Research highlights: laboratory studies of the formation and transformation of atmospheric organic aerosols
Nadine
Borduas†
a and
Vivian S.
Lin
*b
aDepartment of Chemistry, University of Toronto, 80 St George Street, Toronto, Ontario M5S 3H6, Canada
bInstitute of Biogeochemistry and Pollutant Dynamics, ETH Zurich, CH-8092, Zurich, Switzerland. E-mail: vivian.lin@usys.ethz.ch
First published on 6th April 2016
Abstract
Atmospheric particles are emitted from a variety of anthropogenic and natural precursors and have direct impacts on climate, by scattering solar irradiation and nucleating clouds, and on health, by causing oxidative stress in the lungs when inhaled. They may also form from gaseous precursors, creating complex mixtures of organic and inorganic material. The chemical composition and the physical properties of aerosols will evolve during their one-week lifetime which will consequently change their impact on climate and health. The heterogeneity of aerosols is difficult to model and thus atmospheric aerosol research strives to characterize the mechanisms involved in nucleating and transforming particles in the atmosphere. Recent advances in four laboratory studies of aerosol formation and aging are highlighted here.
Introduction
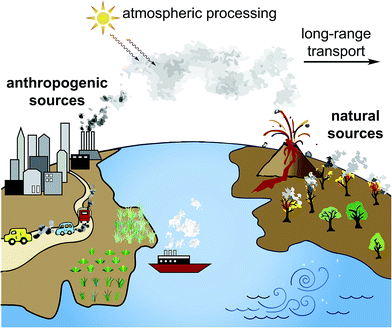
Aerosols are solid or liquid particles suspended in air that have far-reaching impacts on human health as well as influence on local and global climate. They originate from a multitude of anthropogenic sources including agriculture and fossil fuel burning for transportation, electricity, and heat, and biogenic sources such as sea spray, volcanic activity, forest fires, and soil/desert dust (Fig. 1). Primary aerosols are emitted directly into the atmosphere whereas secondary aerosols are formed within the troposphere from precursor gases. A range of minerals, organic compounds, biological material, and water make up the composition of aerosols, causing them to be typically heterogeneous. Aerosols may also have different sizes from nanometers to tens of micrometers and have different shapes depending on their phase and viscosity. This heterogeneity in physical and chemical properties renders it difficult to represent aerosols in models and consequently to predict their impact on climate and health. The characterization of atmospheric aerosols and transformation processes during their lifetime (∼1 week) therefore remains an area of intense investigation (DOI: 10.1021/cr5003485).Aerosols are recognized as important climate forcers since they may scatter solar irradiation and may nucleate clouds, which may result in cooling and warming of the atmosphere (DOI: 10.1021/acs.chemrev.5b00089, DOI: 10.1039/C3EM00178D). However, the mechanisms of their influence on climate remain one of the biggest uncertainties in climate modelling, especially in predicting climate change impacts (DOI: 10.1017/CBO9781107415324). Furthermore, atmospheric processing due to direct photochemistry and transformation by reactive species may alter the chemical and structural composition of organic aerosols, critically altering their ability to act as cloud nuclei and to cause oxidative stress in human lungs (DOI: 10.1029/1999JD900073, DOI: 10.1021/cr5005259, DOI: 10.1021/cr500487s). Efforts to elucidate the behavior of aerosols will assist in model development and reduce uncertainties when considering their influence on a regional and/or global scale.
In this highlight, we focus on four recent publications that seek to better understand the formation and transformation of organic aerosols through laboratory experiments, with emphasis on results that may be extended to the real atmosphere.
