Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Toxicity of dimercaptosuccinate-coated and un-functionalized magnetic iron oxide nanoparticles towards aquatic organisms†
Ya-Qi
Zhang
a,
Ralf
Dringen
bc,
Charlotte
Petters
bc,
Wiebke
Rastedt
bc,
Jan
Köser
a,
Juliane
Filser
d and
Stefan
Stolte
*ae
aUFT - Centre for Environmental Research and Sustainable Technology, Department Sustainable Chemistry, University of Bremen, Leobener Straße, D-28359, Bremen, Germany. E-mail: stefan.stolte@uni-bremen.de
bCBIB - Centre for Biomolecular Interactions Bremen, Neurobiochemistry, Faculty 2 (Biology/Chemistry), University of Bremen, Leobener Straße/NW2, D-28359, Bremen, Germany
cUFT - Centre for Environmental Research and Sustainable Technology, Department Neurobiochemistry, University of Bremen, Leobener Straße, D-28359, Bremen, Germany
dUFT - Centre for Environmental Research and Sustainable Technology, Department General and Theoretical Ecology, Faculty 2 (Biology/Chemistry), University of Bremen, Leobener Straße, D-28359, Bremen, Germany
eDepartment of Environmental Analysis, Faculty of Chemistry, University of Gdańsk, ul. Wita Stwosza 63, 80-308 Gdańsk, Poland
First published on 16th May 2016
Abstract
Magnetic iron oxide nanoparticles (IONP) have gained growing attention in recent years for their promising applications in medical treatment and environmental remediation. Among the IONP, dimercaptosuccinic acid coated-IONP (DMSA-IONP) have great potential because of their rapid uptake into cells and their potential to effectively adsorb heavy metals. The widespread use and potential release of IONP into the environment raises concern about their environmental impact. To date, little is known about the consequences of the exposure of aquatic organisms to such particles. In this context we investigated the colloidal stability of DMSA-IONP in different test media as well as their effects on green algae (Raphidocelis subcapitata), duckweed (Lemna minor) and water fleas (Daphnia magna). Moreover, a comparative analysis of stability and ecotoxicity data of DMSA-IONP with freshly prepared and aged uncoated IONP dispersions was performed, considering the importance of stability of particles in determining their toxicity. The green algae were the most sensitive organism with EC50 values (72 h) ranging between 0.86–2.27 μM Fe (i.e. 0.05 to 0.13 mg Fe L−1) for the three types of IONP. The observed flocculation and (co-)sedimentation of algae with IONP are assumed to reduce the access of cells to nutrients and light. Lemna was not affected by any of the IONP due to the low availability of IONP induced by fast aggregation of IONP in the medium. Minor toxic effects on Daphnia were found for uncoated IONP (EC50 between 374–1181 μM Fe, i.e. 21–66 mg Fe L−1) after 72 h. However, the ingestion and accumulation of coated and uncoated IONP in the gastrointestinal tract of daphnids was observed. Our evaluation of IONP has revealed a certain hazard potential to aquatic organisms. In this light it appears important to prevent the release of large amounts of IONP into the environment, which might limit their applicability.
Nano impactIn recent years iron nanoparticle-based technologies have attracted great interest in the field of wastewater treatment and groundwater remediation. From this application it is very likely that such particles are continuously released into aquatic ecosystems. However, little is known on aquatic toxicity of uncoated and DMSA coated IONP. This study investigates the colloidal stability and ecotoxicity of DMSA-IONP, fresh and aged uncoated IONP to three typical aquatic organisms: Raphidocelis, Lemna and Daphnia. In comparison to ferric and ferrous salts all three types of IONP cause much stronger, short-term toxicological effects to green algae R. subcapitata. The EC50 values for the three types of IONP ranged between 0.86–2.27 μM Fe (i.e. 0.05 to 0.13 mg Fe L−1) whereas the salts at concentrations higher than 1.0 mg Fe L−1 still significantly stimulated algal growth. Thus, IONP do pose a certain risk that needs to be considered when implementing IONP-based wastewater and remediation technologies. |
1. Introduction
In recent decades magnetic iron oxide nanoparticles (IONP), having an iron oxide core of either magnetite (Fe3O4) or maghemite (Fe2O3), have been intensively developed for a wide range of applications in biomedical and environmental technology, due to not only the high surface area, but also their unique magnetic properties and high catalytic abilities.1 These applications include biomedical imaging, drug-loaded targeted delivery, and cell tracking in tumor therapy,2 as well as soil and groundwater remediation3 and wastewater treatment.4 IONP have been reported as a highly efficient, cost-effective and convenient3 adsorbent in the removal of toxic metals from aqueous solutions by adsorption. Nassar found that the adsorption capacity of Fe3O4 NP for Pb(II) ions was 36.0 mg g−1, which is much higher than that reported for other adsorbents.5 Adsorption of Cr(VI) by γ-Fe2O3 was also successful, so that the effect of adsorbate concentration and competing ions in solutions could be ignored.6 Metals can then be readily separated and removed by applying external magnetic fields.7 However, uncoated IONP are susceptible to air oxidation8 and tend to aggregate in solution4 in inhospitable conditions. The reactivity, mobility4 and magnetism1 can subsequently disappear. As a consequence, IONP are often coated with organic or inorganic compounds, such as polymers,2,9 gold,10 dextran11 or humic acid,12 not only to improve their colloidal stability and eventually protect IONP from oxidation in aqueous media, but also to equip IONP with unique and specific surface functionalities for cell labeling and targeting, as well as for ion binding to enhance the capacity for heavy metal adsorption in water treatment procedures.4Among the functionalized IONP, those coated with meso-2,3-dimercaptosuccinic acid (DMSA, Fig. 1A) present great potential in both target-drug delivery13 and remediation of heavy metal pollution.14 DMSA, a dithiol, is a derivative and an analogue of dimercaprol that contains two sulfhydryl groups (–SH).15,16 Oxidation of DMSA molecules around IONP generates a cage of disulfide-cross-linked DMSA molecules around the IONP core, which establishes a negative surface charge of the particles due to an excess of carboxylate groups17 (Fig. 1B).
