano-ZnO in the atmosphere and water are high compared to those of nano-CeO2 and -TiO2. No significant differences can be observed between the distribution across environmental compartments and species of the insoluble nano-CeO2, and -TiO2, (Fig. 2). This indicates that only the difference in dissolution influences fate, whereas the other properties of nano-CeO2, -TiO2 and -ZnO and their differences in emission do not have a large effect on the environmental fate of these three nanoparticles.
On the other hand, the SDPs show some clear differences, i.e. non-overlapping 95% CIs in the extent to which the ENPs occur as free, aggregated with natural colloid particles, or attached to natural coarse particles. Atmospheric ENPs are more prone for coagulation with fine natural aerosol particles than large coarse mode aerosols (Fig. 2B). In water (Fig. 2C), the ENPs that are hetero-aggregated with natural colloid particles represent the dominant fraction (21–100%).
In the sediment compartment (Fig. 2D), the ENPs attached to natural coarse particles are the dominant species for nano-CeO2 and -TiO2, whereas for nano-ZnO there is no dominant species. The fraction of free ENPs in sediment is extremely small for all three ENPs considered (2 × 10−15 to 3 × 10−3%). Still, the free fractions of nano-CeO2 and -TiO2 are even smaller than that of nano-ZnO in sediment (Fig. 2D). The SDPs of ENPs in soil (Fig. 2D) show a sequence: free ENPs make up the smallest fraction (1 × 10−11 to 1.8 × 10−2%), aggregated species are intermediate (9 × 10−7 to 41%), and a dominant fraction of ENPs is attached to the solid grains in soil (61–100%). Here, the 95% CIs for the different species of nano-CeO2 and -TiO2 considered in soil are more distant from each other compared to those of nano-ZnO (Fig. 2E).
Low amounts of engineered nanoparticles in the atmosphere
There are no significant differences between the atmospheric PECs of the three nanomaterials considered, since all of the respective 95% CIs overlap (Fig. 3). It appears that for atmosphere the bioavailable concentrations are almost equal to the total concentrations, because ENPs hetero-aggregated with fine aerosols make up for the dominant fractions (Fig. 2B).Only a small percentage of nano-CeO2, -TiO2, and -ZnO is distributed to the atmosphere as a function of fate and emission (Fig. 2A; 2.7 × 10−3–3.5%). There is relatively little emission of nano-CeO2, -TiO2, and -ZnO to air compared to their emission to soil and water.2,21,45 Airborne ENPs are also effectively removed by rainfall, dry deposition and coagulation with natural aerosol particles.21,46,47 Furthermore, ENPs do not evaporate, so that there is no diffusive transport from the water and soil compartments to the atmosphere.7,21,38 Emission is therefore considered to be the only major mass flow responsible for ENPs entering the air compartment.15 Hence, the relatively low amounts of nano-CeO2, -TiO2, and -ZnO distributed to the atmosphere as calculated by SB4N were to be expected.
In aerosol sciences, the probability of two coagulating particles sticking to each other after a collision event is approached with the Fuchs correction coefficient.48 Since this correction coefficient is a value between 0 and 1, it can be treated in fate simulations as an attachment efficiency.15 In contrast to actual attachment efficiencies between ENPs and natural particles in the water phase, Fuchs correction coefficients can ultimately be calculated as a function of the diameters and specific weights of the two colliding particles.48 It appears that the specific weights of ENPs have little influence on their coagulation with aerosols, because there are no differences between SDPs of the three different ENPs simulated. The diameters of the ENPs are all inserted in the MC simulations to range between 1–100 nm. Hence, the atmospheric coagulation of the different ENPs is calculated to be almost exactly the same (Fig. 2B).
Accumulation in soil
Total concentrations of nano-CeO2 and -TiO2 in soil are calculated to be significantly higher than that of nano-ZnO, whereas free and bioavailable concentrations in soil are comparable for all three nanomaterials considered (Fig. 3). However, the SDPs of nano-ZnO significantly differ from those of nano-CeO2 and -TiO2. Both the free fraction and the hetero-aggregated fraction of nano-ZnO are significantly larger compared to those of nano-CeO2 and -TiO2.The application of ENPs containing sewage sludge in agriculture leads to high emission to soil of nano-CeO2 and -TiO2 which are attached to suspended coarse particles due to the wastewater treatment process.49 These ENPs are calculated to accumulate, because erosion is considered to be their only removal mechanism. ENPs from direct emission accumulate as well, because of their fast attachment to the immobile solid grains of soil.50–52 SB4N calculates the attachment between ENPs and solid grains to occur within minutes (ESI,† Chapter G, Table S2), whereas the removal of attached nano-ZnO by dissolution takes months and removal of the practically insoluble nano-CeO2 and -TiO2 by soil erosion alone even takes centuries. Consequentially, the free and hetero-aggregated fractions of nano-ZnO are relatively large compared to those of nano-CeO2 and -TiO2, because nano-ZnO attached to soil grains accumulates less due to its dissolution. Still, for both the soluble and insoluble ENPs the species attached to the natural coarse particles are calculated to be dominant in soil (61–100%) (Fig. 2E). Stronger, the accumulation of these attached ENPs makes the ED to soil to be the highest for all environmental compartments (Fig. 2A; 46–100%).
Aggregation with natural colloids and particles in water
The bioavailable concentrations appear to be dominant in the water compartment (Fig. 2C and 3).However, this is only significant for nano-ZnO, as the 95% CI calculated for the fraction that is attached to coarse particles, and thus not bioavailable, does not overlap with the dominant fraction of ENPs hetero-aggregated with natural colloid particles (Fig. 2C). The fractions that are still in their free pristine state are calculated to range between 0.0001% and 0.1% for all three ENPs considered (Fig. 2C). The fractions of nano-CeO2 and -TiO2 attached to natural coarse particles are calculated to be significantly larger compared to the free fractions, but this is not the case for nano-ZnO. Except for nano-TiO2, the significant patterns are obscured in the calculation of PECs, i.e. the 95% CI calculated for the free concentration of nano-TiO2 in water does not overlap with those for the bioavailable and total concentrations (Fig. 2).
