Induction of micronuclei by multi-walled carbon nanotubes interacting with humic acids in cultured human lymphocytes†
Maria-Sophia
Vidali
a,
Eleni
Bletsa
bc,
Antonios
Kouloumpis
c,
Charalambos G.
Skoutelis
a,
Yiannis
Deligiannakis
*b,
Dimitrios
Gournis
c and
Dimitris
Vlastos
*a
aDepartment of Environmental and Natural Resources Management, University of Patras, Seferi 2, Agrinio 30100, Greece. E-mail: dvlastos@upatras.gr; Tel: +302641074148
bDepartment of Physics, University of Ioannina, GR-45110 Ioannina, Greece. E-mail: ideligia@cc.uoi.gr
cDepartment of Materials Science and Engineering, University of Ioannina, GR-45110 Ioannina, Greece
First published on 15th September 2015
Abstract
Mixtures of multi-walled carbon nanotubes (MWCNTs) with natural humic acids (Leonardite humic acid, LHA) or humic acid-like polycondensates (HALP) were evaluated, for the first time, about their potential genotoxic and cytotoxic effects in cultured human lymphocytes. The genotoxic evaluation of the tested materials, either separately or in combination, for the detection of micronuclei (MN) in the cytoplasm of interphase cells, was performed using the cytokinesis block micronucleus (CBMN) assay. A comparative analysis of the genotoxicity and cytotoxicity reveals that in the tested concentrations, the [MWCNTs + LHA] mixture is more genotoxic and slightly more cytotoxic than the [MWCNTs + HALP] mixture. MN induction observed in human lymphocytes demonstrates that humic substances enhance the genotoxic effects of MWCNTs. In addition, the present data highlight a – so far unforeseen – potential genotoxic effect as the result of both clastogenic and aneugenic actions of the particular mixtures on human lymphocytes.
Nano impactA genotoxicity mechanism of multi-walled carbon nanotubes (MWCNTs) interacting with humic acids (HAs) is revealed. The interfacial properties of MWCNT-HA formations were characterized with ATR-FTIR, Atomic Force Microscopy and Dynamic Light Scattering. Using natural and synthetic/metal-free humic polymers, the mechanisms of colloidal dispersion are correlated with the revealed genotoxicity. Moreover, the geno- vs. cytotoxicity phenomena are quantitatively distinguished. These phenomena originate from the action of humic macromolecules as “chaperones” that shuttle MWCNTs into the cell compartments. |
1. Introduction
Carbon nanotubes (CNTs) are fiber-shaped particles consisting of graphite hexagonal-mesh planes present as a single layer (single-walled CNTs, SWCNTs) or as multilayers with nest accumulation (multi-walled CNTs, MWCNTs). Their potential applications are broad, e.g. in CNT-enhanced plastics, electromagnetic shielding, antistatic materials, flexible fibers and advanced polymers, medical/health fields, and scanning probe microscopy.1 In 2005, the worldwide CNT production was ~0.2 kilotons (ktn). In 2011, this production increased by more than a factor of 10 and soared to about 4.6 ktn per y.2Nonetheless, despite their already widespread use, there is an increasing need for pertinent information on their implications in human health and the environment.3,4
The main reason of concern about CNTs is related to their fibrous structure which is similar to that of asbestos, and a high-aspect-ratio nanoparticle theory has already been suggested for CNT toxicity.5 Accordingly, as in the case of asbestos, high-aspect-ratio MWCNTs are more toxic and potent to induce mesothelioma than low-aspect-ratio MWCNTs.5 The carcinogenic effect of biopersistent fibers such as asbestos has also been associated with the local generation of reactive oxygen species and inflammatory reactions while genotoxic effects related to these phenomena may also be implicated.6
The small size and high surface-to-volume ratio of CNTs could also affect interactions at the cellular level, leading to enhanced permeability through the cell membrane with a profound influence on cellular dynamics.7 Therefore, it is essential to investigate the potential hazards of CNTs to humans and other biological systems not only at the cellular but also at the subcellular level i.e. on the genetic material for example. While existing literature data primarily focus on the potential cytotoxicity of CNTs with conflicting results, the study on genotoxicity of CNTs is beginning to emerge as an important research area.8
In the recent reviews by Toyokuni9 and Saito et al.,10 it is stated that certain types of MWCNTs are as carcinogenic to mesothelial cells as asbestos fibers, and the cytotoxicity, inflammatogenicity and carcinogenicity of a specific given type of MWCNT are modulated by factors such as its diameter, length, rigidity and surface modification. In addition, distinguishing the mutagenicity and genotoxicity of CNTs remains a challenging research task; some studies judged CNTs to be mutagenic or genotoxic and others did not. The so-far published results vary depending on the cell type even within the same study.
Nowadays, there is a particular concern regarding a possible genotoxic effect of nanomaterials, through their use and release into the environment including soil, as well as their impact to the food chain and especially in humans.11 Engineered Nanoscale Materials (ENMs) are already being used in agriculture as “nanofertilizers”.12 In addition, a recent study has shown that low doses of MWCNTs in normal media, improve water absorption, plant biomass and the concentrations of the essential Ca, Fe nutrients, opening a potential for possible future commercial agricultural applications.13 On the other hand, such use of nanomaterials may be of concern to human health, especially to farmers who may become exposed as a result of MWCNT contamination in soil and/or through the potential interactions between MWCNTs and soil components such as humic acid (HA). In this context, the present study aimed to investigate the potential genotoxic effects of MWCNTs in combination with a main soil component such as soil organic matter on humans.
More specifically, the potential of soil components i.e. humic acid (HA) macromolecules in enhancing the bioavailability of CNTs has to be carefully assessed. Due to their poor solubility and dispersibility – in aqueous or polar solvents – MWCNTs are prone to aggregation and deposition in water due to strong inter-nanotube van der Waals forces.14,15
Humic acids (HAs) represent the most abundant fraction of humic substances, which are the most relevant chemically and biochemically reactive components of soil organic matter.16 HA has been shown to be very efficient in enhancing the water-dispersibility of CNTs.14,15 HA can be associated with CNT surface e.g. either via hydrophobic π–π interactions or/and by a physical wrapping of HA macromolecules around CNTs. The high charge content of HA i.e. typically up to 1–4 meq per 100 mg17 makes the so-formed [HA + CNT] composite highly anionic at pH > 4, and this results in their high dispersibility in water. On the other hand, HAs are natural polyelectrolytes that regulate biological membranes' permeability.18,19 This renders HAs as potent cofactors able to shuttle exogenous entities e.g. such as nanomaterials to the cells' internal compartments. In this context, herein, the effect of the contaminant presence of HA and CNTs on human cell-nucleus has been studied.