How aerosols are formed
Modeling the impact of secondary aerosols on climate and health requires knowing precisely the origin and quantity of precursors necessary for their formation in air. Particles form from individual gas phase molecules colliding together and producing molecular clusters. These clusters then act as a sink for other low-volatility organic compounds and subsequently grow to become nanoparticles. These nanoparticles eventually reach the size to nucleate clouds or/and penetrate lungs to cause oxidative stress. There is growing evidence that different volatile organic compounds (VOCs) have different abilities to nucleate particles, but questions remain as to which ones and from which sources.Field studies in boreal forests have identified and quantified new particle formation events, highlighting the potential ability for biogenic volatile organic compounds (BVOCs) to be good cluster stabilizers in the presence of low sulfuric acid concentrations. Recently, Dal Maso et al. conducted studies at the Jülich plant–atmosphere simulation chamber (DOI: 10.5194/acp-9-4387-2009) to quantify nanoparticle formation rates from BVOCs (DOI: 10.5194/acp-16-1955-2016). Specifically, monoterpenes were measured using a combination of proton transfer reaction mass spectrometry (PTR-MS) and gas chromatography mass spectrometry (GC-MS), while a chemical ionization mass spectrometer (CIMS) was used for sulfuric acid measurements. The researchers employed a set of particle counting instruments to measure particles from three different size classes, as large as 600 nm down to 1 nm, necessary to observe the formation of nanoparticles.
The key experimental parameters in this study were the use of BVOCs from real spruce, pine and birch branches, and sulfuric acid, all at realistic atmospheric concentrations. The authors found a correlation between nanoparticle formation rates (Jd*) and the product of the inflow rate of BVOCs (not the BVOC concentration) and the sulfuric acid concentration (QBVOC-inflow[H2SO4]), as shown in Fig. 2. This linear correlation suggests a mechanism in which BVOC oxidation products are rapidly generated and subsequently lead to particle formation in the presence of more than 2 × 106 molecules per cm3 of sulfuric acid. Oxidized BVOCs, possibly from the ozonolysis of monoterpenes, are presented as a plausible mechanism for generating good cluster stabilizers with realistically low sulfuric acid concentrations. The quality measurements presented by Dal Maso et al. support the role of BVOC oxidation products as important precursors to nanoparticle formation in a boreal forest environment.
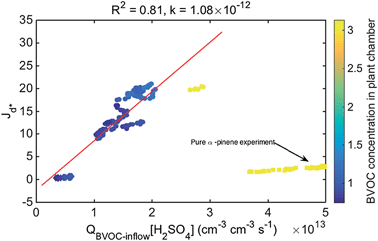 | ||
| Fig. 2 Particle formation rates (J) as function of the product of source rate of the BVOC precursor QBVOC-inflow and sulfuric acid concentration. Marker colors indicate the corresponding monoterpene concentration from the inlet flow of the reaction chamber. Figure reproduced from M. Dal Maso, L. Liao, J. Wildt, A. Kiendler-Scharr, E. Kleist, R. Tillmann, M. Sipilä, J. Hakala, K. Lehtipalo, M. Ehn, V.-M. Kerminen, M. Kulmala, D. Worsnop and T. Mentel, Atmos. Chem. Phys., 2016, 16, 1955–1970 (DOI: 10.5194/acp-16-1955-2016). | ||
Importance of phase and transformation
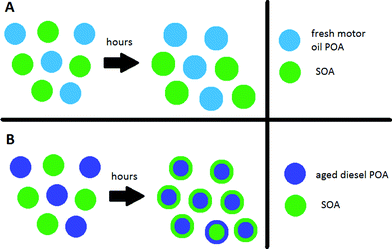
Particles, once emitted or formed in the atmosphere, are expected to have a tropospheric lifetime of approximately one week. During this time, the particles will undergo a variety of physical and chemical changes through atmospheric processing, thereby impacting the particles' ability to nucleate clouds as well as their health effects if inhaled. Atmospheric processing of aerosols includes phase transitions, photochemical transformations, solar irradiation, and water uptake/loss, among others. The use of laboratory generated aerosols with simple compositions aids in understanding the mechanisms of these processes but extending their relevance to ambient air can be more difficult. The next three publications have developed innovative laboratory methods to obtain meaningful conclusions on the mixing state and viscosity of particles in ambient air.Particles may exist as an externally mixed population, where co-existing particles of different sources have different compositions, or as an internally mixed population, where co-existing particles have the same composition regardless of their precursors (Fig. 3). Understanding particle mixing states and gas phase exchange between particles is important for source apportionment in urban environments but also for modelling their impact on climate and air quality. Robinson et al. recently used single particle measurements obtained by an aerosol mass spectrometer to investigate the mixing states of primary organic aerosol (POA) from motor oil and diesel exhaust and from secondary organic aerosol (SOA) made by α-pinene ozonolysis (DOI: 10.1039/C5FD00214A). They measured the timescales and the extent to which different types of fresh POA mix with each other and the extent to which oxidized POA mix thermodynamically with SOA via gas phase exchange of semi-volatiles. The authors conclude that particles with miscible volatiles will form an internally mixed population on shorter time scales, like diesel exhaust POA and squalene POA. Depending on the volatility and miscibility of the organic aerosol, it will take minutes to hours for externally mixed freshly emitted aerosol populations to converge via gas phase exchanges (Fig. 3). Photodegradation and oxidation can suppress this miscibility barrier, affecting the viscosity of particles.
