Open Access Article
Open Access ArticleTrophic transfer of metal-based nanoparticles in aquatic environments: a review and recommendations for future research focus
Stine Rosendal
Tangaa
*ab,
Henriette
Selck
a,
Margrethe
Winther-Nielsen
b and
Farhan R.
Khan
 *a
*a
aDepartment of Science and Environment, Roskilde University, DK-4000 Roskilde, Denmark. E-mail: stangaa@ruc.dk; frkhan@ruc.dk; Tel: +45 4674 2899 Tel: +45 4674 3735
bDepartment of Environment and Toxicology, DHI, Agern Allé 5, DK-2970 Hørsholm, Denmark
First published on 11th August 2016
Abstract
Metal-containing engineered nanoparticles (Me-ENPs) are used in a wide range of products including inks, plastics, personal care products, clothing and electronic devices. The release of Me-ENPs has been demonstrated from some products, and thus, particles are likely to enter the aquatic environment where they have been shown to be taken up by a variety of species. Therefore, there is a possibility that Me-ENPs will enter and pass through aquatic food webs, but research on this topic is limited. In this tutorial review, we discuss the factors contributing to trophic transfer of Me-ENPs, and where this information is scarce, we utilize the existing literature on aqueous metal trophic transfer as a potential starting point for greater mechanistic insight and for setting directions for future studies. We identify four key factors affecting trophic transfer of Me-ENPs: (1) environmental transformations of Me-ENPs, (2) uptake and accumulation in prey organisms, (3) internal fate and localization in the prey, and (4) digestive physiology of the predator. Whilst much research has been conducted on the first two of these factors, key knowledge gaps exist in our understanding of how Me-ENP trophic transfer is affected by the internal distribution in prey organisms and the digestive physiology of the predator. Additionally, we suggest that the ENP association with sediments may be a key process that results in the transfer of intact particles within aquatic food webs.
Nano impactStudies on the trophic transfer of Me-ENPs remain scarce, and the factors leading to this transfer are poorly understood. Here, we describe four processes that influence trophic transfer and suggest that the trophic transfer of aqueous metals is a logical starting point for future research involving Me-ENPs. We suggest that the initial uptake of Me-ENPs through the sediments is a likely source for intact Me-ENPs to enter the aquatic food web, and more focus should be directed here. To fully understand the potential for Me-ENP trophic transfer, future research needs to address the internal fate and localization of Me-ENPs in the prey organisms and the impact of the predator's digestive physiology. |
1. Introduction: trophic transfer, trace metals and metal-containing nanoparticles
Trophic transfer, described as the movement of toxicants up through the food web via ingestion of prey organisms by predators, has been widely recognized and remains a much studied eco-toxicological issue. In the case of trace metals in aqueous form (a term used here to include all metal species (ionic, dissolved, complexed) that exist in the water after addition of the metal salt), high profile incidences affecting both human health (e.g. methylmercury poisoning in the city of Minamata, Japan1) and piscine health have increased public and regulatory awareness. An example of the latter is provided by studies at the Clark Fork River in Montana, USA, which has received inflows of metal-rich mine effluents since the late 19th century. Young rainbow and brown trout readily accumulated Cd, Cu, Pb and As from diets of benthic invertebrates leading to reduced survival, growth and feeding activity.2,3 Moreover, studies show that trace metals biomagnify along the food chain,4–6 where biomagnification is a measure of contaminant transfer from lower to higher trophic levels and a biomagnification factor (BMF) of >1 indicates an increasing concentration up the food chain. Based on such research, the passage of trace metals through aquatic food webs is broken down into two main processes: (1) the accumulation of metals from the surrounding environment by prey organisms (i.e. net accumulation of metal into tissues via all available uptake routes), followed by (2) assimilation of metals in predators (i.e. the efficiency with which the metal is extracted from ingested food and absorbed into the body).7 Whilst the movement of aqueous metals in the aquatic food chain is well known and relatively well understood,2–4,8,9 studies on the potential trophic transfer of particulate metals, metal oxides and metal mixtures in the nano-size range, formulated as engineered nanoparticles (Me-ENPs), are scarce but are increasingly subject to similar concerns.The unique properties of Me-ENPs result from the combination of the inherent properties of the metal and the novel properties related to the nanoscale morphology such as size, shape, high surface to volume ratio, surface functionalization and surface charge.10,11 As such, Me-ENPs have found use in a wide range of products including cosmetics (Ag, TiO2, ZnO), medicine (Ag, CeO2), electronics (Cu, Au, Cd (as quantum dots)), bioactive coatings (Ag, CuO) and inks (Au, Ag, TiO2). Due to the increasing production and use of Me-ENPs, their release into the aquatic environment is inevitable and has already been demonstrated.12,13 Several studies show that metals introduced to organisms as ENPs are taken up from the abiotic compartments from both water and diet,14–17 commonly with ENPs mixed into sediments18–22 or added to food sources such as algae.23,24 This uptake of Me-ENPs creates a link between the abiotic environmental compartments and organisms in the aquatic food chain. Once taken up in the biota, as either an aqueous metal or Me-ENPs retained in the gut or absorbed over the epithelia, predation of these organisms potentially leads to ingestion and retention of Me-ENPs or at least the constituent metal ions in cases of particle dissolution. Describing the movement of intact particles in aquatic food webs is difficult due to the transformations that can occur after particles enter the environment, especially the aquatic environment. There is evidence to indicate that Me-ENP trophic transfer occurs in aquatic food webs,19,22 and although currently there are only a handful of studies on this topic, it warrants further investigation. Furthermore, a few studies have examined trophic transfer of Me-ENPs in terrestrial environments, with movement of intact Au ENPs from tomato and tobacco plants to the tobacco hornworm (Manduca sexta). Au ENPs were significantly accumulated in hornworms when passed on from the lower trophic level (i.e. accumulated in leaves) but not when particles were only sorbed (i.e. added to leaf surfaces) to the leaves.25,26 These studies demonstrate the possible movement of intact Me-ENPs up the food chain, as well as how accumulation in the predator differs based on how Me-ENPs are taken up by the prey.
Based on the current literature on trophic transfer of Me-ENPs in the aquatic environment, we here assess the existing knowledge with the aim of highlighting knowledge gaps and suggesting directions for key future research areas. The trophic transfer of Me-ENPs in aquatic ecosystems is still a topic in its infancy, with <20 studies published to date (summarized in Table 1). Thus, we provide relevant analogies to the wealth of research that already exists regarding trophic transfer of aqueous metals. We recommend areas of research that require greater investigation to better understand how Me-ENPs that enter the aquatic environment may firstly move from the abiotic to biotic compartments and then be subject to food web transfer.
| ENP type | Primary particle size (nm) | Organism(s) | Exposure time | Main findings | Ref. |
|---|---|---|---|---|---|
| CdSe/ZnS QDs | 10–25 | Algae → daphnia | 96 h & 48 h | The coating provides protection against toxicity, leading to increased trophic transfer potential | Bouldin, 2008 (ref. 28) |
| CdSe/ZnS QDs (different surface groups) | 6–12 | Ciliates → rotifers | Up to 7 d | Dietary transfer of QDs important for higher trophic organisms | Holbrook, 2008 (ref. 27) |
| Au (amine coated) | 10 ± 0.5 | Algae → bivalve | 24 h & 7 d | Bioaccumulation & uptake in cells via gill penetration and the intestinal epithelia in bivalve. Biological removal of coating caused oxidative stress | Renault, 2008 (ref. 148) |
| Au (rods) | 65 × 15 | Marine mesocosm | 12 d | Transfer from water & sediment to organisms. Highest bioaccumulation in clams & biofilms | Ferry, 2009 (ref. 37) |
| Entire food web | |||||
| TiO2 | 21 | Daphnia → zebrafish | 24 h & 14 dU + 7 dD | Dietary transfer of TiO2 ENPs from daphnids to zebrafish. No biomagnification | Zhu, 2010 (ref. 145) |
| CdSe/ZnS QDs | — | Daphnia (artemia) → zebrafish | 24 h & 14 dU + 7 dD | Dietary transfer of QDs from daphnids to zebrafish. No biomagnification | Lewinski, 2011 (ref. 146) |
| CdSe QDs (bare) | 5 | Bacteria → protozoa | Up to 16 h | Trophic transfer of QDs led to biomagnification of Cd in protozoans. Non-degraded QDs in protozoans might increase risk of Me-ENP contamination in higher organisms | Werlin, 2011 (ref. 34) |
| ZnO | — | Algae → copepods | 7 d & 7 d | Decreased copepod survival due to trophically transferred ZnO. Impaired fecundity in the highest dietary ZnO concentration | Jarvis, 2013 (ref. 31) |