Two types of well-characterized HAs were used: [i] a reference Leonardite HA (herein codenamed LHA) obtained from the International Humic Substances Society (IHSS) and [ii] a well characterized, metal-free, synthetic humic-acid-like-polycondensate (HALP) produced from simple organic precursors with no use of a co-catalsyst.20 As demonstrated by Giannakopoulos et al.,20 HALP replicates the essential physicochemical parameters e.g. charge, structural carboxy/phenolic content, and radicals that are pertinent in the present study; however, HALP is free of any adventitious ions – in particularly Fe – that might be involved in adverse oxidative stress events.
The aim of the present work is to study the potential genotoxic effects of MWCNTs in combination with LHA or HALP in human lymphocytes in vitro. In general, the genotoxicity is directly linked to the mutagenic and carcinogenic effects of chemicals. To cover the whole spectrum of the genetic damage that can occur, usually a combination of both in vitro and in vivo assays is used. For this purpose, the widely used cytokinesis block micronucleus (CBMN) assay in human lymphocyte cultures, which is a sensitive indicator of the structural and numerical changes in chromosomes, was selected and used herein as a validated method accepted for regulatory purposes.21–23
Micronuclei (MN) may originate from acentric chromosome fragments or whole chromosomes that are unable to migrate to the poles during the anaphase stage of cell division. The simplicity, rapidity and sensitivity of the CBMN assay make it a valuable tool for genotoxicity screening.23
Overall, the main objectives of the present study are: [a] to evaluate the genotoxic and cytotoxic effects of MWCNTs interacting with LHA and HALP in human lymphocytes in vitro, and [b] to understand the physical mechanism of the observed genotoxicity and cytotoxicity in relation to the enhanced water solubility of the LHA + MWCNTs and HALP + MWCNTS.
2. Method
2.1. Synthesis of HALP
HALP was produced by the oxidative polymerization of gallic acid and protocatechuic acid in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 according to Giannakopoulos et al.20 with no use of a co-catalyst. The procedure is described in the ESI.† The so obtained metal-free HALP was fully characterized as detailed by Giannakopoulos et al.20
1 according to Giannakopoulos et al.20 with no use of a co-catalyst. The procedure is described in the ESI.† The so obtained metal-free HALP was fully characterized as detailed by Giannakopoulos et al.20
2.2. Materials
MWCNTs (6–9 nm diameter, 5 μm length) with >95% purity were supplied by Sigma-Aldrich (CAS no. 724769) and purified in a metal-free form according to Georgakilas et al.24 Leonardite humic acid standard (LHAS04) was purchased from the IHSS and used with no further purification.2.3. HA stock solutions
The stock solutions (500 mg L−1) of the LHA and HALP were prepared with Milli-Q water (Millipore-Academic system) and their desired pH was adjusted with small volumes of NaOH and HNO3.2.4. MWCNT-HA suspensions
The stock suspensions of MWCNTs and HAs [LHA or HALP] were prepared as follows: (a) 55 mg of MWCNTs was dispersed in 100 ml of deionized/ultrapure water, (b) 220 mg of LHA was dispersed in 100 ml of deionized/ultrapure water and (c) 88 mg of HALP was dissolved in 100 ml of deionized/ultrapure water. From the stock solutions, appropriate volumes – which correspond to the final concentrations of the tested mixtures – were added in our cultures. The solutions were sonicated using low-power sonication. More particularly, based on the Dynamic Light Scattering data detailed hereafter, we have applied a total of 0.6 kJ power per ml of solution. Typically, this is achieved by applying 50 Watt sonication power for 20 minutes per 10 ml of volume. This can be routinely performed using a commercial bath sonicator. Mild/low-power sonication protocol results in no physical damage of MWCNTs, thus excluding any artificial generation of edge-related radical species that might generate reactive oxygen species. The dispersion was then left under stirring overnight. Water was the only solvent employed, and no organic solvents were used.2.5. Materials' characterization
| Total ultrasound energy delivered per ml = [ultrasound power (Watts) × sonication time]/solution volume (ml) |
We have screened the sonication energies from low (0.2 kJ ml−1) up to high (8 kJ ml−1). Low sonication energies (0.2–0.6 kJ ml−1) were produced using a common path sonicator (Pranson 2000, 50 Watt) by varying the sonication time. A full [energy per ml] scale (0.2 to 8 kJ ml−1) was investigated using a probe sonicator Sonic V500 delivering a maximum of 500 Watts. DLS data show that for the same energy per ml, the method of sonication e.g. bath or probe, gives the same DLS results. Thus, controlling the total energy per ml allows a precise parameterisation of the sonication protocol instead of varying only the sonication time or only the power.
In the present experiments, we have studied suspensions of 10 mg L−1i.e. 10 μg ml−1 MWCNTs in various media: [1] only H2O, pH 6.5, [2] the culture medium, pH 6.5 [6.5 ml of Ham's F-10 medium (Gibco), 1.5 ml of fetal bovine serum (Gibco) and 0.3 ml of phytohaemagglutinin (Gibco)]. The effect of HALP was studied by adding 10 mg L−1 of HALP in the suspension of the MWCNTs.
In all cases, the agglomerate size in solution was determined by DLS at short incubation times [30 minutes] as well as after 72 hours i.e. conditions similar to the biological cultures.
Dynamic Light Scattering (DLS) (Malvern Zetasizer, model Nano ZS) measurements were performed in quartz cuvettes.