 | ||
| Fig. 1 A. Structural formula of meso-2,3-dimercaptosuccinic acid (DMSA); B. DMSA-IONP: DMSA molecules bind to IONP surface via their carboxylate groups and form a cage by disulfide bridges.17 The thiol groups on the surface can effectively capture heavy metal ions to form strong metal–sulfur complexes. | ||
The ability of sulfhydryl-containing compounds to chelate metals, e.g. lead, mercury, arsenic, and cadmium, has been well studied.15,16,18 DMSA is not toxic to humans and has been approved for clinical use for chelation therapy.19 Accordingly, DMSA can be used as a treatment for arsenic18,20 and lead intoxications18,21in vivo and heavy metal removal in the environment14 due to its effectiveness in metal chelation.15,18 Moreover, it shows no toxicity in the treatment of heavy metal intoxications22 such as arsenic in mice and rabbits,23,24 and can be orally administered without causing redistribution of metals from one organ to another.23–25
In addition, DMSA-IONP have a high capacity and selectivity for heavy metals.14 The thiol group on the surface of IONP mainly reacts with heavy metal ions directly to form stable metal–sulfur complexes through chelation.26 In terms of the adsorption coefficient (Kd) values, DMSA-coated Fe3O4 IONP are 3–40 times superior to commercial sorbents such as Duolite GT-73 resins and activated carbon Darco KB-B.14 Moreover, the addition of affinity ligands to the surface of the nanoparticles increases the affinity of the sorbents27 to a wide range of heavy metals with specificity. Compared to uncoated Fe3O4 NP, enhancement of heavy metal ions (e.g. Hg2+, Ag+, Pb2+, and Cu2+) adsorption for DMSA-coated Fe3O4 NP was also observed.14 These ions effectively bind to the DMSA ligands or the iron oxide lattices, and could be separated from solution within a minute with a 1.2 T magnet.14 Singh et al. also found a strong affinity of DMSA-coated Fe3O4 NP to Cr3+, Co2+, Ni2+, Cu2+, Cd2+, Pb2+ and As3+ present in wastewater.26
Although a number of laboratory studies and field trials have indicated IONP-based technology as a promising tool for heavy metal removal in wastewater treatment due to the advantages, such as low cost, strong adsorption capacity, easy separation and enhanced stability,4 it is still at a relatively early stage for wide application.4,7 Many important issues concerning the impact of IONP application on human health and environment remain poorly understood, which may significantly limit the widespread application of IONP as remediation tools.3,7 So far, most studies have focused on the effect of IONP on mammal cells. Related studies have estimated the genotoxicity and cytotoxicity of DMSA-IONP (γ-Fe2O3), such as to cultured brain astrocytes28 and human dermal fibroblasts,29 but weak or no effects were observed in the highest tested concentrations of 4000 and 1791 μM Fe (i.e. 0.22 and 0.10 g Fe L−1), respectively. While uncoated Fe2O3 IONP of 21 nm induced oxidative stress and morphological damage in brain nerve cells of mice when 130 μg of IONP was instilled into mice.30 On the other hand, very few studies have investigated the effects of IONP, particularly in aquatic environments.31 Some data on the effects of IONP on aquatic organisms such as zebrafish (Danio rerio,31 α-Fe2O3) and water flea (Daphnia magna,9,32 Fe3O4) are available but still too few to make comprehensive conclusions, and nothing is known on the effects of DMSA-IONP on aquatic organisms.
In view of the wide range of potential applications of uncoated IONP and particularly of DMSA-IONP it appears very likely that such particles are continuously released into aquatic ecosystems. For example, this could happen through industrial/domestic effluents or discharge of wastewater treatment plants. Therefore, uncertainties about these particles in the aquatic environment need to be investigated and addressed before their widespread application.
In order to anticipate and assess the hazards that DMSA-coated and uncoated IONP might pose in the aquatic environment (Fig. 2), we synthesized magnetic IONP (γ-Fe2O3) and (i) evaluated the stability of DMSA-IONP and compared it to fresh and aged uncoated IONP in different aquatic media; (ii) estimated and compared the potential acute/subchronic toxicity of the IONP with three typical model organisms from freshwater: chlorophyte algae (Raphidocelis subcapitata), duckweed (Lemna minor), and water flea (Daphnia magna); (iii) aimed to discriminate between the potential effect of IONP-derived iron ions and IONP in particulate form, considering the effects that released iron ions cause.
 | ||
| Fig. 2 Outline of the study flow. With MQ = MilliQ water, AM = algae medium, LM = Lemna medium, and DM = Daphnia medium. | ||
2. Materials and methods
2.1 Materials
All chemicals were purchased in the highest purity available from Sigma-Aldrich (Steinheim, Germany), Fluka (Buchs, Switzerland), or Merck (Darmstadt, Germany). Nunc EASY flasks with 25 and 75 cm2 were obtained from VWR (Hannover, Germany). The 6-well plates were standard polystyrene cell culture plates with lids from Greiner Bio-one, Frickenhausen, Germany, and the 24-well tissue culture plates with lids were from Sarstedt, Nümbrecht, Germany. The test kit for immobility/mortality tests of Daphnia magna was Daphtoxkit F magna, MicroBioTests. Glass containers of 20 mL for colloidal stability testing were neo-Lab EPA thread vials (clear glass ND24, Heidelberg, Germany). Syringe filters (Multoclear, 0.45 μm, RC, 13 mm) for medium filtration were purchased from CS Chromatographie Service (Langerwehe, Germany). MilliQ water (18.2 MΩ cm−1 in resistivity) was produced from Millipore MilliQ Plus water purification system (Massachusetts, USA). All deionized water used in this study, if not specifically stated, was previously filtered by Carbonit NFP Premium-9 water filter (Heidenheim, Germany) with pore size 0.45 μm.2.2 Organisms and cultures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark cycle of 14
dark cycle of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 h. The cultures were diluted and checked for cell counts every 3–4 days in order to keep them in exponential growth. Certain amounts of the synchronized cultures were finally diluted in 50 mL medium to reach a count of 2.5 × 104 cells per mL 4 days before the tests. The pH of the medium was adjusted to 8.1 ± 0.2. The flasks were placed on a shaker (IKA®MTS 2/4 digital) at 150 rpm in the chamber. Cultures were illuminated (Sylvania Luxline plus F 15w/865 Daylight de Luxe) at approximate 6600 lux (measured by Panlux Gossen 8 A94256).
10 h. The cultures were diluted and checked for cell counts every 3–4 days in order to keep them in exponential growth. Certain amounts of the synchronized cultures were finally diluted in 50 mL medium to reach a count of 2.5 × 104 cells per mL 4 days before the tests. The pH of the medium was adjusted to 8.1 ± 0.2. The flasks were placed on a shaker (IKA®MTS 2/4 digital) at 150 rpm in the chamber. Cultures were illuminated (Sylvania Luxline plus F 15w/865 Daylight de Luxe) at approximate 6600 lux (measured by Panlux Gossen 8 A94256).
2.3 Synthetic procedures of IONP
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 1.4 M HCl and 4.5% w/v KMnO4 in double-distilled water), and then 30 μL fresh iron-detection reagent (2.5 M ammonium acetate, 1 M ascorbate, 6.5 mM ferrozine and 6.5 mM neocuproine) was added.38 After 30 min reaction at room temperature, 280 μL of the sample was filled in wells of a microtiter plate (Sarstedt, Nümbrecht, Germany), and the absorbance at 540 nm of the iron–ferrozine complex was recorded by a Sunrise RC microtiter plate photometer (Tecan, Crailsheim, Germany). Iron content was finally determined by comparing the absorbance of the samples to defined iron standard solutions (FeCl3 in 10 mM HCl).
1 mixture of 1.4 M HCl and 4.5% w/v KMnO4 in double-distilled water), and then 30 μL fresh iron-detection reagent (2.5 M ammonium acetate, 1 M ascorbate, 6.5 mM ferrozine and 6.5 mM neocuproine) was added.38 After 30 min reaction at room temperature, 280 μL of the sample was filled in wells of a microtiter plate (Sarstedt, Nümbrecht, Germany), and the absorbance at 540 nm of the iron–ferrozine complex was recorded by a Sunrise RC microtiter plate photometer (Tecan, Crailsheim, Germany). Iron content was finally determined by comparing the absorbance of the samples to defined iron standard solutions (FeCl3 in 10 mM HCl).
The aged uncoated IONP used in this study were synthesized by the same method but were produced six months before ecotoxicity testing and stored at 4 °C.