Prior to their inflow to water, nano-CeO2 and -TiO2 are calculated to accumulate in soil by attachment to soil grains and sludge application. The accumulation leads to elevated concentrations of ENPs attached to coarse particles in soil. According to the principles of mass balance equations, the mass flow of these ENPs attached to coarse particles from soil to water will be proportional to the elevated soil concentrations.42 Accumulation in soil is predicted to be the highest for nano-CeO2 and -TiO2, because they do not dissolve, so that the fractions of ENPs in water that are attached to coarse particles are also larger for nano-CeO2 and -TiO2 than for -ZnO (Fig. 2C). Nonetheless, the species of ENPs hetero-aggregated with natural colloid particles is dominant for all three nanomaterials, but this is only significant for nano-ZnO (Fig. 2C). Nano-ZnO is directly emitted to water only and not via wastewater treatment plant (WWTP) effluents, because it dissolves during treatment,34 whereas for nano-CeO2 and -TiO2 indirect emission as ENPs hetero-aggregated with natural colloid particles in WWTP effluents is considerable (ESI,† Chapter B).2 Still for nano-ZnO, the hetero-aggregated species is calculated to be significantly dominant (Fig. 2) as a result of the emitted free ENPs that hetero-aggregate with natural colloids in the water compartment.
The mechanisms dominating the aquatic fate of ENPs, i.e. dissolution and hetero-aggregation with settling natural particles, are considered to be complex and difficult to predict.38,53–56 However, despite all uncertainty, variability and complexity accounted for in the MC simulations of SB4N, it appears that the ENPs hetero-aggregated with natural colloids are likely to represent the dominant fraction of insoluble nano-CeO2 and -TiO2 (21–100%), whereas for the soluble nano-ZnO the dominance of hetero-aggregated species is even significant (59–100%) (Fig. 2C). These findings are supported by those reported by De Klein et al., in a spatially explicit modeling study of nano-CeO2 in the river Dommel validated with field measurements,23 and by Velzeboer et al.56 who demonstrated that in turbulent aquatic systems hetero-aggregation of ENPs with suspended particles governed the sedimentation of ENPs, irrespective of the ENP type studied.
Moreover, despite the differences in emission patterns, the EDs of nano-CeO2, -TiO2 and -ZnO to the water compartment are similar (Fig. 2A; 1 × 10−4 to 49%), since the 95% CIs overlap and are intermediate compared to the other emission compartments, i.e. higher than atmosphere and lower than soil.
Sediment as an environmental sink
SB4N calculates EDs and PECs for the top layers of the sediment, because contaminant concentrations in the deeper layers are considered not to be bioavailable.15,24 Concentrations of free ENPs in sediment are calculated to be extremely low compared to the bioavailable and total concentrations. The total and bioavailable concentrations of nano-TiO2 are calculated to be significantly higher than those of nano-ZnO, whereas the free concentrations are similar for all three ENPs considered. The EDs of ENPs to the sediment compartment appear to be most difficult to predict as the 95% CIs are the largest compared to the other compartments (Fig. 2A). There is no emission to sediment, so that the EDs are dependent on the environmental fate in air, water and soil. It is demonstrated that accumulation of insoluble ENPs in soil eventually leads to elevated concentrations of ENPs attached to natural coarse particles in the water column. The aquatic fate that follows after emission or transport from air and soil to water is important for concentrations in sediment as well, since the ENPs must settle through the water compartment in order to reach the top layer of the sediment.53,55,56 The dominant settling mechanism of ENPs that enter the water as free species is through hetero-aggregation with natural particles.54,56 Aquatic ENPs that are still in the free pristine state reside in the water column as a non-settling fraction.54 Hence, the fraction of free species in the sediment compartment is calculated to be extremely low (2 × 10−15 to 3 × 10−3%) as only very few free ENPs will reach the sediment compartment through sedimentation. Moreover, SB4N's model parameters that govern the hetero-aggregation between ENPs and natural particles in the water column are also influential for the EDs to sediment, e.g. the concentrations and sizes of natural particles in the water and their attachment efficiencies with ENPs. ENPs attached to larger natural particles settle faster56 and therefore are more effectively transported from the water compartment to the sediment compartment. This explains why the ENPs hetero-aggregated with smaller colloids are dominant in the water compartment (Fig. 2C), whereas in the sediment the species attached to natural coarse particles is dominant (Fig. 2D).Sedimentation of natural particles also buries the top layer of the sediment, so that the ENPs residing in the top layers are buried to the deeper layers.57 SB4N considers the deeper layers not to be a part of the bioavailable system.15 However, these layers are regarded as the final environmental sink of ENPs where they will accumulate.18,21,51,58 Although SB4N only calculates concentrations for ENPs in the top layers, the MC simulations demonstrate that only an extremely small fraction of ENPs can reach the deeper layers of the sediment in their pristine and free to disperse state as they cannot even reach the top layer in this state (Fig. 2D).
Sources of uncertainty and variability
Compared to the PECs, the trends in environmental fate are more significant due to the major uncertainty in production volumes, i.e. there is more overlap between the 95% CIs representing the PECs (Fig. 3) compared to those of the environmental distributions (EDs) (Fig. 2). The Spearman rank correlation coefficients confirm that the uncertainty in the estimated production volumes is a major source of the variation in the simulated PECs (ESI,† Chapter F, S30–S36). The uncertainty in production volume of nano-CeO2 is the largest for the three nanomaterials considered. This also applies for the 95% CIs for nano-CeO2 PECs (of 5 to 8 orders of the magnitude) and for the correlation coefficients between production volume and PECs (91–95%, ESI,† Chapter F, Table S1). Uncertainties in production volumes of nano-TiO2 and -ZnO are also the major causes of the variation in their respective PECs in the atmosphere, soil and water. Because the influence of production volumes on the PECs is so large, the influences of other input and model parameters are less prominent.In order to analyze the influences of the other SB4N model and input parameters, the PECs simulated with the production volume set constant at 1 ton per year were used. For all three nanomaterials considered, these PECs1t/y are most influenced by a few parameters that determine emission fractions, compartment volumes and removal rates (ESI,† Chapter F, Table S2). For the various compartments, the parameters with the highest correlation coefficient are (i) for atmosphere: the fraction of the production volume that is emitted to air, atmospheric mixing height, and the wind speed that determines the rate of advective transport of air to outside the system, (ii) for soil: dry weight of soil grains, soil depth, the removal rate by ENP dissolution or erosion, and emission, i.e. the fractions of the production volumes that are emitted to soil directly, the fraction of ENPs taken up in the WWTP sludge and the amount of contaminated sludge eventually applied to soil causing uncertainty in indirect emission, and (iii) for water: the fractions of the production volumes that are either directly emitted to water or through WWTP effluents, removal by ENP dissolution and water outflow (ESI,† Chapter F, Table S2). For the sediment compartment, the relationship between PECs1t/y and model and input parameters is more complex to derive as more parameters show high correlation coefficients. Moreover, these parameters also reflect the interaction between ENPs and natural particles in the water column. The size distribution of suspended natural coarse particles appears to be the most important parameter (ESI,† Chapter F, Table S2). This indicates that attachment of ENPs to larger settling natural particles is indeed the dominant mechanism of transport from the water compartment to the sediment compartment. Hence, it is also an important parameter for the PECs1t/y in water.