2.6. CBMN assay in human lymphocytes in vitro
Blood samples were obtained from two non-smoking, healthy individuals (21 and 25 years old) not undergoing any drug treatment, free of viral infection or X-ray exposure in the recent past. The blood samples were kept under sterile conditions in heparinized tubes. Whole blood (0.5 ml) was added to 6.5 ml of Ham's F-10 medium (Gibco), 1.5 ml of fetal bovine serum (Gibco) and 0.3 ml of phytohaemagglutinin (Gibco) to stimulate cell division. The effect of MWCNTs, LHA and their mixture were studied at three different concentrations (5, 15, 25 μg ml−1), (20, 60, 100 μg ml−1) and (5 + 20, 15 + 60, 25 + 100 μg ml−1), respectively. The reported results in Table 1 represent the pooled data from the two donors' replicated cultures.25 Furthermore, the effect of MWCNTs, HALP and their mixture were studied at four different concentrations (5, 15, 25, 30 μg ml−1), (8, 25, 42, 50 μg ml−1) and (5 + 8, 15 + 25, 25 + 42, 30 + 50 μg ml−1), respectively. The reported results in Table 1 represent the pooled data from two independent experiments. Mitomycin-C (MMC) (Sigma) at a final concentration of 0.05 μg ml−1 served as positive control. 44 h after initiating cultures, 6 μg ml−1 Cytochalasin-B (Cyt-B) (Sigma) was added to the culture medium to block cell division. The use of cytochalasin-B, an inhibitor of actin polymerization, which prevents cytokinesis while permitting nuclear division leads to the formation of binucleated (BN) cells which are scored for the presence of MN.23| Concentration (μg ml−1) | MN MF (‰) ± se | CBPI MF (‰) ± se |
|---|---|---|
| MN, micronuclei; CBPI, Cytokinesis Block Proliferation Index; MF (‰) ± se, mean frequencies (‰) ± standard error; MWCNTs, multi-walled carbon nanotubes; LHA, Leonardite humic acid; MMC, Mitomycin-C; 4000 binucleated cells scored per experimental point.a p < 0.05.b p < 0.01.c p < 0.001 [G-test for MN; χ2 for CBPI]. | ||
| 0 | 5.25 ± 0.48 | 1.78 ± 0.02 |
| MWCNTs | ||
| 5 | 12.25 ± 1.44b | 1.78 ± 0.01 |
| 15 | 10.75 ± 0.63a | 1.77 ± 0.02 |
| 25 | 9.00 ± 0.41 | 1.77 ± 0.02 |
| LHA | ||
| 20 | 8.50 ± 0.50 | 1.79 ± 0.01 |
| 60 | 9.75 ± 0.85 | 1.77 ± 0.02 |
| 100 | 9.50 ± 0.29 | 1.80 ± 0.01 |
| MWCNTs + LHA | ||
| 5 + 20 | 20.25 ± 0.25c | 1.76 ± 0.04 |
| 15+ 60 | 18.50 ± 0.96c | 1.75 ± 0.05 |
| 25 + 100 | 24.00 ± 1.78c | 1.75 ± 0.03 |
| MMC | ||
| 0.05 | 54.75 ± 4.13c | 1.67 ± 0.02b |
The cultures were incubated at 37 °C in a humidified atmosphere of 5% CO2 for 72 h. The procedure for slide preparation is described in the ESI.† The standard criteria were used for scoring MN.26
To determine possible cytotoxic effects, the Cytokinesis Block Proliferation Index (CBPI) was calculated by counting at least 2000 cells for each experimental point. CBPI is given by the equation:
| CBPI = [M1 + 2 M2 + 3(M3 + M4)]/N |
The calculated MN size was also used as an additional parameter to determine whether the activity of the tested substances is clastogenic or aneugenic.27,28 The MN size is expressed as the ratio MNd/CNd (MNd = MN diameter; CNd = cell nucleus diameter).
The MN size was characterized as “small”, when MNd/CNd ≤ 1/10, “medium” when MNd/CNd = 1/3 to 1/9 and “large” when MNd/CNd ≈ 1/3.27
Small-sized MNs are more likely to contain acentric chromosome fragments indicating a clastogenic effect, while large-sized MN may possibly contain whole chromosomes, thus indicating an aneugenic effect.27,28
2.7. Statistical analysis
All results are expressed as mean frequency ± standard error (MF ± se). The statistical analysis of the MN data was conducted using the G-test for independence on 2 × 2 tables. The chi-square test (χ2 test) was used for the analysis of CBPI among each treatment. Statistical decisions were based on a significance level of 0.05. The statistical software used for data analysis was the Statistical Package for Social Sciences (SPSS) for Windows, version 17.0.3. Results
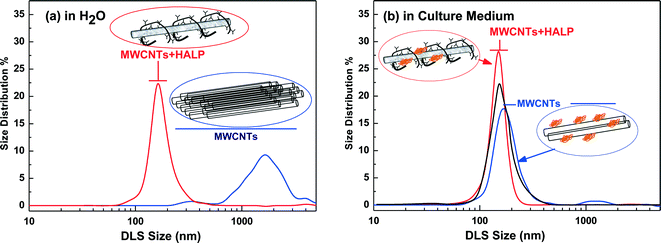
3.1. Dispersion effect of HAs on MWCNTs – Dynamic Light Scattering (DLS)
The sample pictures in Fig. 1 exemplify the dispersibility difference between MWCNTs in H2O in the absence of HAs (Fig. 1a) and in the presence of HAs (Fig. 1c). In the absence of HAs, negligible dispersion of MWCNTs was detected even after 8 kJ ml−1 of sonication (Fig. 1a). In the presence of HAs, under low-energy sonication of 0.6 kJ ml−1, MWCNTs form a non-precipitating suspension which remained practically unaltered for at least two weeks, as illustrated in Fig. 1c, and this is an indication that HAs solubilize the MWCNTs. The complete solubilization of the MWCNTs in the presence of HAs was achieved under 0.6 kJ ml−1 sonication energy, as illustrated in Fig. 1d and e, for HALP and LHA respectively. We noticed a color difference after complete solubilization of the MWCNTs between HALP (grey) and LHA (more brown) in comparison with the HALP (Fig. 1b). These colorimetric observations can be explained using DLS that gives quantitative information on the debundling/disaggregation process. Herein, we discuss the DLS data for 10 μg ml−1 MWCNTs that are within the range of 5–30 μg ml−1, studied in cell cultures, described hereafter. Analogous DLS experiments for higher MWCNT concentrations (25 μg ml−1) show increasing agglomeration (data not shown).Overall, the DLS results show that in both H2O as well as in the cell culture medium HALP macromolecules strongly debundle MWCNTs. The cell culture medium also debundles MWCNTs to some extent. The basis of the debundling effect of HALP is that HALP macromolecules, when associated on the CNT surface, can decrease the stacking energy between the bundled CNTs by introducing hydrophilic interactions via their charged groups. This is in agreement with Ghosh et al.29 who have demonstrated that natural humics enhance the colloidal stability/dispersion of nanotubes and other nanomaterials. Herein, this effect was further investigated in detail for the HALP/MWCNT system using ATR-FTIR spectroscopy.