2.4 Particle characterization and colloidal stability tests
2.5 Toxicity tests
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark cycle of 14
dark cycle of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 h was used instead of a continuous light. The test set-up was validated according to the guideline using the reference chemical 3,5-dichlorophenol. Algae were exposed to DMSA-IONP, fresh and aged uncoated IONP, iron salts ferric ammonium citrate (FAC) or ferrous ammonium sulfate (FAS) in a concentration range of 0.18–1791 μM Fe (0.01–100 mg Fe L−1), as well as to pure DMSA between 0.00326 to 32.6 mg L−1 as a reference. Dispersions were prepared in OECD TG 201 (medium containing 0.24 μM Fe) or iron-free medium (OECD TG 201 but prepared without iron and EDTA). The iron-free medium was prepared in MilliQ water, and the algae were washed with this medium 3 times (3000 rpm, i.e. 1800 g, 10 min; Heraeus™ Labofuge™ 400R centrifuge) before exposure. All glass containers for this treatment were previously rinsed with 10% HNO3 3 times. The vessels for ecotoxcity tests were cell culture EASY flasks of 25 cm2, and working volume for the tests was chosen as 20 mL. Algae cell counts at the start of the incubation were 2.5 × 104 cells per mL. Samples were set on shakers at 150 rpm in the same climate chamber with the same conditions as the culturing for 72 h. Cell counts after 72 h in IONP tests were recorded by a cell counting chamber (Neubauer-improved, depth 0.100 mm, 0.0025 mm2) and light microscope (Zeiss, Germany); while in tests for other non-IONP substances the cell counts were recorded by a cell counter and analyzer system (CASY® Model TTC). Cell morphology was also visually analyzed under a light microscope (Olympus BX60 with Sony DXC-9100P 3CCD color video camera). Results on unimpaired growth are expressed as percentage of cell number counts compared to the controls (absence of test substances). Substances were tested in 3 replicates with 6 controls (pure OECD TG 201 medium or iron-free medium) for each test and each test was performed 2 or 4 times to ensure reproducibility of the data.
10 h was used instead of a continuous light. The test set-up was validated according to the guideline using the reference chemical 3,5-dichlorophenol. Algae were exposed to DMSA-IONP, fresh and aged uncoated IONP, iron salts ferric ammonium citrate (FAC) or ferrous ammonium sulfate (FAS) in a concentration range of 0.18–1791 μM Fe (0.01–100 mg Fe L−1), as well as to pure DMSA between 0.00326 to 32.6 mg L−1 as a reference. Dispersions were prepared in OECD TG 201 (medium containing 0.24 μM Fe) or iron-free medium (OECD TG 201 but prepared without iron and EDTA). The iron-free medium was prepared in MilliQ water, and the algae were washed with this medium 3 times (3000 rpm, i.e. 1800 g, 10 min; Heraeus™ Labofuge™ 400R centrifuge) before exposure. All glass containers for this treatment were previously rinsed with 10% HNO3 3 times. The vessels for ecotoxcity tests were cell culture EASY flasks of 25 cm2, and working volume for the tests was chosen as 20 mL. Algae cell counts at the start of the incubation were 2.5 × 104 cells per mL. Samples were set on shakers at 150 rpm in the same climate chamber with the same conditions as the culturing for 72 h. Cell counts after 72 h in IONP tests were recorded by a cell counting chamber (Neubauer-improved, depth 0.100 mm, 0.0025 mm2) and light microscope (Zeiss, Germany); while in tests for other non-IONP substances the cell counts were recorded by a cell counter and analyzer system (CASY® Model TTC). Cell morphology was also visually analyzed under a light microscope (Olympus BX60 with Sony DXC-9100P 3CCD color video camera). Results on unimpaired growth are expressed as percentage of cell number counts compared to the controls (absence of test substances). Substances were tested in 3 replicates with 6 controls (pure OECD TG 201 medium or iron-free medium) for each test and each test was performed 2 or 4 times to ensure reproducibility of the data.
2.6 Calculation of iron species
The equilibrium iron species present in algae medium (OECD TG 201) were calculated using the software PHREEQCi (v.3)41 using the database file minteq.v4. The addition of 10 μM Fe as ferric ammonium citrate ((NH4)5Fe(C6H4O7)2·2H2O, FAC, Fe3+) and as ferrous ammonium sulfate ((NH4)2Fe(SO4)2·6H2O, FAS, Fe2+) to algae standard medium and iron-free algae medium (OECD TG 201 medium prepared without iron and EDTA) were considered in the speciation calculations. Minor modification of the database file for the calculations was done to uncouple ammonium from redox reactions with nitrite and nitrate by the introduction of the variable Amm as replacement for NH3 in the database. The components of the medium according to the OECD TG 201 and the pH of 8.1 served as input parameters for the calculations. The chloride content served as charge balance in the calculations. The atmospheric partial pressures of CO2 (with saturation index SI −3.41) and O2 (SI −0.678) were used as equilibrium phases in the calculations. As equilibrium phase for iron precipitation ferrihydrite (Fe(OH)3) was chosen, because the SI of this iron phase was the closest to zero in the media and therefore the most realistic precipitation process in the short timeframe of the experiments.42Four scenarios were considered to discuss the iron speciation in the media: a) absence of O2 and no precipitation allowed; b) presence of O2 (8.3 mg L−1, 0.26 mM) and no precipitation allowed; c) absence of O2 and precipitation allowed for ferrihydrite; d) presence of O2 (8.3 mg L−1, 0.26 mM) and precipitation allowed for ferrihydrite.
2.7 Statistical analysis
If not stated otherwise, the data are presented as means ± SD of values from at least two independent experiments with at least three replicates in each. Significance of difference between two groups of data was analyzed by the paired t-test using the software R (version 3.1.1) (https://www.r-project.org/). p > 0.05 was considered as not significant. Dose–response curve and EC50 value estimation for each substance was performed by linlogit or probit model of the ‘drfit’ package in R.3. Results and discussion
3.1 Characterization of IONP
The DLS measurements revealed a hydrodynamic diameter (d) of 50.4 nm for DMSA-IONP in the diluted dispersions (Table 1). A hydrodynamic diameter of 33.3 nm was determined for fresh uncoated IONP, whereas a higher value of 90.2 nm for aged uncoated IONP was observed (Table 1), indicating a small degree of aggregation. Positive zeta-potential values (z, 41.0 and 36.6 mV, respectively) of fresh and aged uncoated IONP in MilliQ water were measured, while DMSA-IONP had a negative surface charge as demonstrated by a zeta-potential of −45.7 mV (Table 1). The negative charge of DMSA-IONP was expected due to negatively charged carboxyl groups of the bonded DMSA molecules on the surface of the IONP.17 Polydispersity indices (PI), a measure of the width of the particle size distribution, ranged from 0.19 to 0.32, indicating an increasing variability in the particle size.| IONP type | d (z-average) [nm] | PI | z [mV] | pH |