The Spearman rank correlation coefficients derived for the SDPs especially reflect the parameters that simulate the interaction between ENPs and the natural particles in the environment, e.g. the highest correlation coefficients for ENPs in air are assigned to the size and number concentrations of aerosol particles and the size of the ENP (ESI,† Chapter F, Table S3).
It is notable that the model parameters that are most important for the SDP reflect the environmental compartment of interest, e.g. the highest Spearman rank coefficients for the SDPs in water either reflect a physicochemical property of the ENP or a model parameter that characterizes the water compartment (ESI,† Chapter F, Table S5). This also applies to nano-CeO2 and -TiO2 in soil, whereas for the SDPs of nano-ZnO in soil, parameters reflecting the atmosphere are important as well. However, this only applies to model parameters and not to emission, since the uncertainty in sludge application to soil yields variation in PECs1t/y for the water and sediment compartments as well.
Emission to atmosphere is low for all three nanomaterials considered. Only for nano-ZnO emission to soil is also low because of the limited contribution of sludge application, i.e. nano-ZnO in sludge is reduced to 0–1% of its initial amount.34 Therefore, although emission of airborne nano-ZnO is low, its atmospheric fate becomes important for concentrations in soil as well (ESI,† Chapter F, Table S4).
For nano-ZnO, the SDPs in sediment mostly depend on model parameters reflecting the aquatic fate of ENPs (ESI,† Chapter F, Table S6). This predicts that the hetero-aggregates of nano-ZnO in sediment are already formed in the water before settling to the sediment compartment. The emission of nano-CeO2 and -TiO2 to soil through sludge application appears to be important for the PECs1t/y in sediment, although it is prior to transport from soil to water and from water to sediment. This indicates that the sediment compartment is an environmental sink of these insoluble ENPs.
It is notable that for all environmental compartments and nanomaterials considered the uncertainty in the characterization of the physicochemical properties of the ENPs, such as size and attachment efficiencies with natural particles, appears to be important for the SDPs and to a lesser extent for the prediction of total concentrations per environmental compartment. However, attachment efficiency may still be an important parameter for the environmental fate of ENPs. Rather, the variation in the attachment efficiencies inserted as input parameters in the MC simulations may not be large enough to yield variation in the outcomes as total concentrations. This would imply that attachment efficiencies for nano-CeO2, -TiO2 and -ZnO can be predicted accurate enough not to contribute to the variation in total concentrations. Furthermore, the hetero-aggregated ENPs are subject to the same aquatic fate processes as the free ENPs, e.g. dissolution, settling and advection. Hence, the aquatic fate of free ENPs is quite similar to that of the total amount of ENPs, since ENPs hetero-aggregated with natural colloids are the dominant species in water (Fig. 2C). Moreover, the influence of the actual attachment efficiencies may also be obscured by emission patterns. ENPs in WWTP effluents are assumed to be hetero-aggregated with natural colloids already, whereas ENPs in sludge applied to soil are assumed to be attached to coarse particles already.
Comparison to previous estimates and measurements
SB4N is a classical multimedia “box model” with a matrix extended with the environmental processes specifically relevant for the fate of ENPs.15 The model's equations specific for the environmental fate processes of ENPs have been validated separately: (i) the process descriptions governing the atmospheric fate of nanoparticles have been validated in traffic emission monitoring,59 (ii) the aquatic fate sub-model has been validated in aggregation, sedimentation and dissolution experiments,23,54–56,60–64 and (iii) the sub-model for filtration of nanoparticles through porous media, e.g. soil and sediment, has been validated in sand column experiments.65–70 SB4N unifies all these sub-models into one overarching “box model”. To date, such “box models” have only been validated for the emission of conventional chemicals through comparison with average measured environmental concentrations.14,71–77 A validation of the integrated SB4N model against measured averaged concentrations, however, still needs to be performed.15A major issue in such a validation is that analytical tools are not yet able to distinguish between the natural and the engineered nanomaterials.22 Moreover, ENPs attached to natural particles cannot be quantified in environmental samples,22 whereas the MC simulations calculate that only a small fraction of ENPs does not attach to natural particles and persist in their free pristine state (Fig. 2). A formal validation by means of a comparison between measured and modeled environmental concentrations is thus hampered14 or even impossible.22 Indicative validations of nano-CeO2, -TiO2, and -ZnO in surface waters23,78,79 and air80 can only be performed by comparing the PECs calculated with SB4N's model simulations with elemental mass concentrations of Ce, Ti, and Zn filtered for <450 nm submicron particles.22 These filtered concentrations actually reflect the sum of elemental mass concentrations able to pass through the <450 nm filter including natural colloids, dissolved elemental species, ENP hetero-aggregates and free species of ENPs.14,22 ENPs attached to natural coarse particles do not pass through the filter and are thus not sampled in field measurements at all.14,22 Hence, indicative validation is only allowed by demonstrating that modeled concentrations for the sum of free and hetero-aggregated ENPs are lower compared to the measured concentrations of the respective ENP's chemical element in filtered field samples.22 Moreover, in such a comparison, it should be noted that SB4N predicts regional background concentrations, whereas field measurements are often performed on locations at which local chemical concentrations are relatively high.81
Recently, the river Dommel (The Netherlands) has been sampled and filtered for <450 nm submicron particles by De Klein et al., 2016. These filtered samples contained mass concentrations of 0.04–0.27 and 0.63–1.15 mg m−3 for elemental Ce and Ti, respectively.23 The order of magnitude of these measured concentrations is comparable to the estimated concentrations of nano-CeO2 and -TiO2 in water calculated as the sum of the free ENPs and ENPs hetero-aggregated with natural colloid particles (Fig. 3; 0.1 μg m−3–10 mg m−3). It is unclear to what extent the measured concentrations of elemental Ce and Ti consists of engineered nano-CeO2 and -TiO2, but a comparison with characteristic elemental ratios of inert geogenic materials suggests at least an anthropogenic source for the measured Ti.23 Markus et al. have estimated concentrations of nano-ZnO (1 mg m−3) in the river Rhine as a fraction of the total measured concentrations of elemental Zn (15–40 mg m−3),79 whereas the background concentration of nano-ZnO predicted with SB4N is predicted to be lower (7 ng m−3–46 μg m−3).