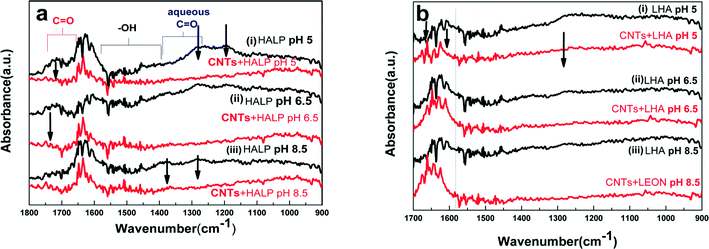
3.2. Interactions of MWCNTs-HALP and MWCNTs-LHA studied by ATR-FTIR spectroscopy
ATR-FTIR spectroscopy is a useful technique for the analysis of organic species adsorbed in the solid–solution interface.30 Although the infrared spectra of pristine MWCNTs are featureless, FTIR spectroscopy using the ATR accessory is very informative for studying the functional groups attached to the sidewalls of the MWCNTs.30,31 The ATR-FTIR spectra for MWCNTs interacting with HALP or LHA are presented in Fig. 3. The pH-dependent features in the ATR-FTIR spectra for the MWCNT–HA interactions were studied at different pH (e.g. pH 8.5, pH 6.5 and 5.0).The ATR-FTIR spectra for HALP (black line) in Fig. 3a show peaks in 1690–1750 cm−1 corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (vC
O (vC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) carboxyl stretches.31 The peaks in 1400–1650 cm−1 correspond to the asymmetric stretching frequencies for aqueous carboxylates while those corresponding to the symmetric stretch are detected in 1300–1420 cm−1. Signals at 1400–1600 cm−1 are assigned to phenyl ring stretches.20 The band at 1155 cm−1 is ascribed to the C–O stretches or O–H deformations of the C–OH groups.31 The ATR-FTIR spectra for LHA (black line) in Fig. 3b show the same features as for HALP, but with inferior resolution of the spectral lines. This is attributed to the less-homogeneous structure of LHA vs. HALP. Upon interaction of MWCNTs with HALP and LHA, severe changes are observed in the ATR-FTIR spectra (red lines in Fig. 3a and b). The interaction of MWCNTs with HALP affects the carboxylate peaks which are significantly diminished in the presence of MWCNTs. Moreover, the carboxylate vibrations at 1622 cm−1 shift to 1634 cm−1. Thus, the ATR-FTIR spectra provide direct evidence of strong interaction of the MWCNTs with the carboxylates of HALP or LHA. The carbonyl band at 1720 cm−1 disappeared, indicating a specific interaction between MWCNTs and the carbonyl O.
O) carboxyl stretches.31 The peaks in 1400–1650 cm−1 correspond to the asymmetric stretching frequencies for aqueous carboxylates while those corresponding to the symmetric stretch are detected in 1300–1420 cm−1. Signals at 1400–1600 cm−1 are assigned to phenyl ring stretches.20 The band at 1155 cm−1 is ascribed to the C–O stretches or O–H deformations of the C–OH groups.31 The ATR-FTIR spectra for LHA (black line) in Fig. 3b show the same features as for HALP, but with inferior resolution of the spectral lines. This is attributed to the less-homogeneous structure of LHA vs. HALP. Upon interaction of MWCNTs with HALP and LHA, severe changes are observed in the ATR-FTIR spectra (red lines in Fig. 3a and b). The interaction of MWCNTs with HALP affects the carboxylate peaks which are significantly diminished in the presence of MWCNTs. Moreover, the carboxylate vibrations at 1622 cm−1 shift to 1634 cm−1. Thus, the ATR-FTIR spectra provide direct evidence of strong interaction of the MWCNTs with the carboxylates of HALP or LHA. The carbonyl band at 1720 cm−1 disappeared, indicating a specific interaction between MWCNTs and the carbonyl O.
The ATR-FTIR spectrum of MWCNTS-LHA is similar to that of MWCNTS-HALP (Fig. 3b). More specifically, in LHA, the peak at 1643 cm−1 is replaced by two peaks shifted to higher wavenumbers (by Δv ~ 5–10 cm−1) indicative of MWCNT interactions with carbonyl-O groups. The enhanced peak at ~1056 cm−1 is due to C–O stretches. Various previous studies have provided evidence that that the carboxyl groups of HA – as well as phenolic and aromatic groups – strongly interact with CNTs.15,32 Overall, the present data in accordance with the literature, are consistent with a structural picture where the COO and R–OH groups of HALP and LHA interact with the sidewalls of MWCNTs, forming a stable embodiment [HA + MWCNT]. This [HA + MWCNT] embodiment bears a pH-dependent charge i.e. due to the chargeable carboxy/phenolics of HAs14,15 and this determines the observed significant effect of pH on the debundling and solubilization of MWCNTs.
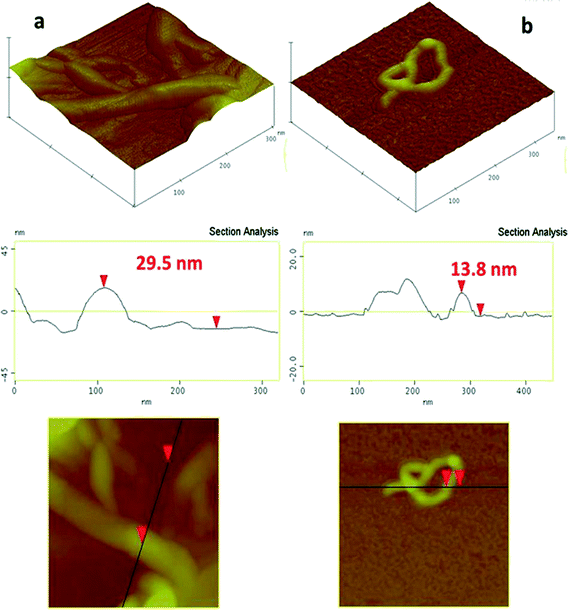
3.3. Atomic Force Microscopy (AFM)
The AFM images of MWCNTs and MWCNTs + HALP deposited on Si-wafer (Fig. 4) allow a comparison of the morphological features of MWCNTs at the nanoscale, before (Fig. 4a) and after (Fig. 4b) the interaction with HA in aqueous solution. A typical AFM image of MWCNTs in the absence of HA (Fig. 4a) shows the aggregated structure of MWCNTs, i.e. despite the sonication treatment, MWCNTS retained a bundled/aggregated form, in agreement with the DLS data. In the HALP + MWCNTs, well-dispersed nanotubes are easily observed, which have a monodisperse structure (Fig. 4b) with an average diameter of 13–14 nm. As seen in Fig. 4b, the configuration of these MWCNTs might be twisted/curled, i.e. not an ideal straight line-shaped tube. The effective diameter of this conformation corresponds to the hydrodynamic diameter detected by DLS.Overall, according to the AFM images and DLS data, the dispersions of MWCNTs in H2O form aggregates, which in the presence of HAs “debundle”, even under low-power sonication, forming monodisperse CNTs in 85–90% of the studied AFM images.