|---|---|---|---|---|
| Fresh uncoated IONP | 33.3 ± 1.1 | 0.19 ± 0.05 | 41.0 ± 8.2 | 3.5 |
| Aged uncoated IONP | 90.2 ± 1.4 | 0.32 ± 0.01 | 36.6 ± 1.2 | 3.2 |
| DMSA-IONP | 50.4 ± 0.3 | 0.25 ± 0.03 | −45.7 ± 0.4 | 6.8 |
3.2 Colloidal stability of IONP
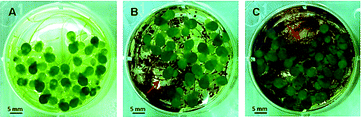
The colloidal stability of DMSA- and uncoated IONP in MilliQ water as well as in the three test media was investigated by DLS and zeta-potential measurements and additionally documented by photographs (Table 2) over a time period of 24 h. As the fresh and aged uncoated IONP behaved similarly in the test media only data for the fresh IONP are presented.During this period, uncoated IONP were stable in MilliQ water (MQ), but precipitated rapidly when brought into the other test media (Table 2), indicating their low colloidal stability in the presence of complex media compositions. In comparison to MilliQ water, the hydrodynamic diameters of uncoated IONP were much larger in all types of media (>5000 nm, t0) (Fig. 3A). Their zeta-potentials were clearly decreased and ranged between +10 to 0 mV in the media for algae (AM), Daphnia (DM) and Lemna (LM) at the beginning of the test (t0) (Fig. 3B). Similar precipitation was also observed by Zhu et al.31 when Fe2O3 NP were added to zebra fish culture medium (consisting of 64.75 mg L−1 NaHCO3, 5.75 mg L−1 KCl, 123.25 mg L−1 MgSO4·7H2O, and 294 mg L−1 CaCl2·2H2O). The average size of the Fe2O3 NP aggregates increased to more than 1 mm from a primary size of 30 nm as measured by DLS.31
DMSA-IONP, however, did not precipitate in MilliQ water and algae medium, but in Lemna and Daphnia media (Table 2). Besides, the precipitation was generally faster in DM than in LM (Table 2). In AM the hydrodynamic diameter was similar to the MilliQ, but much larger in LM (≈1000 nm) and DM (≈3000 nm) (Fig. 3A). The hydrodynamic diameter of dextran-IONP and PVP-IONP (Fe3O4) was reported to have increased from 32 nm and 23 nm in pure water to 52.3 and 82.3 nm in Daphnia medium (Elendt M7, OECD 202 standard medium), respectively.32 In contrast, the hydrodynamic diameter of ascorbate-IONP and citrate-IONP was still very small (17.5 and 17.2 nm respectively) after being transferred into Elendt M7 medium for 3 days.32
The zeta-potential of the DMSA-IONP was less negative in AM (≈−10 mV) in comparison to MilliQ water (≈−46 mV) (Fig. 3B). In DM and LM, particles also appeared less negative (−6 and −19 mV) at the beginning of the test (t0) but precipitated within 2 h (Table 2) and no zeta-potential could be determined by ELS afterwards. In the study of Baumann et al.,32 however, zeta potentials of citrate-IONP were measured as −25 mV in Elendt M7 medium after 5 days that were nearly equal to the one in water which was −27 mV; citrate- and PVP-IONP solutions had stayed transparent without visual agglomeration in Elendt M7 medium after one year.32 In our study, the hydrodynamic diameters (≈46 and 49 nm), polydispersity indices (≈0.26) and zeta-potentials (≈−10 and −44 mV) of DMSA-IONP dispersed in AM or MilliQ water, remained almost constant over a period of up to 7 days (ESI† Fig. S1 and S2), and stayed transparent for one year.
To address the concentration depended colloidal stability test at 25 mg Fe L−1 were performed (ESI† Tables S1 and S2). Similar behaviors compared to IONP at 100 mg Fe L−1 for both types of IONP were observed.
Evidently, DMSA-IONP are colloidally more stable than uncoated IONP in the three types of test media. The ionized and cross-linked DMSA molecules on the surface provide IONP with not only electrostatic stabilization through high negative charges due to the ionized carboxylate groups,13,17 but also steric stabilization due to DMSA polymers.
The decreased surface charges and colloidal stability of uncoated IONP (in the three media) and DMSA-IONP (decreased surface charges in the three media, but decreased stability only in the Daphnia and Lemna media) compared to MilliQ water may be due to the presence of complex ions and ionic strength (IS) in the media (Table 3). The IS has a strong influence on the surface charge and stability of NP dispersion according to DLVO (Derjaguin–Landau–Verwey–Overbeek) theory.43 High ionic strength leads to aggregation of cobalt ferrite43 and TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 nanoparticles by compressing the electrical double layer and decreasing the effective surface potential of particles through formation of a counter ion layer,43,44 thus lowering the repulsive barrier magnitude among particles. In particular, divalent cations such as Ca2+ and Mg2+ have strong effects on bridging between two negatively charged nanoparticles,43 thus aggregating nanoparticles and/or compacting the surface coatings by charge neutralization of the functional groups on NP surface.43 These two cations, when present in concentrations higher than 1 mM, are known to destabilize coated or uncoated NP,43 such as citrate-coated AgNP45,46 and silica NP.47 Accordingly the immediate aggregation of IONP in LM and DM can be explained: the concentrations of divalent cations in the Lemna and Daphnia media are around 7 and 10 times higher compared to algae medium (Table 3).
44 nanoparticles by compressing the electrical double layer and decreasing the effective surface potential of particles through formation of a counter ion layer,43,44 thus lowering the repulsive barrier magnitude among particles. In particular, divalent cations such as Ca2+ and Mg2+ have strong effects on bridging between two negatively charged nanoparticles,43 thus aggregating nanoparticles and/or compacting the surface coatings by charge neutralization of the functional groups on NP surface.43 These two cations, when present in concentrations higher than 1 mM, are known to destabilize coated or uncoated NP,43 such as citrate-coated AgNP45,46 and silica NP.47 Accordingly the immediate aggregation of IONP in LM and DM can be explained: the concentrations of divalent cations in the Lemna and Daphnia media are around 7 and 10 times higher compared to algae medium (Table 3).
| Medium | Main components | Total ionic strength [mM] | Divalent cations (Ca2+, Mg2+) [mM] |
|---|---|---|---|
| OECD TG 201 (R. subcapitata) pH 8.1 | NaHCO3, NH4Cl, CaCl2, MgSO4 | 1.7 | 0.24 |
| ISO 6341 (D. magna) pH 7.0 | CaCl2, MgSO4, NaHCO3, KCl, | 8.4 | 2.5 |
| OECD 221 Steinberg (L. minor) pH 5.5 | KNO3, Ca(NO3)2, MgSO4, KH2PO4 | 9.3 | 1.66 |
Apart of ionic strength the pH value is known to have a strong influence on the colloidal stability of NP. At pH values of the test media (adjusted with NaOH and HCl without additional salts) no aggregation of DMSA-IONP was observed (ESI† Table S3). On the other hand uncoated IONP showed strong aggregation at all three pH values (ESI† Table S3). Therefore, the decreased colloidal stability of DMSA-IONP in LM and DM might be mainly due to the presence of high amounts of divalent cations in the media, while for uncoated IONP, their aggregation appears to be the combined effects of pH and divalent cations.