Elemental Ce has been sampled from urban air and filtered by Park et al. who find it to range between 0.1 and 1 ng m−3.14,80 The background concentrations for nano-CeO2 estimated with SB4N covers this range completely (0.08 pg m−3 to 4 ng m−3), because of the great uncertainty in production volume estimations (ESI,† Chapter F).26 However, 0.1 ng m−3 and 1 ng m−3 are equal to the 75th and 92nd percentiles of the air concentrations predicted with SB4N, respectively. Hence, the modeled background concentrations of nano-CeO2 in air are most likely to be below that of the measured elemental concentrations in urban air.80
To our knowledge, further field data of measured of nano-CeO2, -TiO2 and -ZnO in the compartments air, soil and sediment are not available. However, we argue that the SB4N model yields acceptable results within the context of prospective environmental risk assessment of nanomaterials,16 although a formal and more extensive validation is desired once future measurement techniques allow so.14,16,22 Nonetheless, the model at least appears to be plausible, because (i) the concept of box models in general has been validated with conventional chemicals,14,71–77 (ii) SB4N's model equations are experimentally validated,15 (iii) PECs comply to the order of magnitude of the few environmental measurements available, and (iv) predicted environmental fate, speciation and distributions agree with expected environmental patterns of ENPs as reported in the scientific literature (ESI,† Chapter G, S37–S42).
Model simplifications and limitations
SB4N is developed as a screening level model that is neither temporal nor spatially explicit, whereas complex chemical reactions between ENPs and environmental matrices are only implicitly included in the a priori characterization of attachment efficiencies with natural particles and dissolution rates.15 Such simplifications in environmental exposure modeling of ENPs are inevitable but acceptable if justified scientifically.9 Instead of calculating PECs dynamically with an explicit temporal scale, SB4N calculates steady state concentrations. Such a simplification especially leads to overestimation of PECs for chemicals that are only subject to slow removal processes.82 The majority of the removal processes included in SB4N reflect an annual timescale, but erosion of soil that takes centuries is the exception here.15 Nano-CeO2 and -TiO2 are not expected to dissolve to any significant extent over long periods of time,21 so that once they are attached to grains in soils they are considered to be removed by erosion only.15 Hence, it should be noted that the concentrations of nano-CeO2, and -TiO2 attached to coarse grains of soil are an overestimation if applied within the context of environmental risk assessment guidelines that employ annual timescales.25 Rather, they should be treated as chemicals that are persistent in the environment.82SB4N is also not spatially explicit and does not include landscape details that have proven to be important for the local environmental fate and concentrations of ENPs in close proximity to the emission source.23,30,31 However, multimedia fate models that are spatially explicit only yield better estimates if data on spatial variability in emission intensities are available.81 In regulatory environmental exposure estimation,25 the original SimpleBox24 is not used to simulate PECs for conventional chemicals on a local scale but on a regional scale. On this regional scale, emission intensities are simplified into continuous diffuse volumes.25 The simulated PECs are to be treated as regional background concentrations.25 Since SB4N is an adaptation of the original SimpleBox model,24 the PECs simulated by SB4N are to be treated as regional background concentrations as well.15
The emission estimation of ENPs is further simplified by only considering direct release of pristine ENPs and ENPs attached to suspended particles during wastewater treatment. However, transformation processes such as homoaggregation, surface modification and weathering can alter the pristine state before, during or directly after release as well.7,49 Quantitative data on the release of ENPs are limited,49 so that the extent to which ENPs are physically or chemically altered upon emission is difficult to express.2 Hence, the pristine state of ENPs is chosen as a worst case starting point for the fate simulations after direct release, because most data on physicochemical properties of nano-CeO2, -TiO2 and -ZnO are referring to this state14,83 and because most concerns on the environmental risk of ENPs are related to release in a free to disperse form.36
Finally, complex physical and chemical transformation reactions between ENPs and environmental matrices, such as (re-)oxidation, sulfidication, phosphorization, functionalization and adsorption of natural organic matter, are not explicitly simulated. Rather, these processes are implicitly included by treating the influence that they have on ENP dissolution rates in different aqueous media and the attachment between ENPs and natural particles41,49 as an uncertainty. Hence, the simplifications in SB4N's model simulations do not hamper the environmental exposure estimation of ENPs as long as the PECs are interpreted on a screening level.16
Considerations in the screening level environmental exposure estimation of nanoparticles
The 95% CIs of the PECs derived probabilistically with SB4N for nano-CeO2, -TiO2 and -ZnO account for input parameter uncertainty and natural variability of the environmental system. In regulatory contexts, the purpose of environmental exposure estimation is to ensure safe concentrations at which no adverse effects for organisms in the environment are to be expected.5 Such insurance does not necessarily require complex probabilistic fate models that are difficult to implement in risk assessment frameworks.16 Rather, the upper limits of the confidence intervals for PECs should be below the lower limits of predicted no effect concentrations (PNECs),5 whether or not to use the outcome of this uncertainty study in a regulatory context is a regulatory choice. The simple point estimate multimedia fate models are therefore still fit for environmental exposure estimation as long as they are corrected for model uncertainty and variability. Here, it is discussed to what extent the magnitudes of variation in the PECs calculated for nano-CeO2, -TiO2, and -ZnO are fit for extrapolation to environmental exposure estimation of other nanomaterials at a screening level.Presently, there is no scientific consensus yet on what should be the relevant bioavailable ENP speciation for environmental exposure estimation.16 PNECs are derived from toxicity tests designed for exposure to pristine (free) ENPs,22,84–86 but in practice the ENPs often aggregate with themselves before reaching the exposed organisms.16 According to the guidelines of risk assessment, the bioavailable fraction of metals in the environment is defined as “the fraction of a metal that passes through a filter of 450 nm”.87 If this definition also applies to nanomaterials, then the relevant exposure concentrations would be the sum of the free ENPs and the ENPs hetero-aggregated with natural colloid particles (<450 nm), whereas only the free ENPs are somewhat compatible to the hazard data generated in ecotoxicological studies, because they both fully consist of the same substance.22 Nanomaterials attached to natural coarse particles do not at all fall under this definition of “bioavailable” at all, but they are present in the environment. With a consensus lacking whether ENPs attached to natural coarse particles should be regarded as environmental exposure or not, the conservative approach would be to include them.