3.5. Genotoxic and cytotoxic effects by MWCNTs, LHA and their mixture
The results obtained from human peripheral blood lymphocyte cultures treated with different concentrations of MWCNTs, LHA, their mixture and MMC are shown in Table 1. The data in Table 1 reveal that MWCNTs and LHA, were separately able to induce a minor increase in MN frequencies at all tested concentrations vs. the control. The MWCNTs induced a statistically significant increase (p < 0.01 and p < 0.05) in MN frequencies at concentrations 5 and 15 μg ml−1, respectively. In the case of mixed [MWCNTs + LHA] treatments, our results showed a remarkable three to five-fold increase in MN frequencies vs. the control. More specifically, MWCNTs + LHA induced statistically significant differences (p < 0.001) on MN frequencies vs. the control.The cytotoxic effect was evaluated via the determination of CBPI for MWCNTs, LHA and their mixture. Regarding the cytotoxic index in all tested concentrations, no statistically significant differences were observed between the control and treated cultures. The negative (5.25 ± 0.48‰) and positive (54.75 ± 4.13‰) control frequencies of MN found in our experiments are in accordance with the published values in the used assay.33
The MNd/CNd size ratio of MN in the in vitro CBMN assay is an alerting index i.e. as effective as the Fluorescence in situ Hybridization (FISH) analysis, for the discrimination of clastogenic and aneugenic effects.27,28,34–36
Here, the data on the MNd/CNd size ratio of MN (‰) induced by MWCNTs and their mixtures with LHA are presented in the ESI† (Fig. S1). A statistically significant increase in both small- and large-sized-MN frequencies was observed in all tested mixtures, except for the case of the large-sized MN in the MWCNTs + LHA (5 + 20 μg ml−1) treatment. Moreover, a statistically significant increase in large-sized-MN frequencies was observed in the MWCNTs-treatment (15 μg ml−1).
3.6. Genotoxic and cytotoxic effects by MWCNTs, HALP and their mixture
The results shown in Table 2 reveal that there are no statistically significant differences between the control and MWCNTs- or HALP-treated cultures. In the case of mixed MWCNTs + HALP treatments, an approximately three-fold increase in MN frequencies vs. the control was found. MWCNTs + HALP mixtures induced a statistically significant increase (p < 0.001) in MN frequencies at all tested concentrations vs. the control.Regarding the CBPI index, to evaluate the cytotoxic effect, no statistically significant differences were observed between the control and [MWCNTs/HALP and their mixtures] treated cultures. The reported negative (5.0 ± 0.0‰) and positive (57.0 ± 6.0‰) control frequencies of MN in our experiments are in accordance to published values in a similar assay.33
Regarding the MNd/CNd size ratio of MN (‰) induced by MWCNTs e.g. separately or in combination with HALP, there was a significant increase in both small- and large-sized-MN frequencies at the higher tested concentrations of MWCNTs + HALP mixtures (25 + 42, 30 + 50 μg ml−1, respectively). In the cases of lower concentrations of MWCNTs + HALP mixtures, a statistically significant increase was observed in the small- (5 + 8 μg ml−1) and in large (15 + 25 μg ml−1)-sized-MN frequencies, respectively (see the ESI,† Fig. S2).
4. Discussion
4.1. Significant enhancement of the genotoxic effect by MWCNTs + HA
The present data reveal that natural HA (LHA) resulted in more severe MN enhancement than the synthetic HALP. Since the structural characteristics of HALP are similar to that of LHA, the additional MN increase can be attributed to the adverse effects of heteroatoms, most likely Fe,16 present in natural LHA.Previous studies on the genotoxicity of MWCNTs have been reported, but only for concentrations much higher37 than those used herein. The statistically significant genotoxic induction at MWCNT concentrations of 5 and 15 μg ml−1 as well as the increased MN frequency at 25 μg ml−1 (Table 1) corroborate previous reports with regard to the genotoxic action of CNTs. More precisely, Lindberg et al.38 examined the potential genotoxic effects of carbon nanotubes (CNTs; >50% single-walled, ∼40% other CNTs) in cultured human bronchial epithelial cells (BEAS 2B) for 24, 48 and 72 h with various doses (3.8–380 μg ml−1), using the single cell gel electrophoresis (comet) assay and the micronucleus (MN) assay. In the comet assay, CNTs induced a dose-dependent increase in DNA damage at all treatment times, with a statistically significant effect starting at the lowest dose tested. In the MN assay, no increase in MN frequencies was observed with CNTs after the 24 h and 72 h treatments. The 48 h treatment caused a significant increase in MN frequencies at three doses (lowest 38 μg ml−1) of CNTs. No dose-dependent effects were seen in the MN assay.
Similarly, Ghosh et al.39 demonstrated the genotoxic effect of MWCNTs, using – among others – the comet assay in human lymphocytes. A significant genotoxic response was observed at the concentration of 2 μg ml−1, followed by a gradual decrease at the higher concentrations tested and that may be due to the formation of crosslinks or the agglomeration of MWCNTs.39 The findings of this study39 are consistent with our present results with regard to the genotoxic induction observed in MWCNTs at the concentrations of 5 and 15 μg ml−1, but not at the high concentration of 25 μg ml−1 (Table 1). Taking into account the DLS data, we suggest that the observed decrease at the higher concentrations may be due to the formation or agglomeration of MWCNTs, thus inhibiting their genotoxic action.
A recent study of Tavares et al.40 evaluated, among others, the potential genotoxic effects of six different types of MWCNTs using the CBMN assay on human lymphocytes and clearly indicated MN induction without a dose–effect relationship, in the case of two of the six MWCNTs examined. As commented by Tavares et al.,40 the observed differences in genotoxicity among closely related MWCNTs may be explained not by the morphology and size of MWCNTs but rather by the agglomeration process in correlation with the tested concentrations.