3.3 Toxic effects of IONP on algae, Lemna and Daphnia
Effects of DMSA-IONP, fresh and aged uncoated IONP in the concentration range of 0.18–1791 μM Fe (0.01–100 mg Fe L−1) on algae, Lemna and Daphnia were investigated, and the EC50 values were determined (Table 4). Algal growth was totally inhibited by the three types of IONP when the concentration was ≥17.91 μM Fe (1.0 mg Fe L−1, Fig. 4A). The EC50 of DMSA-IONP for the algal growth after 72 h was 2.27 ± 0.19 μM Fe (0.13 ± 0.01 mg Fe L−1), while fresh and aged uncoated IONP showed stronger effects on algae, with EC50 values of 1.62 and 0.86 μM Fe (0.09 and 0.05 mg Fe L−1), respectively. None of the IONP had significant effects on the growth rate of Lemna within the tested concentration range and the test duration of 7 days (Fig. 4B). For Daphnia, no significant immobilization was observed when exposed to DMSA-IONP for 72 h (NOEC > 1791 μM Fe, i.e. 100 mg Fe L−1), but significant toxicity was found when the Daphnia were exposed to fresh (p < 0.01) or aged (p < 0.001) uncoated IONP at concentrations ≥179.1 μM Fe (10 mg Fe L−1, Fig. 4C) with up to 30–50% inhibition.| Substance | Algae | Lemna | Daphnia | |||
|---|---|---|---|---|---|---|
| EC50 values in μM Fe and mg Fe L−1 (confidence interval) | ||||||
| μM | mg L−1 | μM | mg L−1 | μM | mg L−1 | |
| Fresh uncoated IONP | 1.62 (-) | 0.09 (-) | >1791 | >100 | 1181 (298–18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 771) 771) |
65.94 (16.67–1048) |
| Aged uncoated IONP | 0.86 (-) | 0.05 (-) | >1791 | >100 | 374 (100–2870) | 20.89 (5.60–160.28) |
| DMSA-IONP | 2.27 (2.10–2.47) | 0.13 (0.12–0.14) | >1791 | >100 | >1791 | >100 |
| Pure DMSA | 31.42 (16.56–60.03) | 5.73 (3.02–10.94) | >179.1 | >32.6 | >179.1 | >32.6 |
Pure DMSA was also tested (Fig. 4D) to consider potential ecotoxicological effects that might be caused by unbound or released coating material. No adverse effect of pure DMSA towards Lemna and Daphnia in the tested concentration range was observed, but a significant inhibition on algal growth was determined for DMSA at concentrations >1.79 μM (0.326 mg L−1). The effect at high concentration might be attributed to the chelating effect of DMSA molecules15,16,18 and the masking (removal) of trace elements such as Fe3+ or Zn2+ from the algae medium. The concentration of DMSA in the IONP dispersion is unknown, but based on the synthesis, a concentration between 3.26 μg L−1 to 32.6 mg L−1 DMSA is assumed (this corresponds to one-tenth with regard to the iron content of the IONP, i.e. 0.01–100 mg Fe L−1). Due to the high colloidal stability of DMSA-IONP dispersions (Table 2 and ESI† Fig. S2) in algae medium, an almost complete release of DMSA in the algae medium appears unlikely. Therefore, the contribution of free/released DMSA to the toxicity of DMSA-IONP on algae is assumed to be negligible.
The influence of iron ions on algal growth was further investigated (Fig. 6), aiming to compare and discriminate between the effects of iron ions and particulate IONP. Potentially released iron can occur as ferrous (Fe2+) and ferric (Fe3+) ions. Fe2+ is assumed to be fully oxidized to Fe3+ in the presence of O2 (available in the media). The assumption is supported by speciation calculations (ESI† Table S4). Since these ions might contribute to the observed toxic effects we tested ferric ammonium citrate (Fe3+, FAC) and ferrous ammonium sulfate (Fe2+, FAS) in the same concentration range as IONP (0.18–1791 μM Fe, i.e. 0.01–100 mg Fe L−1) for potential adverse effects on algae (Fig. 6). In a test performed with standard medium that contains 0.24 μM Fe3+ (and EDTA) the two salts stimulated algal growth significantly at concentrations ≤82.4 and 3.9 μM Fe (4.6 and 0.22 mg Fe L−1), respectively (Fig. 6A). Only at concentrations ≥386.8 and 17.9 μM Fe (21.6 and 1.0 mg Fe L−1) could toxic effects of Fe3+ and Fe2+ be observed with a calculated EC50 of 689.2 and 24.5 μM Fe (38.5 and 1.4 mg Fe L−1), respectively. Similar experiments were conducted, but with an iron-free medium. Here the addition of ferric and ferrous species caused 5–12 times higher cell counts (Fig. 6A). Under this condition, even a high concentration of Fe3+ (1791 μM Fe, i.e. 100 mg Fe L−1) greatly promoted algal growth (EC50 > 1791 μM Fe, i.e. 100 mg Fe L−1) , while the Fe2+ only supported the growth at concentrations ≤17.91 μM (1.0 mg Fe L−1) with an EC50 of 82.4 μM Fe (4.6 mg Fe L−1). When algae were exposed to higher concentrations of Fe2+, growth was totally inhibited in both types (standard and iron-free) of algae media. The accelerated growth stimulation by FAC may be due to the combined effects of the presence of both Fe3+ and citrate. Citrates as complex agents have been reported to enhance availability of metal nutrients to algae51 and stimulate growth.52 Moreover, growth stimulation in the presence of iron ions is expected, since iron is the most important trace component for optimum growth of algae53 by involving in photosynthetic carbon and nitrogen reduction.54
In addition, the influence of another component (ammonium tested as NH4Cl) in FAC and FAS on algal growth was also investigated (Fig. 6B) in a concentration range of 0.18–1791 μM considering its important role as a source of nitrogen in plant growth.54 However, no significant stimulation was observed at concentrations of 386.9 μM or lower. Thus the contribution of ammonium ions in promoting algal growth in the presence of FAC/FAS can be neglected.
In subsequent experiments the toxicity of DMSA-coated and uncoated IONP in an iron-free medium was investigated (Fig. 7). Here the only source of iron is IONP which are presented in low concentrations of 1.8 × 10−5–17.91 μM Fe (equal to 1.0 × 10−6–1.0 mg Fe L−1). An additional potential iron ion source, such as DMSA-coated or uncoated IONP, was expected to promote algal growth at low concentrations if iron ions are released. However, neither DMSA-coated nor uncoated IONP significantly increased the algal growth through the tested concentrations. DMSA-IONP of concentrations ≤1.79 μM Fe (0.1 mg Fe L−1) caused a greater suppression on algal growth in the iron-free medium (Fig. 7, red curve) when compared with standard medium (Fig. 4A). Generally, a significantly (p < 0.01) decreased EC50 value of DMSA-IONP in the iron-free medium was observed (0.85 ± 0.40 μM Fe, i.e. 0.05 ± 0.02 mg Fe L−1). Nevertheless, in this case, slightly but significantly decreased toxicity was found at 7.13 and 2.85 μM Fe (0.4 and 0.16 mg Fe L−1) compared to the standard medium. In comparison to DMSA-IONP, uncoated IONP (Fig. 7, blue curve) in the iron-free medium at concentrations of 1.79 and 0.18 μM Fe (0.1 and 0.01 mg Fe L−1) showed lower toxic effects on the algal growth and the cell counts almost reached the control value.
The results indicate that free Fe-species are released from DMSA-coated and uncoated IONP, with higher amounts in the latter due to their comparatively lower stability in algae medium. It might also be possible that the observed stronger inhibition induced by DMSA-IONP is due to the decreased availability (chelation/adsorption) of potentially released iron ions or other trace elements from the medium by DMSA molecules. However, apparently the positive effects of liberated iron ions are compensated by adverse effects of particulate IONP per se, thus strong enhancement in growth is unlikely to be observed.