Viscosity is an important physical property of aerosols and dictates the ability of oxidants, organics and/or water to diffuse into the particle bulk and further react to modify a particle's physical and chemical properties. Atmospheric models tend to assume that organic aerosols are low viscosity liquids in which species can partition under thermodynamic control. However, recent studies have shown that a range of viscosities from solid to semi-solid to liquid exists in the atmosphere, consequently impacting kinetics and thermodynamics of organic aerosols. Two recent publications by Hinks et al. and Hosny et al. provide further evidence for the importance of taking viscosity measurements into account when modelling the fate of atmospheric aerosols.
Exposure to sunlight irradiation and atmospheric oxidants alters the chemical composition of aerosols. These processes photodegrade the molecules present in the aerosol at rates dependent on composition, phase, humidity, etc. Hinks et al. examined the effect of particle viscosity on the degradation of 2,4-dinitrophenol (2,4-DNP), a toxin and a pollutant, in a secondary organic material (SOM) matrix (DOI: 10.1039/C5CP05226B). After exposing limonene and α-pinene SOM material to varying relative humidity (RH) and temperature, the particles were collected on filters and their viscosity analyzed using the poke-flow technique. This technique uses a needle to poke the half-sphere surface of the droplet, and the time to regenerate the original droplet shape translates to a measure of viscosity. Hinks et al. determined that pinene SOM was more viscous than its limonene counterpart, presumably due to the rigid molecular structure of the former. These results are qualitatively consistent with larger apparent activation energies for the photodegradation of 2,4-DNP in pinene SOM versus limonene SOM. Their experimental results reveal that photodegradation rates of photolabile, organic molecules such as 2,4-DNP are slower at lower temperatures and lower RH (Fig. 4). The rheological properties of the organic aerosol therefore slow down photochemical processes in SOM material by limiting the motion of the molecules. At higher RH, water molecules soften the SOM matrix and facilitate faster diffusion of the photoexcited 2,4-DNP molecules to their reaction partners. Experiments where limonene SOA material was allowed to mix with ammonia generated brown carbon, particles which absorb in the actinic region. This work demonstrated that photochemical reactions can potentially destroy or create light-absorbing species in brown carbon, suggesting that brown carbon's absorptive properties can be changed photochemically on atmospherically relevant time scales.
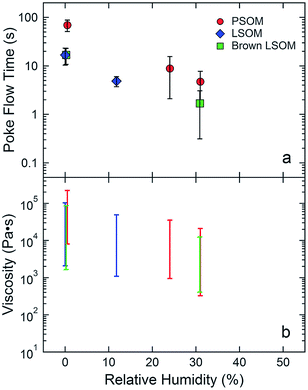 | ||
| Fig. 4 (a) Average experimental flow time from the poke-flow experiments as a function of relative humidity. (b) Simulated ranges of viscosities based on the poke-flow results. Figure reproduced from M. L. Hinks, M. V. Brady, H. Lignell, M. Song, J. W. Grayson, A. K. Bertram, P. Lin, A. Laskin, J. Laskin and S. A. Nizkorodov, Phys. Chem. Chem. Phys., 2016, 18, 8785–8793 (DOI: 10.1039/C5CP05226B) with permission from the PCCP Owner Societies. | ||
Hosny et al. used synthetic dyes, termed molecular rotors, to quantify organic aerosol viscosity using fluorescence lifetime imaging (FLIM) (DOI: 10.1039/C5SC02959G). This imaging technique allows for real time and spatially resolved analyses of aerosol changes inside an environmental chamber mounted on a microscope stage. This method also avoids sample preparation manipulations since the SOA is impacted on filters and directly analyzed.