Our results on the negative genotoxic responses of HAs (Tables 1 and 2) are supported by the research of Ferrara et al.41 who applied the MN assay in human lymphoblastoid cell line (TK6) in order to evaluate the genotoxicity of HAs. The findings reported by Ferrara et al.41 revealed the absence of genotoxic effect in the examined HA samples.
| Concentration (μg ml−1) | MN MF (‰) ± se | CBPI MF (‰) ± se |
|---|---|---|
| MN, micronuclei; CBPI, Cytokinesis Block Proliferation Index; MF (‰) ± se, mean frequencies (‰) ± standard error; MWCNTs, multi-walled carbon nanotubes; HALP, humic-acid-like polycondensates; MMC, Mitomycin-C; 2000 binucleated cells scored per experimental point.a p < 0.01.b p < 0.001 [G-test for MN; χ2 for CBPI]. | ||
| 0 | 5.0 ± 0.0 | 1.84 ± 0.01 |
| MWCNTs | ||
| 5 | 9.5 ± 0.5 | 1.84 ± 0.01 |
| 15 | 9.5 ± 0.5 | 1.83 ± 0.04 |
| 25 | 8.5 ± 0.5 | 1.83 ± 0.01 |
| 30 | 8.5 ± 0.5 | 1.84 ± 0.01 |
| HALP | ||
| 8 | 5.0 ± 0.0 | 1.83 ± 0.01 |
| 25 | 5.5 ± 0.5 | 1.90 ± 0.06 |
| 42 | 6.5 ± 0.5 | 1.86 ± 0.02 |
| 50 | 6.5 ± 0.5 | 1.84 ± 0.03 |
| MWCNTs + HALP | ||
| 5 + 8 | 13.5 ± 0.5a | 1.84 ± 0.02 |
| 15 + 25 | 14.5 ± 0.5b | 1.86 ± 0.03 |
| 25 + 42 | 15.0 ± 1.0b | 1.88 ± 0.02 |
| 30 + 50 | 17.0 ± 0.0b | 1.90 ± 0.01 |
| MMC | ||
| 0.05 | 57.0 ± 6.0b | 1.67 ± 0.02b |
In order to further assess the mechanism of the genotoxicity action of MWCNTs and their mixtures with LHA and HALP, we analyzed the size distribution of induced MN. In general, the tested mixtures that induce MN may do so because they induce chromosome breakage (clastogenic effect), a chromosome loss (aneugenic effect), or a combination of the two. The MN size ratio in the CBMN assay is an alerting index i.e. as effective as the Fluorescence in situ Hybridization (FISH) analysis for the discrimination of clastogenic and aneugenic effects.27,28,34–36 As can be seen in the ESI† (Fig. S1 and S2), the tested mixtures of MWCNTs with HA or HALP induced a statistically significant increase in both small- and large-sized MN. According to the latter, the large MN observed in MWCNTs and their mixtures with LHA and HALP-treated lymphocytes might contain whole chromosomes, thus revealing an evidence of the tested mixtures' aneugenic potency, while the presence of small MN is more likely to contain acentric chromosome fragments, indicating its clastogenic effect. This shows that the genotoxicity of MWCNT-HA is the result of clastogenic as well as aneugenic events. This observation is corroborated by the study of Cveticanin et al.,42 which connects the formation of MN in human lymphocytes with the clastogenic as well as the aneugenic activities of MWCNTs. Also, previous studies in human epithelial cell line (MCF-7) suggested that MWCNTs can induce MN by both clastogenic and aneugenic mechanisms.43
4.2. Absence of cytotoxic effect
The CBPI index showed no statistically significant differences in the CBPI values which were observed between the control and either MWCNTs or MWCNTs + HA treated cultures. Our results are in accordance with the recent findings of Tavares et al.40 which states that CBPI was not significantly affected by any of the six different examined MWCNTs.Kihara et al., demonstrated that HA from Indonesia induced cytotoxicity at concentrations greater than 50 μg ml−1 on human vascular endothelial cells.44 Our observations on the cytotoxicity pattern of the tested HA (IHSS standard LHA and synthetic HALP) indicate that they are not cytotoxic at all tested concentrations in cultured human lymphocytes. The observed differences in the cytotoxicity patterns of the present tested HAs vs. that of Kihara et al.44 could be attributed to their different physicochemical characteristics e.g. metal content or structural profile of the macromolecules, which reflect the differences from one ecosystem type to another.
The concentrations of MWCNTs used in the present study are comparable to the concentrations used in several other studies. Lacerda et al. injected 300 μg of MWCNTs per rat, which translates to a concentration of 20 μg ml−1 (average rat blood volume 15 ml and 200 g of body weight).45 Deng et al. injected 10 μg of MWCNTs per mouse, which translates to a concentration of 10 μg ml−1 (average mouse blood volume 1 ml and 20 g of body weight).46 These animal imaging studies investigated the systemic administration of functionalized and radiolabeled MWCNTs. Recent studies on human cells investigated the effects of MWCNTs on human microvascular endothelial cells at a final concentration of 2.5 μg ml−1 (ref. 47) as well as on human lymphocytes at final treatment concentrations of 1 up to 10 μg ml−1 (ref. 39) and of 1 up to 300 μg ml−1.40
Our rationale for the selection of the low MWCNTs concentrations is also supported by a very recent study which investigated the application of nanobiotechnology to crop-science/agriculture and established the term “nanoagriculture” as a recent development. Tiwari et al. demonstrated that pristine MWCNTs at low concentrations (20 mg l−1) benefit the growth of maize seedlings by enhancing water and nutrient transport, and biomass.13 These findings suggest a potential for the utilization of CNTs for optimizing water transport in arid-zone agriculture and of improving crop biomass yields.
4.3. A physicochemical mechanism
Our ATR-FTIR study shows that HA solubilizes the MWCNT via specific interactions of the COO groups with the CNT surface. DLS provides quantitative data on “debundling” of nanotube aggregates in the presence of HALP. This observation correlates with the observed three to five-fold increase MN-induction by MWCNTs + LHA and MWCNTs + HALP. The straightforward implication of these data is that the formed LHA/HALP-MWCNT embodiments have the potential to penetrate not only the cell membrane but also the nuclear membrane, inducing MN. Here, it is important to notice that despite the partial debundling effect of the cell-culture medium i.e. in the absence of HALP, this is not enough to trigger the MN formation. On the other hand, HALP appears to play a critical role in MWCNTs' debundling and MN induction. This reveals that HALP has a multiple effect that is effectively shuttling MWCNTs into the nucleus, in addition to the debundling effect in solution.Despite the fact that the cellular uptake of CNTs and its underlying mechanisms remain largely unclear,10 it is tempting to comment that association of CNTs with specific proteins may alter their pharmacokinetic and pharmacodynamics behavior48 as well as their genotoxic and/or cytotoxic activity.49 In simulated cell culture conditions, MWCNTs are found to bind the highest number of proteins (133) compared to unmodified nanotubes (<100), suggesting covalent binding to protein amines.48 In addition, Pacurari et al. demonstrated that MWCNTs lead to an increase in cell permeability in human microvascular endothelial cells.47 A recent report suggested that exposure to electromagnetic waves promotes CNT entry not only into the cytoplasm of cells, but also into the nucleus.50
Our present findings suggest a similarity between the modes of action of humic macromolecules with analogous biomacromolecules/proteins. The macromolecule acts as shuttle-like agent to enhance the permeability of [CNT/organic] into the internal cellular compartments. Here, we provide the first evidence that via this mechanism, environmental factors –humics – combined with CNTs, may induce direct genotoxic effects.