In terms of bioavailability it was also expected that the algae would be more susceptible to IONP than Lemna. The surface area to volume ratio of the cell itself is evaluated as 0.959 (surface area60 = 66.96 μm2, biovolume60 = 74.49 μm3) for R. subcapitata, several orders of magnitude higher than for Lemna (4 × 10−6).59
4. Conclusions
In order to assess the aquatic toxicity of IONP we have shown that DMSA-IONP, as well as fresh and aged uncoated IONP, cause strong, short-term toxicological effects to green algae R. subcapitata. The EC50 values for the three types of IONP ranged between 0.86–2.27 μM Fe (0.05 to 0.13 mg Fe L−1). So far no negative effects of IONP have been reported to algae. A recent study investigating two types of zero-valent IONP, bare and coated with Na-acrylic copolymer, has proved that NP support the growth of marine microalgae P. lutheri and I. galbana.69 It has been assumed that this effect is due to an enhanced bioavailability of iron in the form of NP. Our study with DMSA-IONP suggests that neither potentially released Fe2+/Fe3+ nor DMSA molecules from the coating are responsible for the observed toxic effects. Toxicity seems to be caused by interactions of algae cells with particulate IONP. The low colloidal stability of the uncoated IONP in algae medium comes with a rapid precipitation of particles and leads to a co-sedimentation of algae cells, whereas DMSA-IONP exhibit a much higher colloidal stability, but cause the flocculation of algae cells. In both cases the reduced access to nutrients and light might be mainly responsible for the growth inhibition. Moreover, the evolution of ROS (likely to be evolved if iron ions are released) and the NP-corona formed by biomolecules secreted by organisms might be important to explain observed toxic effects, but have not been investigated within this study.None of the investigated particles showed effects on L. minor (EC50s > 1791 μM Fe, i.e. 100 mg Fe L−1), and only for uncoated IONP could minor effects to D. magna be observed (EC50s > 179.1 μM Fe, i.e. 10 mg Fe L−1). However, the ingestion and accumulation of DMSA-coated and uncoated IONP into the gastrointestinal tract was observed. Uncoated IONP were even found at the filtering apparatus, the surface of antenna and the apical spine, which is assumed to hamper the normal growth of Daphnia by decreasing their mobility.
The visible high body-burden in Daphnia might not highly affect their mobility in short-term testing, but most likely will have an influence on chronic toxicity, as it was recently demonstrated that the immobilizing effect of ascorbate-, citrate- and dextran-IONP (Fe3O4) on D. magna increased during a slightly prolonged exposure for 96 h.32 Moreover, considering the accumulation of IONP in Daphnia, organisms at higher trophic levels may be negatively affected due to biomagnification. Given the possibility of IONP affecting aquatic organisms at both individual and population levels, due to not only short-term but also potential long-term exposure, there might be risks to the aquatic ecosystem through application of these NP in water treatment and environmental remediation, which perhaps should be kept in mind. Since there have been no major releases of IONP, due to its not having been widely applied in the real world, little knowledge is available on the distribution level of IONP in aquatic environment.31 Related but too limited information so far is available for zero-valent iron nanoparticles (nZVI), which have been used to remediate contaminated soil and groundwater for decades. Grieger et al. summarized the applied concentrations of nZVI used for environmental remediation at some documented sites in the USA.70 The concentrations ranged from 0.75 to 140 g L−1 which were much higher than the EC50s of the IONP to Raphidocelis and Daphnia in the present study. Although the high concentrations applied were on land, subsequent release of these NP to the aquatic environment is highly likely. The present study covered a wide concentration range of IONP, mainly from 1.0 × 10−6 to 100 mg Fe L−1, which was supposed to provide some information on the acceptable exposure levels of some basic aquatic organisms (including the primary producers) to these IONP. In addition, data presented here were based on laboratory standard tests which were performed in ‘ideal’ conditions, and IONP would experience physical and chemical transformations in the real environment which may further influence their effects on aquatic organisms. The strong negative effects on algae Raphidocelis observed here do raise concerns as these will have pronounced cascade effects on higher trophic levels, due to food limitation. Therefore, in remediation scenarios, benefits of IONP by detoxification should be viewed in comparison to negative side effects via the aquatic food chain.
Acknowledgements
We would like to thank the entire UFT Team for the interdisciplinary cooperation. We thank Dr. Marianne Matzke for helpful discussions and B. Sc. Gizem Alptekin for her experimental support. The authors would like to acknowledge the financial support of the German Federal Ministry of Education and Research (BMBF, funding code 03X0152, project “DENANA”).Notes and references
- D. L. Huber, Synthesis, properties, and applications of iron nanoparticles, Small, 2005, 1, 482–501 CrossRef CAS PubMed.
- A. K. Gupta and M. Gupta, Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications, Biomaterials, 2005, 26, 3995–4021 CrossRef CAS PubMed.
- A. B. Cundy, L. Hopkinson and R. L. D. Whitby, Use of iron-based technologies in contaminated land and groundwater remediation: a review, Sci. Total Environ., 2008, 400, 42–51 CrossRef CAS PubMed.
- P. Xu, G. M. Zeng, D. L. Huang, C. L. Feng, S. Hu, M. H. Zhao, C. Lai, Z. Wei, C. Huang, G. X. Xie and Z. F. Liu, Use of iron oxide nanomaterials in wastewater treatment: a review, Sci. Total Environ., 2012, 424, 1–10 CrossRef CAS PubMed.
- N. N. Nassar, Rapid removal and recovery of Pb(II) from wastewater by magnetic nanoadsorbents, J. Hazard. Mater., 2010, 184, 538–546 CrossRef CAS PubMed.
- J. Hu, G. Chen and I. M. C. Lo, Removal and recovery of Cr(VI) from wastewater by maghemite nanoparticles, Water Res., 2005, 39, 4528–4536 CrossRef CAS PubMed.
- L. Carlos, F. S. G. Einschlag, M. C. González and D. O. Mártire, Applications of magnetite nanoparticles for heavy metal removal from wastewater, Waste Water Treat. Technol. Recent Anal. Dev., INTECH Open Access Publisher, 2013, pp. 63–78 Search PubMed.
- D. Maity and D. C. Agrawal, Synthesis of iron oxide nanoparticles under oxidizing environment and their stabilization in aqueous and non-aqueous media, J. Magn. Magn. Mater., 2007, 308, 46–55 CrossRef CAS.
- J. Filser, D. Arndt, J. Baumann, M. Geppert, S. Hackmann, E. M. Luther, C. Pade, K. Prenzel, H. Wigger, J. Arning, M. C. Hohnholt, K. Köser, A. Kück, E. Lesnikov, J. Neumann, S. Schütrumpf, J. Warrelmann, M. Bäumer, R. Dringen, A. von Gleich, P. Swiderek and J. Thöming, Intrinsically green iron oxide nanoparticles? From synthesis via (eco-)toxicology to scenario modelling, Nanoscale, 2013, 5, 1034–1046 RSC.
- M. Chen, S. Yamamuro, D. Farrell and S. A. Majetich, Gold-coated iron nanoparticles for biomedical applications, J. Appl. Phys., 2003, 93, 7551 CrossRef CAS.
- C. Tassa, S. Y. Shaw and R. Weissleder, Dextran-coated iron oxide nanoparticles: a versatile platform for targeted molecular imaging, molecular diagnostics, and therapy, Acc. Chem. Res., 2011, 44, 842–852 CrossRef CAS PubMed.
- J. Liu, Z. Zhao and G. Jiang, Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water, Environ. Sci. Technol., 2008, 42, 6949–6954 CrossRef CAS PubMed.
- C. R. A. Valois, J. M. Braz, E. S. Nunes, M. A. R. Vinolo, E. C. D. Lima, R. Curi, W. M. Kuebler and R. B. Azevedo, The effect of DMSA-functionalized magnetic nanoparticles on transendothelial migration of monocytes in the murine lung via a β2 integrin-dependent pathway, Biomaterials, 2010, 31, 366–374 CrossRef CAS PubMed.