| TiO2 (heterogeneous) | 6.4–73.8 | Bacteria → ciliates | 24 h | Dietary transfer of TiO2 ENPs led to reduced growth rate and population yield in ciliates. TiO2 NP detected in food vacuoles. No biomagnification | Mielke, 2013 (ref. 30) |
| CuO, ZnO | 40, 10–30 | Brine shrimp → goldfish | 24 h & 21 d | Accumulation of CuO and ZnO in intestine, liver and gills, however no significant increase in concentrations in muscle, heart or brain after dietary (or waterborne) exposure | Ates, 2014 (ref. 147) |
| CeO2 (rods) | 67 ± 8 × 8 ± 31 | Phytoplankton → blue mussel | 5 weeks (37 d) | Trophic transfer of CeO2 ENPs from phytoplankton to mussel. No difference in bioaccumulation in regard to the exposure method (water vs. diet) | Conway, 2014 (ref. 149) |
| SnO2, CeO2, Fe3O4, SiO2 | 61, 50–105, 20–30, 4–40 | Algae → sea urchin larvae | 48 h & 15 d | Decreased larval survival after dietary exposure to SnO2 & CeO2 ENPs. Developmental effects due to trophic transfer of all NPs from algae to larvae | Gambardella, 2014 (ref. 32) |
| Au (citrate or PEG coating) | Differs between media | Algae → blue mussel | 24 h & 24 h | Au only detected in digestive gland after dietary exposure. Coating affected bioaccumulation | Larguinho, 2014 (ref. 102) |
| CdSe/ZnS QDs (polymer coating) | 4.6 | Protozoa → zooplankton → zebrafish | 48 h & 48 h & 48 h | QDs observed in all 3 organisms by IMP-SLM, thus trophic transfer of QDs between the 3 tested levels occurred. No biomagnification in fish | Lee, 2014 (ref. 35) |
| Al2O3 | 40–100 | Algae → daphnia | 48 h & up to 72 h (OECD 202) | Dietary exposure caused alterations in daphnid feeding behavior, which could lead to a disrupted energy flow in the ecosystem | Pakrashi, 2014 (ref. 168) |
| ZnO (bare or octyl-coated) | 30 ± 17 | Daphnia → zebrafish | 24 h & up to 14 dU + 7 dD | Trophic transfer of ZnO from daphnids to zebrafish. Tenfold higher bioaccumulation compared to water exposure | Skjolding, 2014 (ref. 33) |
| TiO2 | 21 (250.5) | Algae → daphnia | 72 h & 35 d | Trophic transfer of TiO2 ENPs from algae to daphnids, with apparent biomagnification (BMF > 1). Addition of SDBS (anionic surfactant) increased ENP dispersion and enhanced accumulation in both species | Chen, 2015 (ref. 29) |
| Ag (PVP, PEG or citrate coating) | ∼11 nm (core size) | Algae → daphnia | 4 h & 40 min to 24 h | Diet is the primary route of uptake for Ag ENPs. Complete depuration of Ag ENPs from daphnids was not obtained, thus trophic transfer to higher levels is possible. Starch granules act as storage sites for ENPs in algae (C. vulgaris) | Kalman, 2015 (ref. 169) |
| Au | 10 ± 1 | 2 algae types → daphnia | 48 h & 24 h | Trophic transfer of Au from both algae types to daphnids. Highest accumulation of Au in E. gracilis probably due to lack of cell wall. Different accumulation patterns in the prey leads to a difference in the amount of Au transferred to the predator | Lee, 2015 (ref. 36) |
2. Current investigations of trophic transfer of Me-ENPs in the aquatic food web
The first reported studies on trophic transfer of Me-ENPs utilized the fluorescence properties of Cd-containing quantum dots (QDs) to visualize transfer in aquatic food webs.27,28 Cd QDs were shown to pass between the ciliate Tetrahymena pyriformis, used as the prey item, and the rotifer Brachionus calyciflorus in a simple two-level invertebrate food chain. Ciliates exposed to a suspension of Cd QDs for up to seven days were offered as a contaminated food source to the rotifers leading to intracellular detection of Cd QDs in ciliates, as well as in the gut and body cavity of the rotifers.27 Similarly, Bouldin et al. (2008)28 exposed a green algae (Pseudokirchneriella subcapitata) to Cd QDs for 96 h and then offered them as feed to a crustacean (Ceriodaphnia dubia). Cd QDs were detected within the algae cells, followed by morphological changes in P. subcapitata, such as altered cell integrity, structure and shape. The dietary transfer of Cd QDs from algae to daphnia was detected within the experimental time frame, with Cd QDs primarily found in the daphnids' digestive tract.28 Both studies revealed transfer of Cd QDs from the lower food chain level (bacteria, algae) to higher organisms (rotifers, daphnia); however, there was no evidence of biomagnification within the experimental time frames used, suggesting that although the QDs did pass to the predating organisms, there was no up-concentration of ENPs in the tissue.Following these initial studies, most research into this topic has been conducted with relatively simple, mainly pelagic food webs consisting of the minimum number of two trophic levels. These studies utilize relatively short exposure durations, typically 24–96 hours for prey and up to 14 days for predators. As shown in Table 1, the most frequently used organisms are algae and daphnids, and the ENPs tested are primarily metal oxides, Cd QDs or Au ENPs. As a general trend, it is reported that transfer of Me-ENPs does occur between the investigated trophic levels; however, the extent of trophic transfer is dependent on various factors including the predator and prey species, the exposure route of the prey, as well as the ENP characteristics, including the constituent metal and the presence of functionalization on the surface (as summarized in Table 1).
Chen et al. (2015)29 observed BMFs of almost 8 for daphnids (Daphnia magna), after dietary exposure to TiO2 ENP contaminated algae (Scenedesmus obliquus). Different sub-lethal effects, such as reduced growth rate,30 impaired fecundity31 and developmental changes,32 also resulted from the transfer of metal-oxide ENPs. Additionally, a 10-times higher body burden was detected in zebrafish (Danio rerio) after dietary transfer of ZnO ENP from daphnids (D. magna) compared to waterborne exposure.33 For Cd QDs, Werlin et al. (2011)34 detected biomagnification from bacteria (Pseudomonas aeruginosa) to protozoans (Tetrahymena thermophila), and since protozoans stayed physically intact after Cd QD accumulation, the authors suggested that non-degraded Cd QDs in protozoans could be transported to higher organisms. However, Lee and An (2014)35 did not detect biomagnification of Cd QDs in fish (D. rerio), after transport of these particles from protozoans (Astasia longa) to zebrafish, highlighting the difficulties in making general conclusions based on single studies. Lee et al. (2015)36 examined whether food type (different algae species) influenced the degree of Au ENP trophic transfer, resulting in the highest accumulation in daphnids (D. magna) when Au ENPs were associated with the algae Euglena gracilis. As the main difference between the food types tested was physiological, the authors suggested that the observed bioaccumulation patterns were likely due to E. gracilis' lack of a cell wall.36 Only a few examples exist in the literature including more trophic levels and complex systems. For instance, Ferry et al. (2009)37 added Au ENPs to a marine mesocosm that included both sediment and water and several trophic levels. Au ENPs accumulated in the food web, with the highest bioaccumulation observed in clams (Mercenaria mercenaria) and biofilms.37 In addition, as organisms such as clams and biofilms constitute a great part of the food for predatory invertebrates and demersal fish, the bioaccumulation of Au ENPs in these organisms could potentially be transferred further up the food web. A comparison of Me-ENP BMFs to that of the corresponding aqueous metal form would indicate whether the particulate metal is more or less biomagnified. Unfortunately, the literature cited does not include a metal reference (e.g. the salt form of the metal), and therefore, direct comparisons are not possible. Biomagnification is considered specific to both abiotic (e.g. environmental parameters) and biotic (e.g. organism physiology, food web structure, feeding relationships, analyses of whole body vs. single organ concentrations) factors, which makes comparison to the published literature on aqueous metals difficult, if not impossible. In fact, metal BMFs vary considerably and depend strongly on these factors, and we encourage readers to visit chapter 7.4.1. in Luoma and Rainbow (2008)38 for a more elaborate discussion. This clearly illustrates the need and importance of including reference treatments in any study of metal ENP uptake kinetics and effects.
The current state of the literature would indicate that the trophic transfer of Me-ENPs appears to occur, but biomagnification factors, when reported, are variable. Where biomagnification does not occur (i.e. BMF < 1), this would suggest that there may be no transfer of ENPs to higher-level organisms. Yet, caution must be taken, as most studies have included relatively short exposure durations and few trophic levels. These studies do highlight the importance of the dietary exposure route, which results in a higher body burden39–42 and differential levels of toxicity40–42 when compared to water-only exposure. Despite the evidence for trophic transfer, the main factors and mechanistic processes that control this, in the case of Me-ENPs, remain largely unknown. It is our contention in this tutorial review that the processes known to be involved in trophic transfer of trace metals in aqueous form may provide insights into the movement of Me-ENPs. Hence, the literature pertaining to the former is considered alongside our review of the Me-ENP trophic transfer literature.