The results from the MC simulations show that although nano-CeO2, -TiO2, and -ZnO differ in chemical composition, their distribution over the water, sediment and soil compartments is quite similar (Fig. 2). The only difference is that nano-ZnO is less accumulative in soil and sediment, because it is prone to dissolution. As a consequence, a higher percentage of nano-ZnO is distributed to the atmosphere and water (Fig. 2). It is plausible that the environmental distributions of nano-CeO2, -TiO2, and -ZnO also apply to ENPs with similar emission patterns and physicochemical properties. The Spearman rank correlation coefficients show that for the total concentrations the most important parameters reflect production volumes, emission fractions, compartment volumes and removal rates by advection or dissolution. We argue that because these parameters are most important and because of the similarity in environmental fate of nano-CeO2, -TiO2 and -ZnO, despite their different chemical compositions and solubility, the magnitudes of variation in the PECs of nano-CeO2, -TiO2, and -ZnO (i.e. the ratio of the 97.5 and 2.5 percentiles), which are (i) a factor of 10 for atmosphere and water, (ii) a factor of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for soluble ENPs and a factor of 100 for insoluble ENPs in sediment, and (iii) a factor of 100 for soil (ESI,† Chapter H, S43–S46), can be used as an assessment factor in screening level environmental exposure estimation of other nanoparticles. Inaccessible production volumes are the major source of uncertainty for total PECs. However, production volumes are linear proportional to PECs and environmental risk assessment of chemicals is performed by individuals with access to the respective production volumes.25 Stronger, the actual production data of the ENPs allow more differentiation in physicochemical properties such as size and a priori characterization of dissolution rates and attachment efficiencies,13 whereas the MC simulations have been performed for nano-CeO2, -TiO2 and -ZnO that are characterized on a generic level. Compartment volumes and advective flows reflect a natural variability of the environmental system that is valid for all chemicals, whereas for emission fractions (high) and dissolution rates (slow) a conservatively chosen value will lead to conservative calculations of the PECs as well. Hence, a conservative point estimated PEC multiplied with the magnitudes of variation identified for nano-CeO2, -TiO2, and -ZnO should provide an exposure concentration that is equal to or higher than the upper limit of the 95% CI of PEC calculated with the complex probabilistic fate model. However, the physicochemical properties and emission patterns of the three nanomaterials evaluated are, besides their dissolution rate, quite similar. There is relatively little emission to the atmosphere.2 Furthermore, they are all metal oxides with a specific weight higher than that of water, so that they do not float but settle,38 whereas the a priori characterized attachment efficiencies with natural particles all appear to be high enough to induce accumulation in soil and hetero-aggregation with natural particles in water. Hence, a screening level multimedia fate model can only be used for conservative environmental exposure estimation under the terms that (i) the most conservative values are chosen for dissolution rates and emission volumes, (ii) physicochemical properties of the ENP are similar to that of nano-CeO2, -TiO2, and -ZnO, i.e. attachment efficiencies are high enough to induce accumulation in soil and hetero-aggregation with natural particles in water, and (iii) the atmosphere is not the dominant compartment of emission.
000 for soluble ENPs and a factor of 100 for insoluble ENPs in sediment, and (iii) a factor of 100 for soil (ESI,† Chapter H, S43–S46), can be used as an assessment factor in screening level environmental exposure estimation of other nanoparticles. Inaccessible production volumes are the major source of uncertainty for total PECs. However, production volumes are linear proportional to PECs and environmental risk assessment of chemicals is performed by individuals with access to the respective production volumes.25 Stronger, the actual production data of the ENPs allow more differentiation in physicochemical properties such as size and a priori characterization of dissolution rates and attachment efficiencies,13 whereas the MC simulations have been performed for nano-CeO2, -TiO2 and -ZnO that are characterized on a generic level. Compartment volumes and advective flows reflect a natural variability of the environmental system that is valid for all chemicals, whereas for emission fractions (high) and dissolution rates (slow) a conservatively chosen value will lead to conservative calculations of the PECs as well. Hence, a conservative point estimated PEC multiplied with the magnitudes of variation identified for nano-CeO2, -TiO2, and -ZnO should provide an exposure concentration that is equal to or higher than the upper limit of the 95% CI of PEC calculated with the complex probabilistic fate model. However, the physicochemical properties and emission patterns of the three nanomaterials evaluated are, besides their dissolution rate, quite similar. There is relatively little emission to the atmosphere.2 Furthermore, they are all metal oxides with a specific weight higher than that of water, so that they do not float but settle,38 whereas the a priori characterized attachment efficiencies with natural particles all appear to be high enough to induce accumulation in soil and hetero-aggregation with natural particles in water. Hence, a screening level multimedia fate model can only be used for conservative environmental exposure estimation under the terms that (i) the most conservative values are chosen for dissolution rates and emission volumes, (ii) physicochemical properties of the ENP are similar to that of nano-CeO2, -TiO2, and -ZnO, i.e. attachment efficiencies are high enough to induce accumulation in soil and hetero-aggregation with natural particles in water, and (iii) the atmosphere is not the dominant compartment of emission.
Conclusions
Although nano-CeO2, -TiO2 and -ZnO differ in chemical composition, their environmental fate and speciation are quite similar. Despite the major uncertainties identified, several significant trends are demonstrated in the fate of the ENPs, such as the dominance of ENPs hetero-aggregated with natural colloid particles in water, accumulation in soil by attachment to grains and the small fraction of ENPs that persists in a free pristine state, especially in the sediment compartment. Moreover, environmental exposure estimation of ENPs with a default point estimate multimedia fate model has become feasible, because the sources of uncertainty have been made explicit. Screening level multimedia fate models, such as SB4N, seem appropriate for screening level estimations of environmental exposure to ENPs, because uncertainties in emission, physicochemical properties of the substance and natural variability in the environmental system only lead to a variation in total PECs that is comparable to that of conventional chemicals, i.e. a factor of 10 in air and water, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for soluble ENPs and 100 for insoluble ENPs in sediment, and 100 in soil.71 However, the bioavailable concentrations in environmental exposure estimation is considered the fraction “that passes through a filter <450 nm”, i.e. the sum of the free ENPs and ENPs hetero-aggregated with natural colloid particles. Physical species concentrations of ENPs as free pristine, hetero-aggregated with natural colloid particles, or attached to coarse particles species are less feasible to extrapolate from nano-CeO2, -TiO2, or -ZnO to other nanomaterials, because they more strongly depend on the physicochemical properties of the nanomaterials. Further, investigation is thus required to determine to what extent environmental fate, speciation, and concentrations are determined by the physicochemical properties of the ENP for two reasons: first, to make explicit to what extent the variation in the bioavailable concentrations predicted for nano-CeO2, -TiO2 and -ZnO is suitable for extrapolation to other nanomaterials and, second, to evaluate the environmental distribution of nanoparticles that do not have physicochemical properties comparable to nano-CeO2, -TiO2 and -ZnO.