- W. Yantasee, C. L. Warner, T. Sangvanich, R. S. Addleman, T. G. Carter, R. J. Wiacek, G. E. Fryxell, C. Timchalk and M. G. Warner, Removal of heavy metals from aqueous systems with thiol functionalized superparamagnetic nanoparticles, Environ. Sci. Technol., 2007, 41, 5114–5119 CrossRef CAS PubMed.
- Thorne Research, Inc., Dimercaptosuccinic Acid, Altern. Med. Rev. Monogr., 2002, pp. 132–136 Search PubMed.
- K. Sompamit, U. Kukongviriyapan, W. Donpunha, S. Nakmareong and V. Kukongviriyapan, Reversal of cadmium-induced vascular dysfunction and oxidative stress by meso-2,3-dimercaptosuccinic acid in mice, Toxicol. Lett., 2010, 198, 77–82 CrossRef CAS PubMed.
- C. Petters, E. Irrsack, M. Koch and R. Dringen, Uptake and metabolism of iron oxide nanoparticles in brain cells, Neurochem. Res., 2014, 39, 1648–1660 CrossRef CAS PubMed.
- H. V. Aposhian, DMSA and DMPS—water soluble antidotes for heavy metal poisoning, Annu. Rev. Pharmacol. Toxicol., 1983, 23, 193–215 CrossRef CAS PubMed.
- M. E. Sears, Chelation: Harnessing and enhancing heavy metal detoxification - A review, Sci. World J., 2013, 2013, 219840, DOI:10.1155/2013/219840.
- D. Mishra and S. J. S. Flora, Differential oxidative stress and DNA damage in rat brain regions and blood following chronic arsenic exposure, Toxicol. Ind. Health, 2008, 24, 247–256 CrossRef CAS PubMed.
- N. Ercal, P. Treeratphan, T. C. Hammond, R. H. Matthews, N. H. Grannemann and D. R. Spitz, In vivo indices of oxidative stress in lead-exposed C57BL/6 mice are reduced by treatment with meso-2,3-dimercaptosuccinic Acid or N-acetylcysteine, Free Radical Biol. Med., 1996, 21, 157–161 CrossRef CAS PubMed.
- N. Fauconnier, J. N. Pons, J. Roger and A. Bee, Thiolation of maghemite nanoparticles by dimercaptosuccinic acid, J. Colloid Interface Sci., 1997, 194, 427–433 CrossRef CAS PubMed.
- H. Muckter, B. Lieb, F.-X. Reich, G. Hunder, U. Walther and B. Fichtl, Are we ready to replace dimercaprol (BAL) as an arsenic antidote, Hum. Exp. Toxicol., 1997, 16, 460–465 CAS.
- H. V. Aposhian, D. E. Carter, T. D. Hoover, C. A. Hsu, R. M. Maiorino and E. Stine, DMSA, DMPS, and DMPA-as arsenic antidotes, Toxicol. Sci., 1984, 4, 58–70 CrossRef CAS.
- H. V. Aposhian, R. M. Maiorino, D. Gonzalez-ramirez, M. Zuniga-charles, Z. Xu, K. M. Hurlbut, P. Junco-munoz, R. C. Dart and M. M. Aposhian, Mobilization of heavy metals by newer, therapeutically useful chelating agents, Toxicology, 1995, 97, 23–38 CrossRef PubMed.
- S. Singh, K. C. Barick and D. Bahadur, Surface engineered magnetic nanoparticles for removal of toxic metal ions and bacterial pathogens, J. Hazard. Mater., 2011, 192, 1539–1547 CrossRef CAS PubMed.
- C. L. Warner, R. S. Addleman, A. D. Cinson, T. C. Droubay, M. H. Engelhard, M. A. Nash, W. Yantasee and M. G. Warner, High-performance, superparamagnetic, nanoparticle-based heavy metal sorbents for removal of contaminants from natural waters, ChemSusChem, 2010, 3, 749–757 CrossRef CAS PubMed.
- M. Geppert, M. C. Hohnholt, K. Thiel, S. Nürnberger, I. Grunwald, K. Rezwan and R. Dringen, Uptake of dimercaptosuccinate-coated magnetic iron oxide nanoparticles by cultured brain astrocytes, Nanotechnology, 2011, 22, 145101 CrossRef PubMed.
- M. Auffan, L. Decome, J. Rose, T. Orsiere, M. De Meo, V. Briois, C. Chaneac, L. Olivi, J. Berge-lefranc, A. Botta, M. R. Wiesner and J. Bottero, In vitro interactions between DMSA-coated maghemite nanoparticles and human fibroblasts: a physicochemical and cyto-genotoxical study, Environ. Sci. Technol., 2006, 40, 4367–4373 CrossRef CAS PubMed.
- B. Wang, W. Feng, M. Zhu, Y. Wang, M. Wang, Y. Gu, H. Ouyang, H. Wang, M. Li, Y. Zhao, Z. Chai and H. Wang, Neurotoxicity of low-dose repeatedly intranasal instillation of nano- and submicron-sized ferric oxide particles in mice, J. Nanopart. Res., 2009, 11, 41–53 CrossRef CAS.
- X. Zhu, S. Tian and Z. Cai, Toxicity assessment of iron oxide nanoparticles in zebrafish (Danio rerio) early life stages, PLoS One, 2012, 7, 1–6 Search PubMed.
- J. Baumann, J. Köser, D. Arndt and J. Filser, The coating makes the difference: acute effects of iron oxide nanoparticles on Daphnia magna, Sci. Total Environ., 2014, 484, 176–184 CrossRef CAS PubMed.
- OECD, Test No. 201: freshwater alga and cyanobacteria, growth inhibition test, OECD guidelines for the testing of chemicals, 2011, section 2, DOI:10.1787/9789264069923-en.
- OECD, Test No. 221: lemna sp. growth inhibition test, OECD guidelines for the testing of chemicals, 2006, section 2, DOI:10.1787/9789264016194-en.
- MicroBioTests Inc., Daphtoxkit F™ magna, http://www.microbiotests.be/toxkits/daphtoxkitf.pdf.
- A. Bee, R. Massart and S. Neveu, Synthesis of very fine maghemite particles, J. Magn. Magn. Mater., 1995, 149, 6–9 CrossRef CAS.
- M. Geppert, M. Hohnholt, L. Gaetjen, I. Grunwald, M. Bäumer and R. Dringen, Accumulation of iron oxide nanoparticles by cultured brain astrocytes, J. Biomed. Nanotechnol., 2009, 5, 285–293 CrossRef CAS PubMed.
- J. Riemer, H. H. Hoepken, H. Czerwinska, S. R. Robinson and R. Dringen, Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells, Anal. Biochem., 2004, 331, 370–375 CrossRef CAS PubMed.
- W. Drost, M. Matzke and T. Backhaus, Heavy metal toxicity to Lemna minor: studies on the time dependence of growth inhibition and the recovery after exposure, Chemosphere, 2007, 67, 36–43 CrossRef CAS PubMed.
- J. Baumann, Y. Sakka, C. Bertrand, J. Köser and J. Filser, Adaptation of the Daphnia sp. acute toxicity test: miniaturization and prolongation for the testing of nanomaterials, Environ. Sci. Pollut. Res., 2014, 21, 2201–2213 CrossRef CAS PubMed.
- S. R. Charlton and D. L. Parkhurst, PhreeqcI - A Graphical User Interface for the Geochemical Computer Program PHREEQC, U.S. Geological Survey, 2016, Available from: http://wwwbrr.cr.usgs.gov/projects/GWC_coupled/phreeqci/index.html Search PubMed.