3. Factors affecting trophic transfer of Me-ENPs
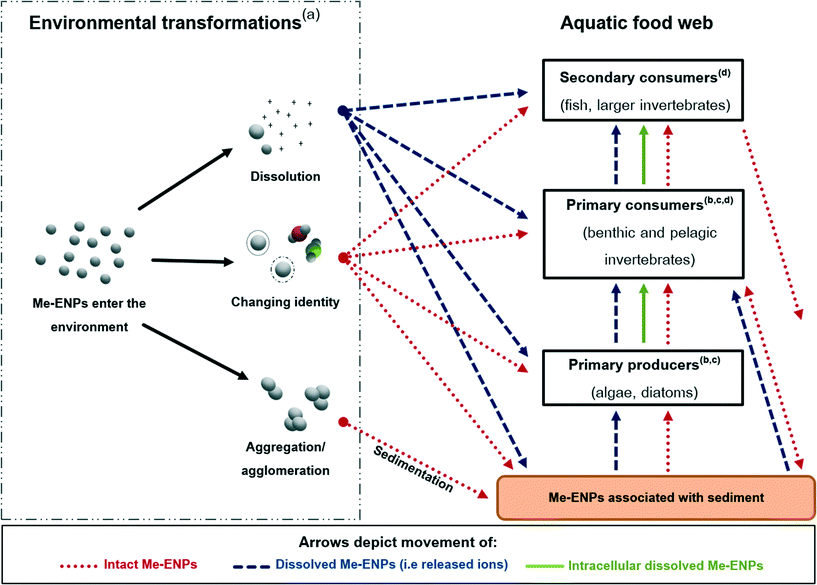
A multitude of factors may affect whether, and in what form, Me-ENPs are transferred between trophic levels. Based on the existing literature that has investigated this directly (described in section 2 and Table 1) as well as the wealth of literature on the trophic transfer of metals, we identify four broad key factors affecting trophic transfer of Me-ENPs. These factors, depicted in Fig. 1, are (1) the environmental transformations of Me-ENPs, (2) the uptake and accumulation in prey organisms, (3) the internal fate and localization in the prey, and (4) the digestive physiology of the predator. The relevance of each of the four factors and their relationship to the trophic transfer of Me-ENPs are detailed in the following sections.3.1. Environmental transformations of Me-ENPs
Me-ENPs enter the environment via several routes including untreated wastewater, accidental spills and intentional usage such as environmental remediation.43,44 Once in the environment, Me-ENPs will undergo a variety of transformation processes that influence their biotic interactions:45–47 (a) dissolution resulting in the release of metal ions,48–50 (b) alterations of the ENPs through association with environmental ligands and/or the formation of possible coatings/bilayers,51–53 and (c) aggregation/agglomeration leading to precipitation and likely sedimentation.54–56 These transformations are likely to occur simultaneously,45 and the combination of these transformative processes will profoundly affect any subsequent trophic transfer.Several studies show that dissolution is of central importance to the accumulation and toxic potential of Me-ENPs. This is particularly the case for ZnO,16,57–59 CuO60–62 and Ag50,63–66 ENPs, although for the latter there is also a weight of evidence to suggest nano-specific uptake mechanisms (i.e. endocytosis).19,23,67 Dissolution of Me-ENPs is affected by the inherent properties of the particle, such as size, constituent metal and surface chemistry as well as the composition of the environmental media. The interaction of these two factors will add to the variety of scenarios under which dissolution occurs, as described by Misra et al. (2012).68 Dissolution in various environmentally relevant media is reported in the range of 1–80% for CuO, Ag and ZnO ENPs, and this wide range again highlights the importance of thorough and exposure-specific characterization during experiments,68 although it is recognized that characterization is not available for all environmental matrices (e.g. sediments) owing to the lack of analytical methodologies. Arguably, however, dissolution is the most important transformation that a Me-ENP can undergo, changing the metal from a nano-scale structure into its ionic form. In terms of environmental safety and risk assessment, it has been suggested that the dissolution of the particle may represent the best case scenario as the ecotoxicological consequences would be likely no different from those of the constituent ion.69 This would be equally true for potential trophic transfer of Me-ENPs as the uptake and accumulation of trace metals by various trophic levels has been widely investigated.70–78
Alterations to the surface of Me-ENPs will take place at different degrees and will depend on factors such as particle coating and the presence of binding or complexing agents in the environment in which particles are released. Surface alterations, as have been shown, affects the behavior of the Me-ENPs in the environment and, subsequently, how they interact with organisms. In terms of changing the ENP surface, sulfidation has been reported to be an important process, particularly in the case of Ag ENPs. It likely causes a decreased dissolution rate and mobility, which would have an impact on the toxicity of the particles and their interactions with organisms.79 The sulfidation of Ag ENPs results in the formation of Ag2S adsorbed to the particles as coating55 or in the formation of new silver-sulfide nanostructures80 similar to those observed in field samples.81 Similarly, salinity, natural organic matter (NOM) and dissolved organic matter (DOM) all affect Me-ENP surfaces. Interactions between particles and NOM or DOM can create new particulate bilayers, which, like sulfidation, would affect the behavior of the particle (i.e. stability in the environment) and its interactions with biota. In their review of how Me-ENPs (termed as inorganic colloids) interact with DOM, Philippe & Schaumann (2014)82 outlined several adsorption mechanisms that control the degree of sorption, stability and aging. Their main findings were that DOM dynamics, bridging, and aggregation–disaggregation mechanisms are all influenced by the presence of humic substances, polysaccharides, and proteins found in natural waters and must be understood to describe colloid stability.82 Studies on ENPs and protein interactions have highlighted that it is not the inert particle that is in contact with biological systems (i.e. epithelial cells of the gill or intestine) during uptake, but in fact the altered particle surface.51,52,83,84 Proteins, and especially apolipoproteins, have shown to adsorb to ENP surfaces creating coatings known as a “protein corona”.85 It is this corona that the epithelial cells “see” and interact with when ENPs are taken up. The presence of a surface layer or corona changes the properties and ‘biological identity’ of the ENP, and in the case of the protein, the corona is likely to promote particle uptake.86 These findings illustrate the importance of characterizing particles in the environment or test media, in particular, as the composition of the media is highly influential in determining the fate of the particle. The chance of finding pristine particles in the aquatic environment is highly unlikely.
Combinations of environmental processes and particle characteristics will cause aggregation and/or agglomeration of Me-ENPs, which results in the likely sedimentation of the particles. For instance, pH, ionic strength, surface coating and surface charge will all influence the degree of aggregation. Furthermore, salinity changes the time taken for sedimentation and aggregation from days in seawater to months in freshwater.87–89 The relatively slow sedimentation in freshwater can result in a greater dispersion time in the water column with possible uptake and effects to pelagic species. Conversely, the faster sedimentation in more saline waters will lead to higher concentrations of ENPs in the sediment, resulting in increased risk for benthic and sediment-dwelling organisms. When natural disturbance is taken into consideration, using setups mimicking turbulent systems, sedimentation rates in the same order of magnitude for different ENPs under different salinity and aging time is seen.90 This is in contrast with the reported data from Garner and Keller (2014)91 highlighting the complexity of determining these factors for Me-ENPs. Furthermore, it illustrates that a greater number of potentially competing processes must be considered when the environmental fate and behavior of Me-ENPs are studied.
Praetorius et al. (2014)92 reported that hetero-aggregation (the interaction between different particle types, both organic and inorganic) is more important than homo-aggregation (interaction of the same particle type) for TiO2 ENPs in natural environments. Furthermore, attachment efficiency, reflecting the likelihood of particles “sticking” together, is highly important and should be implemented in future environmental fate models.92 Attachment efficiency may describe the creation of primary or secondary aggregates, that is aggregates created between primary particles (i.e. TiO2 ENPs) or aggregation of already aggregated particles and other particulates (i.e. organic matter), thereby producing secondary (larger) clusters.92 Primary and secondary aggregates are likely to be found in natural environments, making this an important observation for future studies. Me-ENPs such as TiO2 ENPs will likely exist as aggregates in diverse forms, increasing their sedimentation rates and thereby the likelihood of finding them within the sediment compartment. Again, media composition and turbulence will also play an important role. Dale et al. (2015)93 modelled the environmental fate of ZnO and Ag ENPs in the James River Basin (Virginia) and found that due to high mobility, sediment transport and streamflow, ENPs would be removed downstream from the River Basin. However, estimations also suggest that ENPs would eventually accumulate and persist in sediments. In extreme cases, particles may persist for over a century.93 Hence, depending on the system (static vs. turbulent), media (fresh vs. seawater) and particle type (coated vs. un-coated), sedimentation rates can be highly variable, but sedimentation appears to be a transformation of importance, thus highlighting the sediment compartment as a realistic environmental exposure route to ENPs.
With the differing sedimentation rates in mind, it is important to acknowledge that Me-ENPs will firstly be available for organisms in the water phase, after which particles settle out of the water column. Most studies agree that the final destination for Me-ENPs released into the aquatic environment is the sediment, making this an important sink (and source) for these contaminants.53,90,94 Interactions with sediment-dwelling organisms are therefore important to characterize. The most obvious being the incidental ingestion of particles by these organisms19 and whether (and how) they are subsequently internalized into the tissue. However, sediment dwellers are also likely to influence the distribution of the ENPs through the excretion of unassimilated particles re-entering the environment and via bioturbation, where through the movement through sediment grains and irrigation of burrows these organisms may recycle Me-ENPs back into the water column.95
Whilst a great deal of research has been conducted into the environmental transformations Me-ENPs may undergo in the environment, there are still many knowledge gaps that constitute future research needs including reverse reactions, transformation rates and the implications of aged or altered particles. However, we do know that numerous environmental transformations will occur simultaneously, leading to Me-ENPs in the aquatic environment existing as a mixture of released ions, particles with altered surfaces (potentially altered biological identities), agglomerates and aggregates. Thus, it is of the utmost importance to differentiate between the uptake and trophic transfer of intact Me-ENPs, which, although may have been modified through aggregation/agglomeration, sulfidization or surface alteration, are still nano-scale structures, and the metal which, although may have entered the environment in nano-form, is present in aqueous form. As a significant sink for settled intact Me-ENPs, sediments may be the most important entry point for intact Me-ENPs entering the aquatic food webs.
3.2. Uptake and accumulation by the prey organism
After environmental release, the Me-ENPs in the aforementioned forms (released ions, ENPs with altered surfaces, agglomerates and aggregates, associated with sediments) will be available for uptake by organisms at the base of various food webs. The mechanisms of uptake will highly influence the likelihood of the particles being passed onwards to prey organisms.As mentioned, ENP dissolution has been described as the best case scenario as the risk of aqueous metals are largely established.69 If ions are released, then uptake will be achieved in the same way as ions originating from aqueous metals. Ion uptake is achieved by membrane transporters that can transport metal ions directly into the cell.71 Essential metals, such as Cu, Zn and Fe, use established pathways, whereas non-essential metals often employ ionic mimicry using transporters intended for similarly sized and charged ions. For instance, Ag is taken up by Cu transporters in mammalian cells96 and via Na channels in freshwater fish.97 As Me-ENPs release ions, such mechanisms may also facilitate metal uptake from Me-ENPs. Any subsequent trophic transfer of this metal to the next trophic level would occur in the same ways that have been already studied.2–4,8,9
However, it is internalization of intact ENPs into lower trophic level organism tissues, or adsorption to body surfaces (e.g. on snail shells18,21), accumulation in gut lumen (either adsorbed to gut epithelia or as aggregates/agglomerates), and their subsequent transfer to their predators that presents a novel scenario. Regardless of how ENPs are associated with the prey (sorbed to shell or epithelia layers or internalized), they will be transferred to the predator; however, the availability for assimilation will depend on the ENP association with the prey. One hypothesis is that sorbed ENPs will be more readily available for assimilation than internalized ENPs. Me-ENP uptake into epithelial cells that face the external environment (i.e. those of the gill or gut) can be facilitated via different pathways, yet consensus amongst studies shows that primary uptake mechanisms for intact Me-ENPs is via endocytotic processes.19,65,67,98 Nanomedicine has shown that the different endocytotic processes (i.e. clathrin- and caveolae-mediated endocytosis or pinocytosis) can lead to the internalization of single particles and aggregates in the size range of 10 nm to 5 μm.99 Khan et al. (2014)100 showed that the endocytotic uptake of Ag ENPs presented to a mud snail (Peringia ulvae) through waterborne exposure occurred via multiple routes that included both clathrin- and caveolae-mediated endocytosis, as well as ion channels and/or transporter proteins for the dissolved Ag fraction. Endocytotic processes would lead to uptake of intact particles, which would be followed by different intracellular outcomes such as intracellular ion release, creation of ENP-containing vesicles or disruption of normal cell function.99,100 In primary producers such as unicellular algae, uptake mechanisms depend on both cell wall characteristics and particle size. Plant cell walls are semipermeable, including pores with diameters between 5 and 20 nm, meaning that Me-ENPs within this size range might be allowed to pass the barrier and move into the plasma membrane.101 Again, cellular uptake is predominantly via endocytotic processes;98 however, Me-ENPs could also employ ion channels or protein carriers to cross the membrane.101
As discussed, ENPs have been shown to interact with proteins and ligands within biological fluids, creating a biological surface coating on the particle, possibly enhancing cellular uptake.52 Other particle characteristics can also affect how Me-ENPs are taken up and accumulated. In the study by Bouldin et al. (2008),28 the organic polymer coating on Cd QDs protected the algae against direct toxic effects, leading to an increased transfer of Cd QDs from algae to primary consumers such as daphnids, as the algae continued to be an attractive food source.28 Likewise, coating affected bioaccumulation in a study by Larguinho et al. (2014)102 where a bivalve (Mytilus galloprovincialis) fed algae (Dunaliella salina) pre-exposed to PEGylated Au ENPs showed a higher Au content, compared to algae exposed to citrate-capped particles.102 Cellular uptake mechanisms are also affected by particle characteristics such as size and coating. Smaller particles (5 nm) and organic coated particles (tannic acid and citrate) are taken up in cells to a higher degree than larger (50–100 nm) and PVP-coated Me-ENPs.13,103 Thus, both the physiochemical properties of the ENP and the physiological characteristics of the species will affect the pattern of accumulation and must be considered when investigating the internalization of particles.