000 for soluble ENPs and 100 for insoluble ENPs in sediment, and 100 in soil.71 However, the bioavailable concentrations in environmental exposure estimation is considered the fraction “that passes through a filter <450 nm”, i.e. the sum of the free ENPs and ENPs hetero-aggregated with natural colloid particles. Physical species concentrations of ENPs as free pristine, hetero-aggregated with natural colloid particles, or attached to coarse particles species are less feasible to extrapolate from nano-CeO2, -TiO2, or -ZnO to other nanomaterials, because they more strongly depend on the physicochemical properties of the nanomaterials. Further, investigation is thus required to determine to what extent environmental fate, speciation, and concentrations are determined by the physicochemical properties of the ENP for two reasons: first, to make explicit to what extent the variation in the bioavailable concentrations predicted for nano-CeO2, -TiO2 and -ZnO is suitable for extrapolation to other nanomaterials and, second, to evaluate the environmental distribution of nanoparticles that do not have physicochemical properties comparable to nano-CeO2, -TiO2 and -ZnO.
Acknowledgements
This work is supported by NanoNextNL, a micro- and nanotechnology consortium of the Government of The Netherlands and 130 partners.Notes and references
- L. C. Abott and A. D. Maynard, Risk Anal., 2010, 30, 1634–1644 CrossRef PubMed.
- A. A. Keller, S. McFerran, A. Lazareva and S. Suh, J. Nanopart. Res., 2013, 15 DOI:10.1007/s11051-013-1692-4.
- I. Corsi, G. N. Cherr, H. S. Lenihan, J. Labille, M. Hassellov, L. Canesi, F. Dondero, G. Frenzilli, D. Hristozov, V. Puntes, C. D. Torre, A. Pinsino, G. Libralato, A. Marcomini, E. Sabbioni and V. Matranga, ACS Nano, 2014, 8, 9694–9709 CrossRef CAS PubMed.
- M. E. Pettitt and J. R. Lead, Environ. Int., 2013, 52, 41–50 CrossRef PubMed.
- ECHA, Guidance in a Nutshell Chemical Safety Assessment, 2009, ECHA-09-B-15-EN Search PubMed.
- USEPA, Toxic Substance Control Act Work Plan Chemicals: Methods Document, United States Environmental Protection Agency, 2012 Search PubMed, Published Online.
- J. A. Meesters, K. Veltman, A. J. Hendriks and D. V. D. Meent, Integr. Environ. Assess. Manage., 2013, 9, e15–e26 CrossRef PubMed.
- A. E. Nel, C. J. Brinker, W. J. Parak, J. I. Zink, W. C. W. Chan, K. E. Pinkerton, T. Xia, D. R. Baer, M. C. Hersam and P. S. Weiss, ACS Nano, 2015, 9, 5627–5630 CrossRef CAS PubMed.
- A. Praetorius, R. Arvidsson, S. Molander and M. Scheringer, Environ. Sci.: Processes Impacts, 2013, 15, 161–168 CAS.
- C. O. Hendren, M. Lowry, K. D. Grieger, E. S. Money, J. M. Johnston, M. R. Wiesner and S. M. Beaulieu, Environ. Sci. Technol., 2013, 47, 1190–1205 CrossRef CAS PubMed.
- N. C. Mueller and B. Nowack, Environ. Sci. Technol., 2008, 42, 4447–4453 CrossRef CAS PubMed.
- F. Gottschalk, R. W. Scholz and B. Nowack, Environ. Model Softw., 2010, 25, 320–332 CrossRef.
- T. Y. Sun, F. Gottschalk, K. Hungerbühler and B. Nowack, Environ. Pollut., 2014, 185, 69–76 CrossRef CAS PubMed.
- F. Gottschalk, T. Sun and B. Nowack, Environ. Pollut., 2013, 181, 287–300 CrossRef CAS PubMed.
- J. A. Meesters, A. A. Koelmans, J. T. K. Quik, A. J. Hendriks and D. V. D. Meent, Environ. Sci. Technol., 2014, 48, 5726–5736 CrossRef CAS PubMed.
- A. A. Koelmans, N. J. Diepens, I. Velzeboer, E. Besseling, J. T. K. Quik and D. Van de Meent, Sci. Total Environ., 2015, 535, 141–149 CrossRef CAS PubMed.
- N. Sani-Kast, M. Scheringer, D. Slomberg, J. Labille, A. Praetorius, P. Ollivier and K. Hungerbühler, Sci. Total Environ., 2015, 535, 150–159 CrossRef CAS PubMed.
- H. H. Liu and Y. Cohen, Environ. Sci. Technol., 2014, 48, 3281–3292 CrossRef CAS PubMed.
- H. H. Liu, M. Bilal, Y. Cohen, A. Lazareva and A. A. Keller, 2014 IEEE International Conference on Bioinformatics and Biomedicine, 2014, pp. 10–17 Search PubMed.
- R. Arvidsson, S. Molander, B. A. Sanden and M. Hassellov, Hum. Ecol. Risk Assess., 2011, 17, 245–262 CrossRef CAS.
- K. L. Garner and A. A. Keller, J. Nanopart. Res., 2014, 16, 1–28 CrossRef.
- B. Nowack, M. Baalousha, N. Bornhoft, Q. Chaudhry, G. Cornelis, J. Cotterill, A. Gondikas, M. Hassellov, J. R. Lead, D. M. Mitrano, F. Von der Kammer and T. Wontner-Smith, Environ. Sci.: Nano, 2015, 2, 421–428 RSC.