5. Conclusion
The present study revealed a statistically significant induction of MN frequencies in cultured human lymphocytes treated with mixtures of MWCNTs + HAs. A comparative analysis of the genotoxicity and cytotoxicity in the tested concentrations reveals that the MWCNTs + LHA mixture is more genotoxic and slightly more cytotoxic than the MWCNTs + HALP mixture. The MN induction observed herein in human lymphocytes reveals the ability of [MWCNTs + LHA or HALP] mixtures to enhance genotoxic effects, while there was a first evidence of the potential genotoxic effects as the result of both clastogenic and aneugenic actions of the particular mixtures on human lymphocytes.Taking into account that the examined concentrations were low, MWCNTs should be handled with great care, in order to minimize its environmental and human risk. From that point of view, their potential impact to the environment, the organisms and human health must be further investigated and confirmed.
Ethics statement
The study was approved by the Ethics Committee of the University of Patras. After informed consent, two healthy, non-smoking males (20 and 25 years old) were used as blood donors to establish whole blood lymphocyte cultures. According to the donors' declarations, they had not been exposed to radiation, drug treatment or any viral infection in the recent past.Declaration of interest
The authors report no conflicts of interest.References
- M. Ema, T. Imamura, H. Suzuki, N. Kobayashi, M. Naya and J. Nakanishi, Regul. Toxicol. Pharmacol., 2012, 63, 188–195 CrossRef CAS PubMed.
- M. F. L. De Volder, S. H. Tawfick, R. H. Baughman and A. J. Hart, Science, 2013, 339, 535–539 CrossRef CAS PubMed.
- L. Braydich-Stolle, S. Hussain, J. J. Schlager and M. C. Hofmann, Toxicol. Sci., 2011, 88, 412–419 CrossRef PubMed.
- S. M. Hussain, K. L. Hess, J. M. Gearhart, K. T. Geiss and J. J. Schlager, Toxicol. In Vitro, 2005, 19, 975–983 CrossRef CAS PubMed.
- IARC, Monographs on the evaluation of carcinogenic risk to chemicals on man, Asbestos, 1977, 14, 1–106 Search PubMed.
- J. S. Kim, K. Lee, Y. H. Lee, H. S. Cho, K. H. Kim, K. H. Choi, S. H. Lee, K. S. Song, C. S. Kang and I. J. Yu, Arch. Toxicol., 2011, 85, 775–786 CrossRef CAS PubMed.
- R. H. Hurt, M. Monthioux and A. Kane, Carbon, 2006, 44, 1028–1033 CrossRef CAS.
- A. M. Schrand, J. Johnson, L. Dai, S. M. Hussain, J. J. Schlager, L. Zhu, Y. Hong and E. Osawa, in Safety of Nanoparticles, Nanostructure Science and Technology, ed. T. J. Webster, Springer Science+Business Media, New York, 2009, ch. 8, pp. 159–187 Search PubMed.
- S. Toyokuni, Adv. Drug Delivery Rev., 2013, 65, 2098–2110 CrossRef CAS PubMed.
- N. Saito, H. Haniu, Y. Usui, K. Aoki, K. Hara, S. Takanashi, M. Shimizu, N. Narita, M. Okamoto, S. Kobayashi, H. Nomura, H. Kato, N. Nishimura, S. Taruta and M. Endo, Chem. Rev., 2014, 114, 6040–6079 CrossRef CAS PubMed and references therein.
- UWE (University of the West of England), Science Communication Unit, Bristol, 2013, Science for Environment Policy In-depth Report: Soil Contamination: Impacts on Human Health. Report produced for the European Commission DG Environment, http://ec.europa.eu/environment/integration/research/newsalert/pdf/IR5.pdf, (accessed August 2014).
- IATP (Institute for Agriculture and Trade Policy), Suppan S. 2013. Nanomaterials In Soil. Our Future Food Chain?, http://www.iatp.org/files/2013_04_23_Nanotech_SS.pdf (accessed June 2015) Search PubMed.
- D. K. Tiwari, N. Dasgupta-Schubert, L. M. Villasenor Cendejas, J. Villegas, L. Carreto Montoya and S. E. Borjas Garcia, Appl. Nanosci., 2014, 4, 577–591 CrossRef CAS.
- B. Pan and B. S. Xing, Environ. Sci. Technol., 2008, 42, 9005–9013 CrossRef CAS PubMed.
- X. L. Wang, S. Tao and B. S. Xing, Environ. Sci. Technol., 2009, 43, 6214–6219 CrossRef CAS PubMed.
- N. Senesi and E. Loffredo, in Soil Physical Chemistry, ed. D. L. Sparks, 2nd edn, CRC Press, Boca Raton FL, 1999, pp. 239–370 Search PubMed.
- F. J. Stevenson, in Humus Chemistry: Genesis, Composition, Reactions, 2nd edn., Wiley & Sons Inc., Canada, 1994 Search PubMed.
- B. Vigneault, A. Percot, M. Lafleur and P. G. C. Campbell, Environ. Sci. Technol., 2000, 34, 3907–3913 CrossRef CAS.
- L. M. Ojwang and R. L. Cook, Environ. Sci. Technol., 2013, 47, 8280–8287 CAS.
- E. Giannakopoulos, M. Drosos and Y. Deligiannakis, J. Colloid Interface Sci., 2009, 336, 59–66 CrossRef CAS PubMed.
- D. Ziech, R. Franco, A. Pappa, V. Malamou-Mitsi, S. Georgakila, A. G. Georgakilas and M. I. Panayiotidis, Chem.-Biol. Interact., 2010, 188, 340–349 CrossRef CAS PubMed.