SOA generated from the oxidation of myrcene showed decreasing viscosity with increasing RH exposure (Fig. 5a). During a fast increase in RH from 0–98%, the viscosity at the particle surface declines rapidly and a diffusion front forms as the highly viscous aerosol is hydrated, giving the particle a transitional core–shell morphology. In addition, atmospheric aging through exposure to ozone increases the viscosity of oleic acid aerosol. The study shows that the kinetics of the ozonolysis are dependent on particle size, suggesting surface limitations to ozone uptake. Hosny et al.'s measurements visually demonstrate that atmospheric chemical aging processes such as ozone exposure can increase the viscosity of particles. In turn, this may slow heterogeneous reaction kinetics. Hosny et al.'s unique viscosity probe technique allows real-time visualization of sub-micrometer scale changes of organic aerosol particles, spanning over four orders of magnitude.
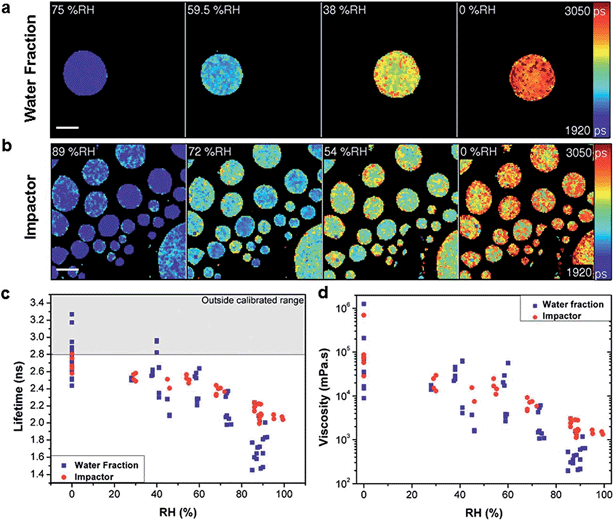 | ||
| Fig. 5 Measuring viscosity of oxidised myrcene aerosol during RH change. Fluorescence lifetime images of (a) water extracted fraction and (b) impactor collected samples at decreasing RH (scale bar 40 μm). Images show the increase of fluorescence lifetime with decreasing RH. (c) Averaged fluorescence lifetimes of individual aerosol particles that were either water extracted (blue) or impactor collected (red), measured at various RHs. (d) Lifetimes converted into viscosity. The lifetime values above the threshold in (c) (grey shaded area) are outside the calibrated range for the molecular rotor and were not converted in (d). Figure and caption reproduced from N. A. Hosny, C. Fitzgerald, A. Vyšniauskas, A. Athanasiadis, T. Berkemeier, N. Uygur, U. Pöschl, M. Shiraiwa, M. Kalberer, F. D. Pope and M. K. Kuimova, Chem. Sci., 2016, 7, 1357–1367 (DOI: 10.1039/C5SC02959G) with permission from the Royal Society of Chemistry. | ||
Concluding remarks
Aerosols are complex mixtures of inorganic and organic compounds emitted into the atmosphere from anthropogenic and natural sources. They play crucial roles in the climate by scattering solar radiation and by nucleating clouds, which in turn can also scatter solar radiation or trap the Earth's radiative heat. Aerosols smaller than 2.5 microns may also have serious health effects and have been linked to morbidity and mortality. All these effects, however, depend on the physical and chemical properties of aerosols, and how they are formed as well as how they are transformed in the atmosphere. The four articles covered in this highlight have very recently contributed to our further understanding of formation and transformation of aerosols using innovative laboratory techniques. These articles also provide quantitative data on aerosol properties relevant to aerosol modelling, towards a better representation of their impacts on climate and health.Footnote |
| † Current address: Natural Resources and the Environment Unit, Council of Scientific and Industrial Research, Meiring Naude Road, Pretoria, 0001, South Africa. |
| This journal is © The Royal Society of Chemistry 2016 |