Several factors influence the particle fate after organism uptake, when the route of uptake is dietary. Feeding rate affects the time a metal or Me-ENP is retained in the gut (the gut passage time (GPT)) of the prey, and therefore, the time allowed to, for example, disaggregation/dissolution and absorption over the gut epithelia. GPT is inversely related to feeding rate, and the assimilation efficiency (AE) increases with increasing GPT until a ‘steady-state’ level is reached.104–107 Metal assimilation efficiencies (AE) from diets are generally high (ranging from 65 to 97%) and depend on both the test organism and the selected metal.75,108,109 Examples of AE values for Me-ENPs are reported in the same range, from 41 to 93%.23,110,111 If metal or Me-ENP assimilation is high in the prey, the possible transfer of the accumulated and assimilated metal to the predator is increased.
Daphnia magna is a well-established laboratory species and natural prey to many aquatic organisms. Thus, a number of studies have investigated how daphnids accumulate metals and Me-ENPs from the surrounding media and food.112–115 Daphnids filter particles between 0.1–5 μm,116 making aggregated ENPs available for ingestion in these organisms. Recent studies on uptake and depuration mechanisms in D. magna after short-term exposure to Au ENPs demonstrated that the elimination rate is increased when daphnids have access to food117 and a bi-phasic elimination pattern during the depuration phase with food present results in more than two thirds of the ingested Au being excreted within the first hour of depuration.115 Thus, the presence of food enhances the elimination of Me-ENPs from daphnids, whereas limited or no access to food decreases particle excretion.118 The retention of intact Me-ENPs in the gut of daphnids may not constitute uptake in the sense of being incorporated into the tissue (and nor would ENPs adsorbed to the external carapace118), but if daphnids containing ENPs are predated upon, then those intact particles are subject to transfer to the predator as discussed above.
Pelagic zooplankton, however, whilst well studied are perhaps not where investigations of trophic transfer should focus. As previously discussed, the likely eventual fate for Me-ENPs released into the environment is to associate with sediments, although the time to sedimentation may vary with environmental conditions. Nereid polychaetes provide an example of deposit-feeding animals that ingest sediments to consume nutritious organic matter but will also incidentally ingest sediment-associated contaminants. Up to half the silver uptake in Nereis diversicolor resulted from the ingestion of sediments,119 whereas for Nereis succinea the figure was 95%.120 Such organisms are an important prey item for a variety of large invertebrates, fish and bird species and thus provide a pathway for ENPs to move from the abiotic compartment into the aquatic food web. The caveat with this is of course whether the ENPs remain intact or whether they are prone to dissolution within the worm tissue or gut cavity. García-Alonso et al. (2011)19 visualized the ENPs in endosomes and small vesicles in gut epithelial cells at the base of the microvilli upon exposure to citrate-coated Ag NPs mixed in estuarine sediments. These ENPs appeared to have been endocytosed as intact particles, but as described in the following section different endocytotic mechanisms can lead to different intracellular fates, including lysosomal degradation leading to particle dissolution. Although the exact nature of endocytosis was not investigated by those authors, the presence of intact ENPs in the tissue of common prey items does dramatically increase the probability of Me-ENP trophic transfer.
The biological processes utilized in the uptake of intact Me-ENPs can influence the internal fate of the particles. Intracellular dissolution in prey organisms will negate the transfer of intact Me-ENPs to predators, whereas slower dissolution could result in a relatively higher transfer of ENPs through the food chain. Thus, it is important to distinguish the trophic transfer of ions released by Me-ENPs internally and the movement of the ENPs themselves up the food chain. In order for actual trophic transfer of Me-ENPs to occur, the uptake by or adsorption to the prey followed by further transfer to predators should be of the intact Me-ENPs. In this regard, the uptake route that starts with the incidental ingestion of ENPs from food sources, such as algae or from sediment, may present the greatest likelihood of intact ENPs passing up the food chain.
3.3. Internal fate and subcellular localization in the prey
When Me-ENPs are taken up by prey organisms, different processes will occur depending on species- and tissue-specific physiology and the mechanism of Me-ENP uptake. For example, the interactions between gastric acid and Ag ENPs show accelerated dissolution,64 whereas the release of silver ions from Ag ENPs in simulated lung media is negligible after 96 h, and aggregation of particles increases with ionic strength.121 Thus, whilst the former may limit the potential for trophic transfer, the latter in which Ag NPs remain intact may offer greater potential for food web passage. Me-ENPs might degrade or form complexes with substances present within biological media, such as gut or cellular fluids, altering their toxicity and bioavailability63,122 to both the organism in question and those that predate it.As mentioned, the primary mechanism for intact particles to enter tissues appears to be endocytotic in nature and could potentially take place at the epithelia of the digestive or respiratory systems. The exact mechanism may be an important determinant of the Me-ENP's intracellular fate: NPs endocytosed through the clathrin-mediated pathway are likely destined for lysosomal degradation.123 This pathway may be responsible for the much described nanoparticle “Trojan horse” effect in which intracellular toxicity results in the presence of high concentrations of labile metal ions.124 Conversely, if uptake is achieved via caveolae-mediated endocytosis or macropinocytosis, the ENP is not directed to the lysosome. Instead, intracellular vesicles (known as caveosomes in the case of the caveolae pathway) may fuse with the cell membrane and deliver the NPs out of the cell (exocytosis), so that NPs pass through the cell (transcytosis98). ENPs within macropinosome vesicles are not directed to the lysosome either but may remain in the cell in particulate form. Whilst internalization via these mechanisms may lead ENPs to novel intracellular locations where they might induce toxic responses,98 this does suggest that intact ENPs do remain in the tissue and potentially may be bioavailable to the predatory organisms.
Whilst different uptake mechanisms potentially lead to different internal fates, the key aspect of this topic is to address whether intact Me-ENPs in the tissue are trophically available. Within this review, we have proposed that previous research into the trophic availability of aqueous metals provides a guide to understand whether and how Me-ENPs move in aquatic food chains. It is perhaps in this topic area that studies with aqueous metals are most useful. Subcellular fractionation (differential centrifugation) protocols have been used to examine the internal distribution of metals and in a few studies with Me-ENPs. Commonly, the following subcellular fractions are collected from tissue homogenates: metal-rich granules (MRG), cellular debris, organelles (i.e. lysosomes and mitochondria), cytosolic heat sensitive proteins (‘enzymes’) and cytosolic heat stable proteins (‘metallothionein-like proteins’ (MTLP)).125–127 These fractions can be grouped according to solubility (insoluble MRG, debris and organelles vs. soluble enzymes and MTLP), toxic potential (detoxified metal within the MRG and MTLP fractions and other fractions considered as metal sensitive), and on the basis of trophic bioavailability of metals (‘trophically available metal’ (TAM), considered to include MTLP, enzymes and organelles).128 This latter division has been shown to be largely consistent amongst a variety of prey organisms and predators when exposing the prey to aqueous metals,129–136 but TAM is not a universally defined fraction and differences occur based on the metal in question, the physiology and internal metal handling of the food item and the feeding animal.128,137
The described fractionation method was employed on the endo-benthic ragworm, N. diversicolor, following exposure to citrate-coated Ag ENP spiked sediments.19 Ag ions were used as reference, and tissue homogenates from different exposure scenarios were examined and showed differences between Ag forms. Ag ions were detected in the MTLP fraction, whereas Ag ENPs were found in MRG, organelles and enzyme fractions. The difference in the distribution of Ag administered as particulate and aqueous forms was demonstrated and indicated that Ag ENPs did not follow the same subcellular distribution as Ag+, suggesting that the Ag ENPs did not dissolve internally.19 Similarly, sediment exposure of N. diversicolor to different forms of Cu (Cu ions, CuO micro- and nanoparticles) resulted in differential distribution of Cu between the subcellular fractions. Following exposure to sediment spiked with Cu ions, Cu was primarily found in MRG, to sediment spiked with CuO-micro, Cu was distributed equally among all five fractions and to sediment spiked with CuO-nano, Cu was primarily present in cellular debris.22 Thus, the subcellular fractionation protocol established for aqueous metals may also work for Me-ENPs, but nano-specific considerations need to be taken into account. The drawback is that such operational processes might introduce artefacts, as shown for trace metals.138 For instance, ENPs might combine with fractions based on mass during centrifugation rather than biological association, giving a false impression of the actual subcellular distribution of the Me-ENPs. Yet, given the importance of metal localization in prey organisms in regard to bioavailability to the next trophic level, this method, with appropriate nano-specific considerations, may provide initial guidelines into determining internal fate and trophic availability.
Additional tools for characterizing Me-ENPs in different sample types offer approaches for determining internal fate. Qualitative analysis via transmission electron microscopy (TEM) or scanning electron microscopy (SEM) has been used to visualise the location of ENPs in tissue,19,139 and even light microscopy and TEM have been used to detect Me-ENPs in D. magna. Au ENPs were observed in the midgut of organisms with no cellular uptake detected, indicating that particles were not moving past the intestinal barrier.115 Synchrotron X-ray radiation tools have been applied to nanomaterials science to measure ENP size, agglomeration state and surface structure in situ.140 This technique appears very promising for investigating the internal fate of Me-ENPs in tissue samples, as well as ENP behaviour in different media such as water or sediment. Other visualization techniques include the use of fluorescent particles (e.g. QDs) together with flow cytometry35 and confocal laser scanning microscopy (CLSM).141 The advantage of these newer techniques compared to TEM and SEM is that particles can be tracked inside whole organisms, diminishing the artefacts related to sample preparation. Furthermore, imaging particles in vivo will increase our qualitative understanding of how Me-ENPs are accumulated and handled within tissues.