- H. D. Schulz and M. Kölling, DVWK-Schriften 100: Anwendung hydrogeochemischer Modelle, Verlag Paul Parey, Hamburg and Berlin, 1992, p. 45 Search PubMed.
- M. Pavlin and V. B. Bregar, Stability of nanoparticle dispersions in different biologically relevant media, Dig. J. Nanomater. Biostruct., 2012, 7, 1389–1400 Search PubMed.
- Z. Ji, X. Jin, S. George, T. Xia, H. Meng, X. Wang, E. Suarez, H. Zhang, E. M. V. Hoek, H. Godwin, A. E. Nel and J. I. Zink, Dispersion and stability optimization of TiO2 nanoparticles in cell culture media, Environ. Sci. Technol., 2010, 44, 7309–7314 CrossRef CAS PubMed.
- K. A. Huynh and K. L. Chen, Aggregation kinetics of citrate and polyvinylpyrrolidone coated silver nanoparticles in monovalent and divalent electrolyte solutions, Environ. Sci. Technol., 2011, 45, 5564–5571 CrossRef CAS PubMed.
- S. L. Chinnapongse, R. I. MacCuspie and V. A. Hackley, Persistence of singly dispersed silver nanoparticles in natural freshwaters, synthetic seawater, and simulated estuarine waters, Sci. Total Environ., 2011, 409, 2443–2450 CrossRef CAS PubMed.
- C. O. Metin, L. W. Lake, C. R. Miranda and Q. P. Nguyen, Stability of aqueous silica nanoparticle dispersions, J. Nanopart. Res., 2011, 13, 839–850 CrossRef CAS.
- E. Navarro, A. Baun, R. Behra, N. B. Hartmann, J. Filser, A.-J. Miao, A. Quigg, P. H. Santschi and L. Sigg, Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi, Ecotoxicology, 2008, 17, 372–386 CrossRef CAS PubMed.
- C. Wei, Y. Zhang, J. Guo, B. Han, X. Yang and J. Yuan, Effects of silica nanoparticles on growth and photosynthetic pigment contents of Scenedesmus obliquus, J. Environ. Sci., 2010, 22, 155–160 CrossRef CAS.
- V. Aruoja, H.-C. Dubourguier, K. Kasemets and A. Kahru, Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata, Sci. Total Environ., 2009, 407, 1461–1468 CrossRef CAS PubMed.
- O. Errecalde, M. Seidl and P. G. C. Campbell, Influence of a low molecular weight metabolite (citrate) on the toxicity of cadmium and zinc to the unicellular green alga Selenastrum capricornutum: an exception to the free-ion model, Water Res., 1998, 32, 419–429 CrossRef CAS.
- W. A. Kratz and J. Myers, Nutrition and growth of several blue-green algae, Am. J. Bot., 1955, 42, 282–287 CrossRef CAS.
- M. Chen, H. Tang, H. Ma, T. C. Holland, S. K. Y. Ng and S. O. Salley, Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta, Bioresour. Technol., 2011, 102, 1649–1655 CrossRef CAS PubMed.
- C. Reynolds, Ecology of Phytoplankton, Cambridge University Press, 2006 Search PubMed.
- Z. Wang, J. Li, J. Zhao and B. Xing, Toxicity and internalization of CuO nanoparticles to prokaryotic alga Microcystis aeruginosa as affected by dissolved organic matter, Environ. Sci. Technol., 2011, 45, 6032–6040 CrossRef CAS PubMed.
- G. Song, Y. Gao, H. Wu, W. Hou, C. Zhang and H. Ma, Physiological effect of anatase TiO2 nanoparticles on Lemna minor, Environ. Toxicol. Chem., 2012, 31, 2147–2152 CrossRef CAS PubMed.
- F. Perreault, R. Popovic and D. Dewez, Different toxicity mechanisms between bare and polymer-coated copper oxide nanoparticles in Lemna gibba, Environ. Pollut., 2014, 185, 219–227 CrossRef CAS PubMed.
- G. Juhel, E. Batisse, Q. Hugues, D. Daly, F. N. A. M. van Pelt, J. O'Halloran and M. A. K. Jansen, Alumina nanoparticles enhance growth of Lemna minor, Aquat. Toxicol., 2011, 105, 328–336 CrossRef CAS PubMed.
- K. K. Jessing, M. Andresen and N. Cedergreen, Temperature-dependent toxicity of artemisinin toward the macrophyte Lemna minor and the algae Pseudokirchneriella subcapitata, Water, Air, Soil Pollut., 2014, 225, 2010 CrossRef.
- J. A. Weiner, M. E. DeLorenzo and M. H. Fulton, Relationship between uptake capacity and differential toxicity of the herbicide atrazine in selected microalgal species, Aquat. Toxicol., 2004, 68, 121–128 CrossRef CAS PubMed.
- A. Baun, N. B. Hartmann, K. Grieger and K. O. Kusk, Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing, Ecotoxicology, 2008, 17, 387–395 CrossRef CAS PubMed.
- X. Zhu, Y. Chang and Y. Chen, Toxicity and bioaccumulation of TiO2 nanoparticle aggregates in Daphnia magna, Chemosphere, 2010, 78, 209–215 CrossRef CAS PubMed.
- S. B. Lovern, H. A. Owen and R. Klaper, Electron microscopy of gold nanoparticle intake in the gut of Daphnia magna, Nanotoxicology, 2008, 2, 43–48 CrossRef CAS.
- P. Rosenkranz, Q. Chaudhry, V. Stone and T. F. Fernandes, A comparison of nanoparticle and fine particle uptake by Daphnia magna, Environ. Toxicol. Chem., 2009, 28, 2142–2149 CrossRef CAS PubMed.
- D. Ebert, Ecology, Epidemiology, and Evolution of Parasitism in Daphnia [Internet], Chapter 2: Introduction to Daphnia Biology, Bethesda (MD): National Center for Biotechnology Information (US), 2005, Available from: http://www.ncbi.nlm.nih.gov/books/NBK2042/ Search PubMed.
- S. B. Lovern, J. R. Strickler and R. Klaper, Behavioral and physiological changes in Daphnia magna when exposed to nanoparticle dispersions (titanium dioxide, nano-C60, and C60HxC70Hx), Environ. Sci. Technol., 2007, 41, 4465–4470 CrossRef CAS PubMed.
- A. Okamoto, M. Yamamuro and N. Tatarazako, Acute toxicity of 50 metals to Daphnia magna, J. Appl. Toxicol., 2015, 35, 824–830 CrossRef CAS PubMed.
- A. Dabrunz, L. Duester, C. Prasse, F. Seitz, R. Rosenfeldt, C. Schilde, G. E. Schaumann and R. Schulz, Biological surface coating and molting inhibition as mechanisms of TiO2 nanoparticle toxicity in Daphnia magna, PLoS One, 2011, 6, e20112 CAS.
- E. Kadar, P. Rooks, C. Lakey and D. A. White, The effect of engineered iron nanoparticles on growth and metabolic status of marine microalgae cultures, Sci. Total Environ., 2012, 439, 8–17 CrossRef CAS PubMed.
- K. D. Grieger, A. Fjordbøge, N. B. Hartmann, E. Eriksson, P. L. Bjerg and A. Baun, Environmental benefits and risks of zero-valent iron nanoparticles (nZVI) for in situ remediation: risk mitigation or trade-off?, J. Contam. Hydrol., 2010, 118, 165–183 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5en00222b |
| This journal is © The Royal Society of Chemistry 2016 |