- J. De Klein, J. T. K. Quik, P. Bauerlein and A. A. Koelmans, Environ. Sci.: Nano, 2016, 3, 434–441 RSC.
- L. J. Brandes, H. D. Hollander and D. V. D. Meent, Simplebox 2.0: a nested multimedia fate model for evaluating the environmental fate of chemicals, Report 719101029, 1996 Search PubMed.
- ECHA, Guidance on information requirements and chemical safety assessment Chapter R.16: Environmental Exposure Estimation, 2012 Search PubMed.
- F. Piccinno, F. Gottschalk, S. Seeger and B. Nowack, J. Nanopart. Res., 2012, 14, 1–11 CrossRef.
- E. I. D. T. Oracle, Crystal Ball User's Guide, Report 11.1.2.3, Oracle America, Inc., Redwood City, 2013 Search PubMed.
- D. V. D. Meent, SIMPLEBOX: a generic multimedia fate evaluation model, National Institute of Public Health and Environment (RIVM), Bilthoven, The Netherlands, 1993 Search PubMed.
- P. Westerhoff, G. Song, K. Hristovski and M. A. Kiser, J. Environ. Monit., 2011, 13, 1195–1203 RSC.
- A. L. Dale, E. A. Casman, G. V. Lowry, J. R. Lead, E. Viparelli and M. Baalousha, Environ. Sci. Technol., 2015, 49, 2587–2593 CrossRef CAS PubMed.
- A. L. Dale, G. V. Lowry and E. A. Casman, Environ. Sci. Technol., 2015, 49, 7285–7293 CrossRef CAS PubMed.
- R. Kaegi, A. Voegelin, C. Ort, B. Sinnet, B. Thalmann, J. Krismer, H. Hagendorfer, M. Elumelu and E. Mueller, Water Res., 2013, 47, 3866–3877 CrossRef CAS PubMed.
- G. Brunetti, E. Donner, G. Laera, R. Sekine, K. G. Scheckel, M. Khaksar, K. Vasilev, G. De Mastro and E. Lombi, Water Res., 2015, 77, 72–84 CrossRef CAS PubMed.
- E. Lombi, E. Donner, E. Tavakkoli, T. W. Turney, R. Naidu, B. W. Miller and K. G. Scheckel, Environ. Sci. Technol., 2012, 46, 9089–9096 CrossRef CAS PubMed.
- J. Struijs, SimpleTreat 3.0: a model to predict the distribution and elimination of chemicals by sewage treatment plants, Report no. 719101025, National Institute of the Public Health and Environment (RIVM), Bilthoven, The Netherlands, 1996 Search PubMed.
- A. R. Kohler and C. Som, Hum. Ecol. Risk Assess., 2008, 14, 512–531 CrossRef.
- E. A. J. Bleeker, W. H. D. Jong, R. E. Geertsma, M. Groenewold, E. H. W. Heugens, M. Koers-Jacquemijns, D. V. D. Meent, J. R. Popma, A. G. Rietveld, S. W. P. Wijnhoven, F. R. Cassee and A. G. Oomen, Regul. Toxicol. Pharmacol., 2013, 65, 119–125 CrossRef PubMed.
- J. T. K. Quik, J. A. Vonk, S. F. Hansen, A. Baun and D. V. D. Meent, Environment International, 2011, 37, 1068–1077 CrossRef CAS PubMed.
- B. V. Derjaguin and L. D. Landau, Acta Physicochim. URSS, 1941, 14, 633–642 Search PubMed.
- E. J. W. Verwey and J. T. G. Overbeek, Theory of the Stability of Lyophobic Colloids, Elsevier, Amsterdam, The Netherland, 1948 Search PubMed.
- A. R. Petosa, D. P. Jaisi, I. R. Quevedo, M. Elimelech and N. Tufenkji, Environ. Sci. Technol., 2010, 44, 6532–6549 CrossRef CAS PubMed.
- D. Van de Meent, A. Hollander, W. Peijnenburg and T. Breure, ed. F. Sanchez-Bayo, P. J. van den Brink and R. M. Mann, Fate and transport of contaminants, Bentham Science Publishers, Oak Park, 2011, pp. 13–42 Search PubMed.
- R. K. Rosenbaum, M. A. J. Huijbregts, A. D. Henderson, M. Margni, T. E. McKone, D. V. D. Meent, M. Z. Hauschild, S. Shaked, D. S. Li, L. S. Gold and O. Jolliet, Int. J. Life Cycle Assess., 2011, 16, 710–727 CrossRef CAS.
- B. Salieri, S. Righi, A. Pasteris and S. I. Olsen, Sci. Total Environ., 2015, 505, 494–502 CrossRef CAS PubMed.
- A. A. Keller and A. Lazareva, Environ. Sci. Technol. Lett., 2014, 1, 65–70 CrossRef CAS.
- A. J. Tiwari and L. C. Mar, J. Environ. Qual., 2010, 39, 1883–1895 CrossRef CAS PubMed.
- P. Kumar, P. Fennell and A. Robins, J. Nanopart. Res., 2010, 12, 1523–1530 CrossRef CAS PubMed.
- E. Otto, H. Fissan, S. H. Parkt and K. W. Leet, J. Aerosol Sci., 1999, 30, 17–34 CrossRef CAS.
- B. Nowack, J. F. Ranville, S. Diamond, J. A. Gallego-Urrea, C. Metcalfe, J. Rose, N. Horne, A. A. Koelmans and S. J. Klaine, Environ. Toxicol. Chem., 2012, 31, 50–59 CrossRef CAS PubMed.
- D. Lin, X. Tian, F. Wu and B. Xing, J. Environ. Qual., 2010, 39, 1896–1908 CrossRef PubMed.
- G. E. Batley, J. K. Kirby and M. J. McLaughlin, Acc. Chem. Res., 2013, 854–862 CrossRef CAS PubMed.
- G. Cornelis, L. Pang, C. Doolette, J. K. Kirby and M. J. McLaughlin, Sci. Total Environ., 2013, 463–464, 120–130 CrossRef CAS PubMed.
- A. Praetorius, M. Scheringer and K. Hungerbühler, Environ. Sci. Technol., 2012, 46, 6705–6713 CrossRef CAS PubMed.
- J. T. K. Quik, M. C. Stuart, M. Wouterse, W. Peijnenburg, A. J. Hendriks and D. V. D. Meent, Environ. Toxicol. Chem., 2012, 31, 1019–1022 CrossRef CAS PubMed.