Determining the internal fate of Me-ENPs is still a relatively novel research area. Protocols known to work for aqueous metals, such as differential centrifugation, could also be applicable for Me-ENPs, whilst nano-specific methods will undoubtedly build on initial data. In combination, these tools should be employed to understand the mechanisms controlling internal localization of Me-ENPs. Within prey organisms, this is likely key to determining whether and how Me-ENPs move through aquatic food chains.
3.4. Digestive physiology and accumulation mechanisms of the predator
The preceding sections have described how Me-ENPs may be subject to transformations both following environmental release and within prey organisms. With the assumption that some ENPs persist in particulate form, the remaining barrier to achieving trophic transfer will be how those ENPs within the tissue of the prey are handled once ingested by the predator. At this point, it is important to consider what constitutes dietary uptake and/or assimilation efficiency when dealing with particulate contaminants. For non-particulate contaminants, including trace metals, the common understanding is that the term includes the proportion of the ingested contaminant that crosses the gut lumen and is present in the tissue.7,8 This is predominantly determined by measuring tissue burdens following a suitable depuration period. However, it is not clear whether this requirement also applies to Me-ENPs. Many studies with Me-ENPs determine the presence of metal ions in tissue digests, where the metal has been introduced as an Me-ENP,14–24 but this is not the same as determining the presence of the nanoparticle itself. Thus, in many cases where ENPs are introduced via food, it is not possible to determine whether i) the ENP has crossed the intestinal epithelium, ii) the intact ENP remains in the lumen or iii) the ENP undergoes complete or partial dissolution in the lumen and the ions are translocated into the tissue. Included within this is the possibility that particulate forms may associate with luminal material and persist beyond the depuration period as seen in some invertebrate models.115,118 Thus, for the purposes of our discussion on trophic transfer, we suggest the widest definition of uptake and assimilation, which also encompasses the retention without assimilation of ENPs in the gut lumen of the higher trophic level organisms.Like prey, predatory organisms differ in their feeding mode, gut residence time and digestive physiology, all affecting how metals are taken up and assimilated within the organism.142 Gut pH varies among different organisms, with invertebrates having a somewhat neutral pH, most fish having acidic gut conditions (pH<2) and some polychaetes experiencing higher gut pH (>8).38 This leads to an enhanced or decreased metal uptake, as pH is believed to influence ion release.68 Whilst the trophic transfer of metals has been shown to be affected by factors such as assimilation, internal localization, gut physiology and concentration of metals within both prey and predator,7,143,144 much less is known for Me-ENPs. Some studies with Me-ENPs have included secondary consumers, such as zebrafish33,35,145–147 or bivalves,102,148,149 when investigating trophic transfer (Table 1). Based on the published results to date, evidence suggests that biomagnification is not of concern at this level, thereby decreasing the contamination risk for higher, predatory organisms such as carnivorous fish or humans. However, knowledge at these trophic levels is limited, and studies describing factors and processes responsible for trophic transfer of Me-ENP to higher organisms are scarce.
The major predator in the pelagic food web is fish, and studies have looked into how metals and Me-ENPs are being taken up and accumulated in these organisms.17,150,151 Fish can, like daphnids, accumulate metals and Me-ENPs in their gut from the surrounding media, as they drink metal-contaminated water.152,153 Dietary uptake of trace metal ions may result in physiological alterations of the gut,154,155 affect reproductive output156 and possibly cause cell damage.135,136 The mechanism(s) responsible for metal transport in predatory fish have been shown via in vitro and in vivo exposures of the African catfish (Clarias gariepinus), revealing that mucosal cells within the intestinal regions were responsible for the highest Cu accumulation.157,158 Fish were able to eliminate metals by increasing their intestinal mucus production and excrete mucosal cells. Metals can also be translocated from gut cells to organs such as intestine, brain and gills,74,159 thereby increasing the metal concentration within internal organs. With regard to Me-ENPs, the same kind of translocation was observed in a freshwater fish (Cyprinus carpio) exposed to waterborne Ag ENPs. A significant Ag uptake in liver, intestine and gallbladder was due to translocation of Ag ENPs from the gastrointestinal tract.112 Hence, the mechanisms responsible for trace metal accumulation in predatory fish could be applicable for Me-ENPs, but many factors remain unclear.
Besides fish, bivalve mollusks are considered a top predator, primarily in the benthic food web. They are often used as bio-indicators in aquatic ecosystems, and several studies have examined metal accumulation and effects on these organisms,160–162 including subcellular distribution.125–127,163 As suspension feeders, bivalves are at high risk of Me-ENP exposure. Due to their enhanced processes of cellular internalization of natural particles in the micro- and nano-size ranges, their physiological system is susceptible to ENP uptake.164 For example, the bivalves Mytilus edulis and Crassostrea virginica capture and retain natural particles <100 μm in size during certain times of the year, making aggregated ENPs highly available for uptake.165 As reviewed by Canesi et al. (2012),164 bivalve mollusks are valuable model organisms for understanding the risks and effects of ENPs on aquatic invertebrates. In vivo and in vitro studies show that ENPs may target the immune system, and agglomerates and aggregates translocated from gill to the digestive gland lead to intracellular uptake and oxidative stress.166,167 This makes these organisms sensitive to the increasing ENP contamination and, due to their placement in the food web, also an important predatory organism to encounter in trophic transfer studies.
Amongst the four key processes we outline as factors that may affect the potential for Me-ENP trophic transfer, the role played by the digestive physiology of the predator is the least studied. The likelihood of intact ENPs moving to this level of the food chain decreases at each step, due to environmental and in vivo transformations that take place before and after uptake by primary consumers. However, given the effects caused by dietary trace metals, more research needs to be conducted on potential outcomes following Me-ENP passage up the food chain. Future studies should aim at describing the fate of Me-ENPs at this food chain level in more detail, in order to increase the understanding of mechanisms responsible for transport to higher trophic levels.
4. Recommendations for future research & conclusions
Trophic transfer of Me-ENPs has become an increasingly researched area, yet many factors remain unknown. As shown in Fig. 1, numerous processes and mechanisms are likely to influence Me-ENP transfer, and these can be grouped into the four broad categories that we propose, (1) environmental transformations of Me-ENPs, (2) uptake and accumulation in prey organisms, (3) internal fate and localization in the prey, and (4) digestive physiology of the predator. Most research has been conducted within the first step(s) of the food web. Primary producers and consumers have been thoroughly investigated with regard to uptake, bioaccumulation and nano-specific effects. These organisms create the largest pool of knowledge for further ENP studies, but as we continue up the food web, less information is available and we rely more and more on indications and qualified guesses.Currently, little is known about the trophic transfer of ENPs; therefore, we propose that mechanisms, processes and factors controlling trophic transfer of trace metals may provide a good starting point for increasing our understanding with the acknowledgement that nano-scale specificities must also be considered. Examining the species-specific characteristic of lower and higher trophic-level organisms, including uptake routes, accumulation characteristics and subcellular distribution could provide the first steps towards a better description of trophic transfer of Me-ENPs in aquatic food webs. The internal fate and behavior of Me-ENPs, particularly in those organisms that constitute food items, are understudied, yet highly important. Subcellular fractionation can give an indication of where particles reside within organisms following uptake and bioaccumulation; however, the link between subcellular distribution and trophic availability requires verification for Me-ENPs. Moreover, very little research has focused on how the digestive physiology of the predator influences the uptake of Me-ENPs at the higher trophic levels, and related to this, it may become necessary to revise our understanding of what constitutes uptake for particulate contaminants if they remain within the digestive system without necessarily achieving trans-epithelial uptake.
Amongst the relevant accumulation routes, sediment exposures arguably provide the greatest likelihood of intact ENPs being subject to trophic transfer. Although pelagic zooplankton has been shown to take up particles from the water column via filter feeding, the contact with water-borne particles is time-limited since particles are generally assumed to sediment. Thus, both through sediments being an eventual sink for ENPs and the potential persistence of the particle, sediment dwelling-organisms have the greatest exposure duration. Furthermore, it has been demonstrated that benthic organisms may incidentally ingest ENPs during their consumption of sediment, and that, at least in some cases, these particles can be endocytosed within the gut and remain relatively untransformed for a period of time. From this scenario, the potential for Me-ENP food web transfer is maximal but will ultimately depend on the fate of the particle in the prey and the digestive physiology of the predator. These two factors constitute areas where more research focus is required, but sediment exposures could be regularly employed as the most likely exposure route to consistently load prey food items with Me-ENPs.
In this tutorial review, we highlight four broad key factors in describing trophic transfer of Me-ENPs, which all should be studied further to give a better understanding of this phenomenon. Trophic transfer of Me-ENPs occurs under some circumstances, but the underlying processes responsible are poorly understood. Emphasis on digestive physiology of predators is needed, as well as studies including several trophic levels and more complex systems. For both greater scientific understanding and risk assessment needs, the present research into the trophic availability of trace metals is likely to be an important guide. However, nano-specific deviations from this must be recognized and understood.
Acknowledgements
SRT is jointly funded by Roskilde University and DHI (Denmark) through the performance contract of DHI with the Danish Ministry of Higher Education and Science.References
- M. Harada, Crit. Rev. Toxicol., 1995, 25, 1–24 CrossRef CAS PubMed.
- D. F. Woodward, W. G. Brumbagh, A. J. DeLonay, E. E. Little and C. E. Smith, Trans. Am. Fish. Soc., 1994, 123, 51–62 CrossRef.
- D. F. Woodward, A. M. Farag, H. L. Bergman, A. J. DeLonay, E. E. Little, C. E. Smith and F. T. Barrows, Can. J. Fish. Aquat. Sci., 1995, 52, 1994–2004 CrossRef CAS.
- M.-N. Croteau, S. N. Luoma and A. R. Stewart, Limnol. Oceanogr., 2005, 50, 1511–1519 CrossRef CAS.