- S. Bonassi, R. El-Zein, C. Bolognesi and M. Fenech, Mutagenesis, 2011, 26, 93–100 CrossRef CAS PubMed.
- OECD, Test No. 487: In Vitro Mammalian Cell Micronucleus Test, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, 2014, DOI:10.1787/9789264224438-en.
- V. Georgakilas, A. Bourlinos, D. Gournis, T. Tsoufis, C. Trapalis, A. Mateo-Alonso and M. Prato, J. Am. Chem. Soc., 2008, 130, 8733–8740 CrossRef CAS PubMed.
- M. Kirsch-Volders, T. Sofuni, M. Aardema, S. Albertini, D. Eastmond, M. Fenech, M. Ishidate Jr., S. Kirchner, E. Lorge, T. Morita, H. Norppa, J. Surralles, A. Vanhauwaert and A. Wakata, Mutat. Res., 2003, 540, 153–163 CAS.
- M. Fenech, W. P. Chang, M. Kirsch-Volders, N. Holland, S. Bonassi and E. Zeiger, Mutat. Res., 2003, 534, 65–75 CAS.
- P. Papapaulou, D. Vlastos, G. Stephanou and N. A. Demopoulos, Fresenius Environ. Bull., 2001, 10, 421–437 Search PubMed.
- K. Hashimoto, Y. Nakajima, S. Matsumura and F. Chatani, Toxicol. In Vitro, 2010, 24, 208–216 CrossRef CAS PubMed.
- S. Ghosh, H. Mashayekhi, B. Pan, P. Bhowmik and B. Xing, Langmuir, 2008, 24, 12385–12391 CrossRef CAS PubMed.
- L. D. Tickanen, M. I. Tejedor-Tejedor and M. A. Anderson, Langmuir, 1991, 7, 451–456 CrossRef CAS.
- M. B. Hay and S. C. B. Myneni, Geochim. Cosmochim. Acta, 2007, 71, 3518–3532 CrossRef CAS.
- D. Lin and B. Xing, Environ. Sci. Technol., 2008, 42, 7254–7259 CrossRef CAS PubMed.
- G. Clare, G. Lorenzon, L. Akhurst, D. Marzin, J. van Delft, R. Montero, A. Botta, A. Bertens, S. Cinelli, V. Thybaud and E. Lorge, Mutat. Res., 2006, 607, 37–60 Search PubMed.
- D. Vlastos, C. G. Skoutelis, I. T. Theodoridis, D. R. Stapleton and M. I. Papadaki, J. Hazard. Mater., 2010, 177, 892–898 CrossRef CAS PubMed.
- C. G. Skoutelis, D. Vlastos, M. C. Kortsinidou, I. T. Theodoridis and M. I. Papadaki, J. Hazard. Mater., 2011, 197, 137–143 CrossRef CAS PubMed.
- E. Toufexi, V. Tsarpali, I. Efthimiou, M.-S. Vidali, D. Vlastos and S. Dailianis, J. Hazard. Mater., 2013, 260, 593–601 CrossRef CAS PubMed.
- L. Gonzalez, B. J. S. Sanderson and M. Kirsch-Volders, Mutagenesis, 2011, 26, 185–191 CrossRef CAS PubMed.
- H. K. Lindberg, G. C. M. Falck, S. Suhonen, M. Vippola, E. Vanhala, J. Catalán, K. Savolainen and H. Norppa, Toxicol. Lett., 2009, 186, 166–173 CrossRef CAS PubMed.
- M. Ghosh, A. Chakraborty, M. Bandyopadhyay and A. Mukherjee, J. Hazard. Mater., 2011, 197, 327–336 CrossRef CAS PubMed.
- A. M. Tavares, H. Louro, S. Antunes, S. Quarré, S. Simar, P.-J. De Temmerman, E. Verleysen, J. Mast, K. A. Jensen, H. Norppa, F. Nesslany and M. J. Silva, Toxicol. In Vitro, 2014, 28, 60–69 CrossRef CAS PubMed.
- G. Ferrara, E. Loffredo, N. Senesi and R. Marcos, Mutat. Res., 2006, 603, 27–32 CAS.
- J. Cveticanin, G. Joksic, A. Leskovac, S. Petrovic, A. V. Sobot and O. Neskovic, Nanotechnology, 2010, 21, 015102 CrossRef PubMed.
- I. Muller, I. Decordier, P. Hoet, N. Lombaert, I. Thomassen, F. Huaux, D. Lison and M. Kirsch-Volders, Carcinogenesis, 2008, 29, 427–433 CrossRef PubMed.
- Y. Kihara, Yustiawati, M. Tanaka, S. Gumiri, Ardianor, T. Hosokawa, S. Tanaka, T. Saito and M. Kurasaki, Environ. Toxicol., 2014, 29, 916–925 CrossRef CAS PubMed.
- L. Lacerda, A. Soundararajan, R. Singh, G. Pastorin, K. T. Al-Jamal, J. Turton, P. Frederik, M. A. Herrero, S. L. A. Bao, D. Emfietzoglou, S. Mather, W. T. Phillips, M. Prato, A. Bianco, B. Goins and K. Kostarelos, Adv. Mater., 2008, 20, 225–230 CrossRef CAS.
- X. Deng, G. Jia, H. Wang, H. Sun, X. Wang, S. Yang, T. Wang and Y. Liu, Carbon, 2007, 45, 1419–1424 CrossRef CAS.
- M. Pacurari, Y. Qian, W. Fu, D. Schwegler-Berry, M. Ding, V. Castranova and N. L. Guo, J. Toxicol. Environ. Health, Part A, 2012, 75, 112–128 CrossRef CAS PubMed.
- J. H. Shannahan, J. M. Brown, R. Chen, P. C. Ke, X. Lai, S. Mitra and F. A. Witzmann, Small, 2013, 9, 2171–2181 CrossRef CAS PubMed.
- L. Gonzalez, M. Lukamowicz-Rajska, L. C. J. Thomassen, C. E. A. Kirschhock, L. Leyns, D. Lison, J. A. Martens, A. Elhajouiji and M. Kirsch-Volders, Nanotoxicology, 2014, 8, 876–884 CrossRef CAS PubMed.
- V. Raffa, L. Gherardini, O. Vittorio, G. Bardi, A. Ziaei, T. Pizzorusso, C. Riggio, S. Nitodas, T. Karachalios, K. T. Al-Jamal, K. Kostarelos, M. Costa and A. Cuschieri, Nanomedicine, 2011, 6, 1709–1718 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5en00138b |
| This journal is © The Royal Society of Chemistry 2016 |