- J. T. K. Quik, D. Van de Meent and A. A. Koelmans, Water Res., 2014, 62, 193–201 CrossRef CAS PubMed.
- I. Velzeboer, J. T. K. Quik, D. Van de Meent and A. A. Koelmans, Environ. Toxicol. Chem., 2014, 33, 1766–1773 CrossRef CAS PubMed.
- A. A. Koelmans, B. Nowack and M. R. Wiesner, Environ. Pollut., 2009, 157, 1110–1116 CrossRef CAS PubMed.
- A. A. Koelmans, J. T. K. Quik and I. Velzeboer, Environ. Pollut., 2015, 196, 171–175 CrossRef CAS PubMed.
- M. Ketzel and R. Berkowicz, Atmos. Environ., 2004, 38, 2639–2652 CrossRef CAS.
- L. E. Barton, M. Auffan, M. Bertrand, M. Barakat, C. Santaella, A. Masion, D. Borschneck, L. Olivi, M. R. Wiesner and J. Y. Bottero, Environ. Sci. Technol., 2014, 48, 7289–7296 CrossRef CAS PubMed.
- L. E. Barton, M. Auffan, L. Olivi and M. R. Wiesner, Environ. Pollut., 2015, 203, 122–129 CrossRef CAS PubMed.
- A. A. Keller, H. Wang, D. Zhou, H. S. Lenihan, G. Cherr, B. J. Cardinale, R. Miller and Z. Ji, Environ. Sci. Technol., 2010, 44, 1962–1967 CrossRef CAS PubMed.
- A. Praetorius, J. Labille, M. Scheringer, A. Thill, K. Hungerbühler and J.-Y. Bottero, Environ. Sci. Technol., 2014, 48, 10690–10698 CrossRef CAS PubMed.
- J. T. K. Quik, PhD, Radboud University Nijmegen, 2013 Search PubMed.
- E. H. Jones and C. Su, Water Res., 2012, 46, 2445–2456 CrossRef CAS PubMed.
- E. H. Jones and C. Su, J. Hazard. Mater., 2014, 275, 79–88 CrossRef CAS PubMed.
- Z. Li, E. Sahle-Demessie, A. A. Hassan and G. A. Sorial, Water Res., 2011, 45, 4409–4418 CrossRef CAS PubMed.
- A. R. Petosa, S. J. Brennan, F. Rajput and N. Tufenkji, Water Res., 2012, 46, 1273–1285 CrossRef CAS PubMed.
- I. G. Godinez and C. J. G. Darnault, Water Res., 2011, 45, 839–851 CrossRef CAS PubMed.
- I. G. Godinez, C. J. G. Darnault, A. P. Khodadoust and D. Bogdan, Environ. Pollut., 2013, 174, 106–113 CrossRef CAS PubMed.
- J. Bakker, L. J. Brandes, H. A. den Hollander, D. van de Meent and J. Struijs, Validating SimpleBox-Computed Steady-state Concentration Ratios, National Institute of Public Health and the Environment (RIVM), Bilthoven, The Netherlands, 2003 Search PubMed.
- M. Hauck, M. A. J. Huijbregts, J. A. Armitage, I. T. Cousins, A. M. J. Ragas and D. Van de Meent, Chemosphere, 2008, 72, 959–967 CrossRef CAS PubMed.
- A. M. J. Ragas, R. S. Etienne, F. H. Willemsen and D. Van de Meent, Environ. Toxicol. Chem., 1999, 18, 1856–1867 CrossRef CAS.
- M. Mayo, Z. A. Collier, V. Hoang and M. Chapell, Sci. Total Environ., 2014, 494–495, 104–112 CrossRef CAS PubMed.
- J. M. Armitage, I. T. Cousins, M. Hauck, J. V. Harbers and M. J. Huijbregts, J. Environ. Monit., 2007, 9, 572–581 RSC.
- A. Franco and S. A. Trapp, Environ. Toxicol. Chem., 2010, 29, 789–799 CrossRef CAS PubMed.
- B. M. Pederson, L. J. Thibodeaux, K. T. Valsaraj and D. D. Reible, Environ. Toxicol. Chem., 2001, 20, 2114–2121 CrossRef CAS PubMed.
- A. P. Gondikas, F. Von der Kammer, R. B. Reed, S. Wagner, J. F. Ranville and T. Hofmann, Environ. Sci. Technol., 2014, 48, 5415–5422 CrossRef CAS PubMed.
- A. A. Markus, J. R. Parsons, E. W. M. Roex, P. De Voogt and R. W. P. M. Laane, Water Res., 2016, 91, 214–224 CrossRef CAS PubMed.
- B. Park, K. Donaldson, R. Duffin, L. Tran, F. Kelly, I. Mudway, J.-P. Morin, R. Guest, P. Jenkinson, Z. Samaras, M. Giannoulig, H. Kouridis and P. Martin, Inhalation Toxicol., 2008, 20, 547–566 CrossRef CAS PubMed.
- A. Hollander, M. Hauck, I. T. Cousins, M. A. J. Huijbregts, A. Pistocchi, A. M. J. Ragas and D. Van de Meent, Environ. Model. Assess., 2012, 17, 5770587 Search PubMed.
- D. Mackay and E. Webster, Environ. Sci. Pollut. Res., 2006, 13, 43–49 CrossRef CAS PubMed.
- F. Gottschalk and B. Nowack, J. Environ. Monit., 2011, 13, 1145–1155 RSC.
- B. K. Gaiser, T. F. Fernandes, M. A. Jepson, J. R. Lead, C. R. Tyler and V. Stone, Environ. Health, 2009, 8 DOI:10.1186/1476-069X-8-S1-S2.
- F. Gottschalk, E. Kost and B. Nowack, Environ. Toxicol. Chem., 2013, 32, 1278–1287 CrossRef CAS PubMed.
- E. J. Petersen, S. A. Diamond, A. J. Kennedy, G. G. Goss, K. Ho, J. R. Lead, S. K. Hanna, N. B. Hartmann, K. Hund-Rink, B. Mader, N. Manier, P. Pandard, E. R. Salinas and P. Sayre, Environ. Sci. Technol., 2015, 49, 9532–9547 CrossRef CAS PubMed.
- ECHA, Guidance for the implementation of REACH Appendix R.7.13-2: Environmental risk assessment for metals and metal compounds, 2008 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6en00081a |
| This journal is © The Royal Society of Chemistry 2016 |