- L. Zhao, F. Yang and X. Yan, Chem. Ecol., 2013, 29, 197–207 CrossRef CAS.
- P. G. Cardoso, E. Pereira, A. C. Duarte and U. M. Azeiteiro, Mar. Pollut. Bull., 2014, 87, 39–47 CrossRef CAS PubMed.
- P. S. Rainbow, L. Poirier, B. D. Smith, K. V. Brix and S. N. Luoma, Mar. Ecol.: Prog. Ser., 2006, 308, 91–100 CrossRef CAS.
- P. S. Rainbow, L. Poirier, B. D. Smith, K. V. Brix and S. N. Luoma, Mar. Ecol.: Prog. Ser., 2006, 321, 167–181 CrossRef CAS.
- T. Mathews and N. S. Fisher, Mar. Ecol.: Prog. Ser., 2008, 367, 23–33 CrossRef CAS.
- G. V. Lowry, E. M. Hotze, E. S. Bernhardt, D. D. Dionysiou, J. A. Pedersen, M. R. Wiesner and B. Xing, J. Environ. Qual., 2010, 39, 1867–1874 CrossRef CAS PubMed.
- C. E. H. Beaudrie, M. Kandlikar, R. Gregory, G. Long and T. Wilson, Environ. Syst. Decis., 2015, 35, 88–109 CrossRef.
- T. M. Benn and P. Westerhoff, Environ. Sci. Technol., 2008, 42, 4133–4139 CrossRef CAS PubMed.
- J. Farkas, P. Christian, J. A. Gallego-Urrea, N. Roos, M. Hassellöv, K. E. Tollefsen and K. V. Thomas, Aquat. Toxicol., 2011, 101, 117–125 CrossRef CAS PubMed.
- P.-E. Buffet, O. F. Tankoua, J.-F. Pan, D. Berhanu, C. Herrenknecht, L. Poirier, C. Amiard-Triquet, J.-C. Amiard, J.-B. Bérard, C. Risso, M. Guibbolini, M. Roméo, P. Reip, E. Valsami-Jones and C. Mouneyrac, Chemosphere, 2011, 84, 166–174 CrossRef CAS PubMed.
- F. R. Khan, S. K. Misra, J. García-Alonso, B. D. Smith, S. Strekopytov, P. S. Rainbow, S. N. Luoma and E. Valsami-Jones, Environ. Sci. Technol., 2012, 46, 7621–7628 CrossRef CAS PubMed.
- F. R. Khan, A. Laycock, A. Dybowska, F. Larner, B. D. Smith, P. S. Rainbow, S. N. Luoma, M. Rehkämper and E. Valsami-Jones, Environ. Sci. Technol., 2013, 47, 8532–8539 CAS.
- B. J. Shaw, G. Al-Bairuty and R. D. Handy, Aquat. Toxicol., 2012, 116–117, 90–101 CrossRef CAS PubMed.
- C. Pang, H. Selck, S. K. Misra, D. Berhanu, A. Dybowska, E. Valsami-Jones and V. E. Forbes, Aquat. Toxicol., 2012, 106–107, 114–122 CrossRef CAS PubMed.
- J. García-Alonso, F. R. Khan, S. K. Misra, M. Turmaine, B. D. Smith, P. S. Rainbow, S. N. Luoma and E. Valsami-Jones, Environ. Sci. Technol., 2011, 45, 4630–4636 CrossRef PubMed.
- M. Cozzari, A. C. Elia, N. Pacini, B. D. Smith, D. Boyle, P. S. Rainbow and F. R. Khan, Environ. Pollut., 2015, 198, 32–40 CrossRef CAS PubMed.
- T. Ramskov, V. E. Forbes, D. Gilliland and H. Selck, Aquat. Toxicol., 2015, 166, 96–105 CrossRef CAS PubMed.
- A. Thit, G. T. Banta and H. Selck, Environ. Pollut., 2015, 202, 50–57 CrossRef CAS PubMed.
- M.-N. Croteau, S. K. Misra, S. N. Luoma and E. Valsami-Jones, Environ. Sci. Technol., 2011, 45, 6600–6607 CrossRef CAS PubMed.
- F. R. Khan, K. Schmuecking, S. H. Krishnadasan, D. Berhanu, B. D. Smith, J. C. Demello, P. S. Rainbow, S. N. Luoma and E. Valsami-Jones, Environ. Toxicol. Chem., 2013, 32, 2621–2629 CAS.
- J. D. Judy, J. M. Unrine and P. M. Bertsch, Environ. Sci. Technol., 2011, 45, 767–781 CrossRef PubMed.
- J. D. Judy, J. M. Unrine, W. Rao and P. M. Bertsch, Environ. Sci. Technol., 2012, 46, 12672–12678 CrossRef CAS PubMed.
- R. D. Holbrook, K. E. Murphy, J. B. Morrow and K. D. Cole, Nat. Nanotechnol., 2008, 3, 352–355 CrossRef CAS PubMed.
- J. L. Bouldin, T. M. Ingle, A. Sengupta, R. Alexander, R. E. Hannigan and R. A. Buchanan, Environ. Toxicol. Chem., 2008, 27, 1958–1963 CrossRef CAS PubMed.
- J. Chen, H. Li, X. Han and X. Wei, Bull. Environ. Contam. Toxicol., 2015, 95, 145–149 CrossRef CAS PubMed.
- R. E. Mielke, J. H. Priester, R. A. Werlin, J. Gelb, A. M. Horst, E. Orias and P. A. Holden, Appl. Environ. Microbiol., 2013, 79, 5616–5624 CrossRef CAS PubMed.
- T. A. Jarvis, R. J. Miller, H. S. Lenihan and G. K. Bielmyer, Environ. Toxicol. Chem., 2013, 32, 1264–1269 CrossRef CAS PubMed.
- C. Gambardella, L. Gallus, A. M. Gatti, M. Faimali, S. Carbone, L. V. Antisari, C. Falugi and S. Ferrando, Chem. Ecol., 2014, 30, 308–316 CrossRef CAS.
- L. M. Skjolding, M. Winther-Nielsen and A. Baun, Aquat. Toxicol., 2014, 157, 101–108 CrossRef CAS PubMed.
- R. Werlin, J. H. Priester, R. E. Mielke, S. Krämer, S. Jackson, P. K. Stoimenov, G. D. Stucky, G. N. Cherr, E. Orias and P. A. Holden, Nat. Nanotechnol., 2011, 6, 65–71 CrossRef CAS PubMed.
- W.-M. Lee and Y.-J. An, Nanotoxicology, 2015, 9, 407–412 CrossRef CAS PubMed.
- W.-M. Lee, S.-J. Yoon, Y.-J. Shin and Y.-J. An, Environ. Pollut., 2015, 201, 10–16 CrossRef CAS PubMed.
- J. L. Ferry, P. Craig, C. Hexel, P. Sisco, R. Frey, P. L. Pennington, M. H. Fulton, I. G. Scott, A. W. Decho, S. Kashiwada, C. J. Murphy and T. J. Shaw, Nat. Nanotechnol., 2009, 4, 441–444 CrossRef CAS PubMed.
- S. N. Luoma and P. S. Rainbow, Metal contamination in aquatic environments – science and lateral management, Cambridge University Press, 2008 Search PubMed.
- H. Selck, V. E. Forbes and T. L. Forbes, Mar. Ecol.: Prog. Ser., 1998, 164, 167–178 CrossRef CAS.
- H. A. Roditi and N. S. Fisher, Limnol. Oceanogr., 1999, 44, 1730–1749 CrossRef CAS.
- S. E. Hook and N. S. Fisher, Environ. Toxicol. Chem., 2001, 20, 568–574 CrossRef CAS PubMed.
- E. C. Irving, D. J. Baird and J. M. Culp, Environ. Toxicol. Chem., 2003, 22, 1058–1064 CrossRef CAS PubMed.
- B. Nowack and T. D. Bucheli, Environ. Pollut., 2007, 150, 5–22 CrossRef CAS PubMed.
- S. J. Klaine, P. J. J. Alvarez, G. E. Batley, T. F. Fernandes, R. D. Handy, D. Y. Lyon, S. Mahendra, M. J. McLaughlin and J. R. Lead, Environ. Toxicol. Chem., 2008, 27, 1825–1851 CrossRef CAS PubMed.
- G. V. Lowry, K. B. Gregory, S. C. Apte and J. R. Lead, Environ. Sci. Technol., 2012, 46, 6893–6899 CrossRef CAS PubMed.
- A. G. Schultz, D. Boyle, D. Chamot, K. J. Ong, K. J. Wilkinson, J. C. McGeer, G. Sunahara and G. G. Goss, Environ. Chem., 2014, 11, 207–226 CrossRef CAS.
- R. K. Cross, C. Tyler and T. S. Galloway, Environ. Chem., 2015, 12, 627–642 CrossRef CAS.
- P. J. A. Borm, F. C. Klaessig, T. D. Landry, B. Moudgil, J. Pauluhn, K. Thomas, R. Trottier and S. Wood, Toxicol. Sci., 2006, 90, 23–32 CrossRef CAS PubMed.
- N. M. Franklin, N. J. Rogers, S. C. Apte, G. E. Batley, G. E. Gadd and P. S. Casey, Environ. Sci. Technol., 2007, 41, 8484–8490 CrossRef CAS PubMed.
- S. Elzey and V. H. Grassian, J. Nanopart. Res., 2009, 12, 1945–1958 CrossRef.
- M. Lundqvist, J. Stigler, G. Elia, I. Lynch, T. Cedervall and K. A. Dawson, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 14265–14270 CrossRef CAS PubMed.
- I. Lynch, A. Salvati and K. A. Dawson, Nat. Nanotechnol., 2009, 4, 546–547 CrossRef CAS PubMed.
- A. A. Keller, H. Wang, D. Zhou, H. S. Lenihan, G. Cherr, B. J. Cardinale, R. Miller and J. I. Zhaoxia, Environ. Sci. Technol., 2010, 44, 1962–1967 CrossRef CAS PubMed.
- S. L. Chinnapongse, R. I. MacCuspie and V. A. Hackley, Sci. Total Environ., 2011, 409, 2443–2450 CrossRef CAS PubMed.
- A. L. Dale, G. V. Lowry and E. A. Casman, Environ. Sci. Technol., 2013, 47, 12920–12928 CrossRef CAS PubMed.
- T. J. Baker, C. R. Tyler and T. S. Galloway, Environ. Pollut., 2014, 186, 257–271 CrossRef CAS PubMed.
- R. J. Miller, H. S. Lenihan, E. B. Muller, N. Tseng, S. K. Hanna and A. A. Keller, Environ. Sci. Technol., 2010, 44, 7329–7334 CrossRef CAS PubMed.
- N. Adam, C. Schmitt, J. Galceran, E. Companys, A. Vakurov, R. Wallace, D. Knapen and R. Blust, Nanotoxicology, 2014, 8, 709–717 CAS.
- S. W. Y. Wong and K. M. Y. Leung, Nanotoxicology, 2014, 8, 24–35 CrossRef CAS PubMed.
- S. K. Misra, S. Nuseibeh, A. Dybowska, D. Berhanu, T. D. Tetley and E. Valsami-jones, Nanotoxicology, 2014, 8, 422–432 CrossRef CAS PubMed.
- S. K. Misra, A. Dybowska, D. Berhanu, M. N. Croteau, S. N. Luoma, A. R. Boccaccini and E. Valsami-Jones, Environ. Sci. Technol., 2012, 46, 1216–1222 CrossRef CAS PubMed.
- P. Cronholm, H. L. Karlsson, J. Hedberg, T. A. Lowe, L. Winnberg, K. Elihn, I. O. Wallinder and L. Möller, Small, 2013, 9, 970–982 CrossRef CAS PubMed.
- A. P. Gondikas, A. Morris, B. C. Reinsch, S. M. Marinakos, G. V. Lowry and H. Hsu-Kim, Environ. Sci. Technol., 2012, 46, 7037–7045 CrossRef CAS PubMed.
- J. Liu, Z. Wang, F. D. Liu, A. B. Kane and R. H. Hurt, ACS Nano, 2012, 6, 9887–9899 CrossRef CAS PubMed.
- R. Behra, L. Sigg, M. J. D. Clift, F. Herzog, M. Minghetti, B. Johnston, A. Petri-Fink and B. Rothen-Rutishauser, J. R. Soc., Interface, 2013, 10, 1–15 CrossRef PubMed.
- A. R. Harmon, A. J. Kennedy, A. R. Poda, A. J. Bednar, M. A. Chappell and J. A. Steevens, Environ. Toxicol. Chem., 2014, 33, 1783–1791 CrossRef CAS PubMed.
- F. R. Khan, S. K. Misra, N. R. Bury, B. D. Smith, P. S. Rainbow, S. N. Luoma and E. Valsami-Jones, Nanotoxicology, 2015, 9, 493–501 CrossRef CAS PubMed.
- S. K. Misra, A. Dybowska, D. Berhanu, S. N. Luoma and E. Valsami-Jones, Sci. Total Environ., 2012, 438, 225–232 CrossRef CAS PubMed.
- E. Valsami-Jones and I. Lynch, Science, 2015, 350, 388–389 CrossRef CAS PubMed.
- D. A. Wright, Mar. Pollut. Bull., 1995, 31, 8–18 CrossRef CAS.
- P. S. Rainbow, Estuarine, Coastal Shelf Sci., 1997, 44, 169–175 CrossRef CAS.
- R. Guan and W.-X. Wang, Environ. Toxicol. Chem., 2004, 23, 2689–2698 CrossRef CAS PubMed.
- M.-N. Croteau and S. N. Luoma, Environ. Sci. Technol., 2009, 43, 4915–4921 CrossRef CAS PubMed.
- B. J. Shaw and R. D. Handy, Aquat. Toxicol., 2006, 76, 111–121 CrossRef CAS PubMed.
- M.-N. Croteau and S. N. Luoma, Environ. Sci. Technol., 2008, 42, 1801–1806 CrossRef CAS PubMed.
- Z. A. Awrahman, P. S. Rainbow, B. D. Smith, F. R. Khan, N. R. Bury and W. Fialkowski, Aquat. Toxicol., 2015, 161, 196–207 CrossRef CAS PubMed.
- M. C. Casado-Martinez, B. D. Smith, S. N. Luoma and P. S. Rainbow, Aquat. Toxicol., 2010, 98, 34–43 CrossRef CAS PubMed.
- P. S. Rainbow and S. N. Luoma, Aquat. Toxicol., 2011, 105, 455–465 CrossRef CAS PubMed.
- C. Levard, B. C. Reinsch, F. M. Michel, C. Oumahi, G. V. Lowry and G. E. Brown, Environ. Sci. Technol., 2011, 45, 5260–5266 CrossRef CAS PubMed.
- J. Liu, K. G. Pennell and R. H. Hurt, Environ. Sci. Technol., 2011, 45, 7345–7553 CrossRef CAS PubMed.
- B. Kim, C. S. Park, M. Murayama and M. F. Hochella, Environ. Sci. Technol., 2010, 44, 7509–7514 CrossRef CAS PubMed.
- A. Philippe and G. E. Schaumann, Environ. Sci. Technol., 2014, 48, 8946–8962 CrossRef CAS PubMed.
- I. Lynch, T. Cedervall, M. Lundqvist, C. Cabaleiro-Lago, S. Linse and K. A. Dawson, Adv. Colloid Interface Sci., 2007, 134–135, 167–174 CrossRef CAS PubMed.
- I. Lynch and K. A. Dawson, Nano Today, 2008, 3, 40–47 CrossRef CAS.
- T. Cedervall, I. Lynch, M. Foy, T. Berggård, S. C. Donnelly, G. Cagney, S. Linse and K. A. Dawson, Angew. Chem., Int. Ed., 2007, 46, 5754–5756 CrossRef CAS PubMed.
- H. R. Kim, K. Andrieux, C. Delomenie, H. Chacun, M. Appel, D. Desmaële, F. Taran, D. Georgin, P. Couvreur and M. Taverna, Electrophoresis, 2007, 28, 2252–2261 CrossRef CAS PubMed.
- V. K. Sharma, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2009, 44, 1485–1495 CrossRef CAS PubMed.
- R. A. French, A. R. Jacobson, B. Kim, S. L. Isley, L. Penn and P. C. Baveye, Environ. Sci. Technol., 2009, 43, 1354–1359 CrossRef CAS PubMed.
- T. J. MacCormack and G. G. Goss, J. Ind. Ecol., 2008, 12, 286–296 CrossRef CAS.
- I. Velzeboer, J. T. K. Quik, D. van de Meent and A. A. Koelmans, Environ. Toxicol. Chem., 2014, 33, 1766–1773 CrossRef CAS PubMed.
- K. L. Garner and A. A. Keller, J. Nanopart. Res., 2014, 16, 1–28 CrossRef.
- A. Praetorius, J. Labille, M. Scheringer, A. Thill, K. Hungerbühler and J.-Y. Bottero, Environ. Sci. Technol., 2014, 48, 10690–10698 CrossRef CAS PubMed.
- A. L. Dale, G. V. Lowry and E. A. Casman, Environ. Sci. Technol., 2015, 49, 7285–7293 CrossRef CAS PubMed.
- M. Tella, M. Auffan, L. Brousset, J. Issartel, I. Kieffer, C. Pailles, E. Morel, C. Santaella, A. Bernard, E. Artells, J. Rose, A. Thiéry and J.-Y. Bottero, Environ. Sci. Technol., 2014, 48, 9004–9013 CrossRef PubMed.
- G. Graf and R. Rosenberg, J. Mar. Syst., 1997, 11, 269–278 CrossRef.
- J. Bertinato, L. Cheung, R. Hoque and L. J. Plouffe, J. Trace Elem. Med. Biol., 2010, 24, 178–184 CAS.
- N. R. Bury and C. M. Wood, Am. J. Physiol., 1999, 277, R1385–R1391 CAS.
- M. N. Moore, Environ. Int., 2006, 32, 967–976 CrossRef CAS PubMed.
- G. Sahay, D. Y. Alakhova and A. V. Kabanov, J. Controlled Release, 2010, 145, 182–195 CrossRef CAS PubMed.
- F. R. Khan, S. K. Misra, N. R. Bury, B. D. Smith, P. S. Rainbow, S. N. Luoma and E. Valsami-Jones, Nanotoxicology, 2015, 9, 493–501 CrossRef CAS PubMed.
- E. Navarro, A. Baun, R. Behra, N. B. Hartmann, J. Filser, A. J. Miao, A. Quigg, P. H. Santschi and L. Sigg, Ecotoxicology, 2008, 17, 372–386 CrossRef CAS PubMed.
- M. Larguinho, D. Correia, M. S. Diniz and P. V. Baptista, J. Nanopart. Res., 2014, 16, 1–11 CrossRef CAS.
- W.-C. Hou, B. Y. Moghadam, C. Corredor, P. Westerhoff and J. D. Posner, Environ. Sci. Technol., 2012, 46, 1869–1876 CrossRef CAS PubMed.
- G. L. Taghon, R. F. L. Self and P. A. Jumars, Limnol. Oceanogr., 1978, 23, 752–759 CrossRef.
- G. R. Lopez and J. S. Levinton, Q. Rev. Biol., 1987, 62, 235–260 CrossRef.
- L. Kofoed, V. Forbes and G. Lopez, in Ecology of Marine Deposit Feeders, 1989, pp. 129–148 Search PubMed.
- P. G. C. Campbell and A. Tessier, in Ecotoxicology: A Hierarchical Treatment, 1996, pp. 11–51 Search PubMed.
- H. Selck, A. W. Decho and V. E. Forbes, Environ. Toxicol. Chem., 1999, 18, 1289–1297 CrossRef CAS.
- P. S. Rainbow and W.-X. Wang, Mar. Ecol.: Prog. Ser., 2001, 218, 239–248 CrossRef CAS.
- M.-N. Croteau, S. K. Misra, S. N. Luoma and E. Valsami-Jones, Environ. Sci. Technol., 2014, 48, 10929–10937 CrossRef CAS PubMed.
- A. L. S. Oliver, M.-N. Croteau, T. L. Stoiber, M. Tejamaya, I. Römer, J. R. Lead and S. N. Luoma, Environ. Pollut., 2014, 189, 87–91 CrossRef CAS PubMed.
- B. K. Gaiser, T. F. Fernandes, M. A. Jepson, J. R. Lead, C. R. Tyler, M. Baalousha, A. Biswas, G. J. Britton, P. A. Cole, B. D. Johnston, Y. Ju-Nam, P. Rosenkranz, T. M. Scown and V. Stone, Environ. Toxicol. Chem., 2012, 31, 144–154 CrossRef CAS PubMed.
- N. B. Hartmann, S. Legros, F. Von der Kammer, T. Hofmann and A. Baun, Aquat. Toxicol., 2012, 118–119, 1–8 CrossRef CAS PubMed.
- J. Hu, D. Wang, J. Wang and J. Wang, Environ. Pollut., 2012, 162, 216–222 CrossRef CAS PubMed.
- F. R. Khan, G. M. Kennaway, M.-N. Croteau, A. Dybowska, B. D. Smith, A. J. A. Nogueira, P. S. Rainbow, S. N. Luoma and E. Valsami-Jones, Chemosphere, 2014, 100, 97–104 CrossRef CAS PubMed.
- M. Gophen and W. Geller, Oecologia, 1984, 64, 408–412 CrossRef.
- L. M. Skjolding, K. Kern, R. Hjorth, N. Hartmann, S. Overgaard, G. Ma, J. G. C. Veinot and A. Baun, Ecotoxicology, 2014, 23, 1172–1183 CrossRef CAS PubMed.
- E. J. Petersen, J. Akkanen, J. V. K. Kukkonen and W. J. Weber Jr, Environ. Sci. Technol., 2009, 43, 2969–2975 CrossRef CAS PubMed.
- P. S. Rainbow, B. D. Smith and S. N. Luoma, Mar. Ecol.: Prog. Ser., 2009, 390, 145–155 CrossRef CAS.
- W. X. Wang, I. Stupakoff and N. S. Fisher, Mar. Ecol.: Prog. Ser., 1999, 178, 281–293 CrossRef CAS.
- L. V. Stebounova, E. Guio and V. H. Grassian, J. Nanopart. Res., 2011, 13, 233–244 CrossRef CAS.
- E. Navarro, F. Piccapietra, B. Wagner, F. Marconi, R. Kaegi, N. Odzak, L. Sigg and R. Behra, Environ. Sci. Technol., 2008, 42, 8959–8964 CrossRef CAS PubMed.
- H. Y. Nam, S. M. Kwon, H. Chung, S.-Y. Lee, S.-H. Kwon, H. Jeon, Y. Kim, J. H. Park, J. Kim, S. Her, Y.-K. Oh, I. C. Kwon, K. Kim and S. Y. Jeong, J. Controlled Release, 2009, 135, 259–267 CrossRef CAS PubMed.
- L. K. Limbach, P. Wick, P. Manser, R. N. Grass, A. Bruinink and W. J. Stark, Environ. Sci. Technol., 2007, 41, 4158–4163 CrossRef CAS PubMed.
- W. G. Wallace, B.-G. Lee and S. N. Luoma, Mar. Ecol.: Prog. Ser., 2003, 249, 183–197 CrossRef CAS.
- W. G. Wallace and S. N. Luoma, Mar. Ecol.: Prog. Ser., 2003, 257, 125–137 CrossRef CAS.
- E. Bonneris, O. Perceval, S. Masson, L. Hare and P. G. C. Campbell, Environ. Pollut., 2005, 135, 195–208 CrossRef CAS PubMed.
- P. S. Rainbow, S. N. Luoma and W.-X. Wang, Environ. Pollut., 2011, 159, 2347–2349 CrossRef CAS PubMed.
- W. G. Wallace and G. R. Lopez, Estuaries, 1996, 19, 923–930 CrossRef CAS.
- S. Il Chang and J. R. Reinfelder, Environ. Sci. Technol., 2000, 34, 4931–4935 CrossRef.
- I. H. Ni, W. X. Wang and Y. K. Tam, Mar. Ecol.: Prog. Ser., 2000, 194, 203–210 CrossRef CAS.
- G. M. Stewart and N. S. Fisher, Limnol. Oceanogr., 2003, 48, 2011–2019 CrossRef CAS.
- H. Selck and V. E. Forbes, Mar. Environ. Res., 2004, 57, 261–279 CrossRef CAS PubMed.
- L. Zhang and W.-X. Wang, Limnol. Oceanogr., 2006, 51, 2008–2017 CrossRef CAS.
- F. R. Khan, N. R. Bury and C. Hogstrand, Aquat. Toxicol., 2010, 99, 466–472 CrossRef CAS PubMed.
- F. R. Khan, N. R. Bury and C. Hogstrand, Aquat. Toxicol., 2010, 96, 124–129 CrossRef CAS PubMed.
- M. Cheung and W. X. Wang, Mar. Ecol.: Prog. Ser., 2005, 286, 155–166 CrossRef CAS.
- V. Bragigand and B. Berthet, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 2003, 134, 55–61 CrossRef.
- G. Pletikapić, V. Žutić, I. V. Vrček and V. Svetličić, J. Mol. Recognit., 2012, 25, 309–317 CrossRef PubMed.
- Y. F. Li, J. Zhao, Y. Qu, Y. Gao, Z. Guo, Z. Liu, Y. Zhao and C. Chen, Nanomedicine, 2015, 11, 1531–1549 CAS.
- S. W. Kim, J. Il Kwak and Y. J. An, Chemosphere, 2016, 144, 1763–1770 CrossRef CAS PubMed.
- S. E. Shumway, T. L. Cucci, R. C. Newell and C. M. Yentsch, J. Exp. Mar. Biol. Ecol., 1985, 91, 77–92 CrossRef.
- D. Goto and W. G. Wallace, Can. J. Fish. Aquat. Sci., 2009, 66, 836–846 CrossRef CAS.
- F. Guo, J. Yao and W.-X. Wang, Environ. Toxicol. Chem., 2013, 32, 2109–2116 CrossRef CAS PubMed.
- X. Zhu, J. Wang, X. Zhang, Y. Chang and Y. Chen, Chemosphere, 2010, 79, 928–933 CrossRef CAS PubMed.
- N. A. Lewinski, H. Zhu, C. R. Ouyang, G. P. Conner, D. S. Wagner, V. L. Colvin and R. A. Drezek, Nanoscale, 2011, 3, 3080–3083 RSC.
- M. Ates, Z. Arslan, V. Demir, J. Daniels and I. O. Farah, Environ. Toxicol., 2015, 30, 119–128 CrossRef CAS PubMed.
- S. Renault, M. Baudrimont, P. Mesmer-Dudons, P. Gonzalez, S. Mornet and A. Brisson, Gold Bull., 2008, 41, 116–126 CrossRef CAS.
- J. R. Conway, S. K. Hanna, H. S. Lenihan and A. A. Keller, Environ. Sci. Technol., 2014, 48, 1517–1524 CrossRef CAS PubMed.
- G. Federici, B. J. Shaw and R. D. Handy, Aquat. Toxicol., 2007, 84, 415–430 CrossRef CAS PubMed.
- T. M. Blickley, C. W. Matson, W. N. Vreeland, D. Rittschof, R. T. Di Giulio and P. D. McClellan-Green, Aquat. Toxicol., 2014, 148, 27–39 CrossRef CAS PubMed.
- N. R. Bury, P. A. Walker and C. N. Glover, J. Exp. Biol., 2003, 206, 11–23 CrossRef CAS PubMed.
- R. D. Handy, T. B. Henry, T. M. Scown, B. D. Johnston and C. R. Tyler, Ecotoxicology, 2008, 17, 396–409 CrossRef CAS PubMed.
- C. N. Glover and C. Hogstrand, J. Exp. Biol., 2002, 205, 141–150 CAS.
- F. R. Khan and J. C. McGeer, Aquat. Toxicol., 2013, 142–143, 17–25 CrossRef CAS PubMed.
- D. Boyle, K. V. Brix, H. Amlund, A.-K. Lundebye, C. Hogstrand and N. R. Bury, Environ. Sci. Technol., 2008, 42, 5354–5360 CrossRef CAS PubMed.
- R. D. Handy, M. M. Musonda, C. Phillips and S. J. Falla, J. Exp. Biol., 2000, 203, 2365–2377 CAS.
- I. Hoyle, B. J. Shaw and R. D. Handy, Aquat. Toxicol., 2007, 83, 62–72 CrossRef CAS PubMed.
- C. S. Ramsden, T. J. Smith, B. J. Shaw and R. D. Handy, Ecotoxicology, 2009, 18, 939–951 CrossRef CAS PubMed.
- N. S. Fisher, J.-L. Teyssié, S. W. Fowler and W.-X. Wang, Environ. Sci. Technol., 1996, 30, 3232–3242 CrossRef CAS.
- M.-N. Croteau, S. N. Luoma, B. R. Topping and C. B. Lopez, Environ. Sci. Technol., 2004, 38, 5002–5009 CrossRef CAS PubMed.
- W.-X. Wang, Environ. Toxicol. Chem., 2001, 20, 1367–1373 CrossRef CAS PubMed.
- E. Bonneris, A. Giguère, O. Perceval, T. Buronfosse, S. Masson, L. Hare and P. G. C. Campbell, Aquat. Toxicol., 2005, 71, 319–334 CrossRef CAS PubMed.
- L. Canesi, C. Ciacci, R. Fabbri, A. Marcomini, G. Pojana and G. Gallo, Mar. Environ. Res., 2012, 76, 16–21 CrossRef CAS PubMed.
- J. E. Ward and D. J. Kach, Mar. Environ. Res., 2009, 68, 137–142 CrossRef CAS PubMed.
- L. Canesi, C. Ciacci, D. Vallotto, G. Gallo, A. Marcomini and G. Pojana, Aquat. Toxicol., 2010, 96, 151–158 CrossRef CAS PubMed.
- C. Barmo, C. Ciacci, B. Canonico, R. Fabbri, K. Cortese, T. Balbi, A. Marcomini, G. Pojana, G. Gallo and L. Canesi, Aquat. Toxicol., 2013, 132–133, 9–18 CrossRef CAS PubMed.
- S. Pakrashi, S. Dalai, N. Chandrasekaran and A. Mukherjee, Aquat. Toxicol., 2014, 152, 74–81 CrossRef CAS PubMed.
- J. Kalman, K. B. Paul, F. R. Khan, V. Stone and T. F. Fernandes, Environ. Chem., 2015, 12, 662–672 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |