 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A passive dosing method to determine fugacity capacities and partitioning properties of leaves†
Damien Johann
Bolinius
*a,
Matthew
MacLeod
a,
Michael S.
McLachlan
a,
Philipp
Mayer
b and
Annika
Jahnke
c
aDepartment of Environmental Science and Analytical Chemistry (ACES), Stockholm University, SE-114 18 Stockholm, Sweden. E-mail: damien.bolinius@aces.su.se
bDepartment of Environmental Engineering, Technical University of Denmark, Bygningstorvet B 115, DK-2800 Kongens Lyngby, Denmark
cDepartment Cell Toxicology, Helmholtz Centre for Environmental Research (UFZ), Permoserstr. 15, DE-04318 Leipzig, Germany
First published on 15th September 2016
Abstract
The capacity of leaves to take up chemicals from the atmosphere and water influences how contaminants are transferred into food webs and soil. We provide a proof of concept of a passive dosing method to measure leaf/polydimethylsiloxane partition ratios (Kleaf/PDMS) for intact leaves, using polychlorinated biphenyls (PCBs) as model chemicals. Rhododendron leaves held in contact with PCB-loaded PDMS reached between 76 and 99% of equilibrium within 4 days for PCBs 3, 4, 28, 52, 101, 118, 138 and 180. Equilibrium Kleaf/PDMS extrapolated from the uptake kinetics measured over 4 days ranged from 0.075 (PCB 180) to 0.371 (PCB 3). The Kleaf/PDMS data can readily be converted to fugacity capacities of leaves (Zleaf) and subsequently leaf/water or leaf/air partition ratios (Kleaf/water and Kleaf/air) using partitioning data from the literature. Results of our measurements are within the variability observed for plant/air partition ratios (Kplant/air) found in the literature. Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kleaf/air from this study ranged from 5.00 (PCB 3) to 8.30 (PCB 180) compared to log
Kleaf/air from this study ranged from 5.00 (PCB 3) to 8.30 (PCB 180) compared to log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kplant/air of 3.31 (PCB 3) to 8.88 (PCB 180) found in the literature. The method we describe could provide data to characterize the variability in sorptive capacities of leaves that would improve descriptions of uptake of chemicals by leaves in multimedia fate models.
Kplant/air of 3.31 (PCB 3) to 8.88 (PCB 180) found in the literature. The method we describe could provide data to characterize the variability in sorptive capacities of leaves that would improve descriptions of uptake of chemicals by leaves in multimedia fate models.
Environmental impactThis study presents a proof of concept for a straightforward method to measure the capacity of leaves to hold chemicals. Leaves play an important role in the cycling of semi-volatile organic chemicals in the environment, yet much remains unknown about the sorptive capacities of leaves and how they differ between plant species. Measurements made with our method can be incorporated into multimedia fate and transport models that contain a vegetation compartment and thus provide more powerful tools to explore processes such as the forest filter effect and the bioaccumulation of organic pollutants in edible plants. In addition, leaf/air partitioning data are essential for monitoring studies that use leaves as passive samplers. |
Introduction
Leaves are a sink for semi-volatile organic chemicals in the atmosphere and a vector for transfer of air pollutants to soil with litter1,2 and into the terrestrial food chain.3 Studies comparing forested areas with nearby clearings have found that deposition of some organic pollutants to forested soils was significantly higher because of the shedding of leaves and waxes loaded with chemicals.2,4 Enhanced deposition of chemicals under forest canopies is known as the forest filter effect and is thought to be most pronounced for chemicals with octanol/air partition ratios (KOA) between 107 and 1011 and air/water partition ratios (KAW) greater than 10−6.5 Multimedia models indicate that the forest filter effect can have a substantial impact on the fate of chemicals in the environment, in particular in boreal regions characterized by cool temperatures and dense forest coverage.6,7Understanding the forest filter effect requires knowledge of the fugacity capacities of leaves (Zleaf), which are important parameters in multimedia fate and transport models such as CoZMo-POP8 and BETR.9 By definition, leaf/air and leaf/water partition ratios (Kleaf/air and Kleaf/water) are the ratios Zleaf/Zair and Zleaf/Zwater respectively. Available measurements indicate that plant/air partition ratios (Kplant/air) can differ by up to 3 orders of magnitude between plant species.2,5,10,11 An interesting observation is that the partition ratios are plant species-dependent in the case of whole leaves, but one regression curve could be fitted to a wide range of literature data for partitioning to isolated cuticles, which are the waxy outermost part of the leaves.12 There is a need for consistent data on how species diversity influences the partitioning of semi-volatile pollutants to leaves, and how partitioning to whole leaves relates to partitioning to isolated cuticles.
In this study we illustrate the use of sheets of polydimethylsiloxane (PDMS) as passive dosing devices for determining leaf/PDMS partition ratios (Kleaf/PDMS) of whole leaves for polychlorinated biphenyls (PCBs). PDMS is a convenient reference phase to calculate Zleaf and subsequently Kleaf/air and Kleaf/water.13 We further calculate cuticle/water partition ratios from our measurements that are consistent with measurements derived from isolated cuticles reported in the literature.
Material and methods
All native and labeled standards used in this study were purchased from Larodan (Solna, Sweden). Vendor specifications and purities for the solvents can be found in Table s1.† PDMS sheets of the type SSP-M823 with a thickness of 610 μm were purchased from Shielding Solutions Ltd (Essex, U.K.), the European distributor for Specialty Silicone Products, Inc.Experimental setup
PDMS disks 18 mm in diameter were punched from the sheets, soaked in 200 mL of methanol for at least a week and then soaked overnight in acetone. The disks were loaded with PCBs in a flask by adding 100 mL of methanol spiked with 1 mg of each of the PCB congeners 3, 4, 28, 52, 101, 118, 138 and 180 dissolved in isooctane. In total 8 mL of isooctane was spiked to the loading solution. The PDMS disks and the loading solution were left to equilibrate in the dark for a week, then 50 to 100 mL aliquots of water from a MilliQ purification unit (Merck, Darmstadt, Germany) were successively added over the course of several hours up to a final volume of 400 mL to force the chemicals into the PDMS as described by Birch et al.14 The PDMS disks were then transferred to MilliQ water and left for one hour to remove traces of methanol, after which they were dried overnight in a fume hood to remove traces of isooctane. No measurable amounts of isooctane were found in the PDMS disks after this drying step in a trial study.Rhododendron leaves (R. ponticum L.) were picked from a bush on the Frescati campus of Stockholm University. Rhododendron leaves were chosen because of their year-round availability and their rigid structure. Care was taken to select leaves that had similar exposure to sunlight. All leaves were wiped clean using wet paper tissues and blotted dry. In total, 15 leaves were collected. Disks 18 mm in diameter were punched from the leaves and shuffled randomly to avoid a possible bias of the leaf source. A subset of 17 disks was dried in the oven at 60 °C for 3 days to determine the dry weight (DW) of the leaves. The volume of the leaf disks was measured by volume displacement of water using a separate batch of 54 disks.
Our passive dosing apparatus is similar to that of Kim et al.,12 which is a modified version of the setup used by Mayer et al.15 and Trapp et al.16 to measure the kinetics of chemical transport through different matrices. Leaf disks were sandwiched between two loaded PDMS disks (Fig. 1) and the assembly was pressed together with glass plates held by metal clamps. Blanks consisting of cleaned PDMS disks in contact with leaves were kept in a closed jar to avoid cross-contamination from the loaded PDMS. Leaves were sampled in triplicates before the leaves and PDMS were brought into contact (at 0 hours) and after 2, 4, 8, 24, 48 and 96 hours contact time. The temperature and relative humidity in the fume hood were not monitored during this experiment but were later measured to be 20 ± 0.5 °C and 32.1 ± 4.8%.
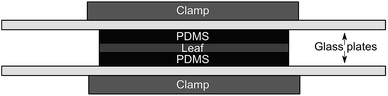 | ||
| Fig. 1 Schematic of the experimental setup. The top and bottom “clamp” represent one clamp assembly. | ||
Measurement of total solvent-extractable organic matter
The extractable organic matter of a batch of Rhododendron leaves was measured using the modified Jensen extraction method (mod. 2).17 The extracts were then left in the fume hood until they reached a constant weight (within 24–48 h).Extraction of PCBs from leaves
After terminating exposure, the leaf disks were immediately transferred to pre-weighed microcentrifuge tubes containing 1 mL of acetonitrile, spiked with 100 ng of 13C labeled PCBs 28, 101, 138 and 180, and a 5 mm diameter stainless steel ball was added. The disks were then homogenized using a Mini G 1600 tissue homogenizer (SPEX, Stanmore, U.K.) and extracted using QuEChERS (Quick-Easy-Cheap-Effective-Rugged-Safe, Phenomenex, California, U.S.A.). A detailed description of the method can be found in Text s1.† The final extracts in acetonitrile were solvent-exchanged to isooctane, reduced to 0.5 mL using a gentle stream of nitrogen, and spiked with 50 ng of PCB 53 before being analyzed. The concentrations in the leaves are given on a dry volume basis (ng m−3), with the volume of a batch of dry leaves measured by the displacement of water.PDMS extraction
The top and bottom PDMS disks of each sandwich were pooled, transferred to a test tube containing 4 mL of acetone and 100 ng of 13C labeled PCBs and left overnight. The extract was then transferred to a new vial and solvent-exchanged to isooctane. The final extract was reduced to 0.5 mL using a gentle stream of nitrogen, spiked with 50 ng of PCB 53 and then diluted by a factor 10 before analysis.Instrumental analysis
The samples were analyzed on a gas chromatograph (Trace 1310, Thermo Scientific, U.S.) coupled to a single quadrupole mass spectrometer (ISQ LT, Thermo Scientific, U.S.). The ions used to quantify each of the analytes can be found in Table s2† and an overview of the GC/MS program in Text s2.† The analyte concentrations were quantified using the labeled internal standards in all samples except the leaf samples collected between 0 and 48 hours. In these samples we used PCB 53 as an internal standard and corrected the concentrations with the average recoveries of the labeled standards determined in the 96 hour samples.Quality assurance/quality control
All the blanks in this study (n = 8 for the leaves to 13 for the PDMS) underwent the same extraction procedure as the actual samples with at least one blank processed at every sampling time point. Method quantification limits (MQLs) were calculated using the average concentration of the analyte in the blanks + 9 times the standard deviation (Table s3†). When the concentration in the blank was too low to be measured, the lowest concentration in the calibration curve (4.84 ± 2.01 pg μL−1, stddev.) was used. In those cases where analytes in the blanks were only detected in one of the replicates, we calculated the MQL conservatively as ten times the concentration found in the blanks. As the concentration in the blanks did not show any correlation with time, all blanks were combined to derive MQLs.Results
Unless specified otherwise, all uncertainties provided in this section are standard deviations and all concentration ratios (Cleaf/CPDMS) are given on a dry volume basis.Quality assurance/quality control
The MQLs ranged from 27 to 197 ng g−1 DW for the leaves and 10 to 886 ng g−1 for the PDMS (Table s3†). All the samples in this study had concentrations > MQL.The recoveries of the labeled PCBs from the QuEChERS-based leaf extraction ranged from 69 to 80% (Table s4†) which is 10% lower than the recoveries reported for a similar extraction method for PCBs from catfish.18 The recoveries of the labeled internal standards from the PDMS ranged from 105% to 120% for the extractions from blank PDMS and from 135% to 155% for the extractions from loaded PDMS. One possible explanation for the high recoveries from loaded PDMS is the dilution by a factor 10 of the loaded PDMS samples before analysis. Peak areas of the volumetric standard PCB 53 were roughly 20 times lower in diluted samples than in the standards and the undiluted samples. Therefore the apparently high recoveries may reflect an artefact associated with extrapolation from our calibration series which is based on a series of replicates with a specific concentration of labeled internal standards and PCB 53. The recoveries of labeled PCBs from PDMS do not affect the concentration measurements used to determine uptake kinetics in the passive dosing experiments since all analytes were quantified using labeled internal standards.
Water loss from leaves
The leaves gradually dried out during the experiment (Fig. s1†). While fresh leaves weighed on average 65 mg per disk, their average weight was reduced to 27 mg (a loss of 58%) at the end of the experiment, which is comparable to the DW (29 mg).Leaf/PDMS partitioning
The concentrations of individual PCBs in the loaded PDMS disks varied by a factor of 2 to 4 between the lowest and highest measured values. No correlation was found between exposure time and concentration of the analytes (Pearson, P > 0.7).Due to the variability in the PCB concentrations in the PDMS disks, the concentrations in leaves were normalized by calculating the concentration ratio between each individual leaf and the pooled PDMS disks it was exposed to. This calculation was done on a volume/volume basis.
A one-phase association curve fitted the data well for all congeners, with R2 values ranging from 0.86 to 0.91 (Fig. 2). This association curve was used to estimate the leaf/PDMS concentration ratio at equilibrium (Kleaf/PDMS). According to the curve fits, the chemicals had reached between 76% (±17, PCB 4) and 99% (±4, PCB 3) of equilibrium after 4 days. The estimated time to reach 95% of equilibrium ranged between 3 and 6 days for all congeners except PCB 4 (8 days). Upon reaching equilibrium, between 2 and 6% of the analytes in each PDMS/leaf/PDMS system would have been transferred to the leaves (Table s5†).
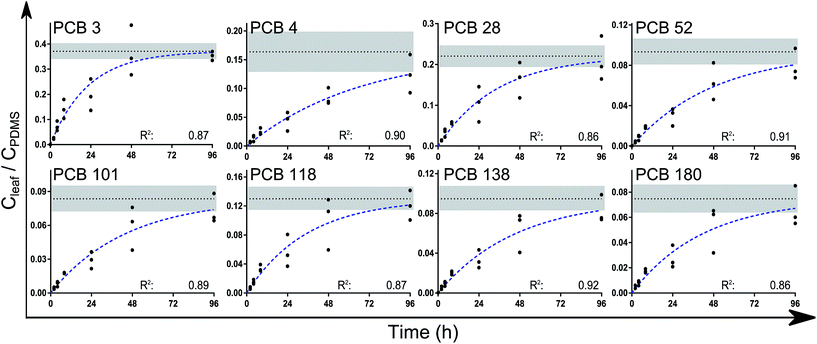 | ||
| Fig. 2 The leaf/PDMS concentration ratios (on a volume/volume basis) of the analytes over time. Extrapolation to equilibrium (Kleaf/PDMS) was done using a one-phase association curve in Graphpad Prism of the form Y = Ymax(1 − e(−kt)). The broken blue lines show the fitted curves while the dotted black lines show Ymax = Kleaf/PDMS (±std error, shaded area). No difference was found between the measured k values (P > 0.15). Values for k and Kleaf/PDMS are reported in Table 1. | ||
Z leaf (Table 1) was estimated by multiplying Kleaf/PDMS with ZPDMS obtained from measurements of the PDMS/water partition ratios (KPDMS/water) multiplied by Henry's law constants (H) from the literature measured at 25 °C.13,19,20Kleaf/water was then calculated by dividing Zleaf by Zwater (KPDMS/water × H) and Kleaf/air by dividing Zleaf with Zair (1/RT = 0.00040 mol m−3 Pa−1, with R being the gas constant and T the temperature in Kelvin).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kleaf/PDMS and values for Zleaf, log
Kleaf/PDMS and values for Zleaf, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kleaf/water and log
Kleaf/water and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kleaf/air for dry leaves. Values of k and Zleaf are given with standard deviations which show the uncertainty of the extrapolation using the nonlinear regression. For log
Kleaf/air for dry leaves. Values of k and Zleaf are given with standard deviations which show the uncertainty of the extrapolation using the nonlinear regression. For log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kleaf/PDMS, log
Kleaf/PDMS, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kleaf/water and log
Kleaf/water and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kleaf/air, the uncertainty was propagated from the input data and is given as a range of ±1 standard deviation of the measured value (in brackets). A more detailed description of the error propagation can be found in Text s3
Kleaf/air, the uncertainty was propagated from the input data and is given as a range of ±1 standard deviation of the measured value (in brackets). A more detailed description of the error propagation can be found in Text s3
| Compound | k (h−1) | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kleaf/PDMS Kleaf/PDMS |
Z leaf (mol m−3 Pa−1) | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kleaf/water Kleaf/water |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kleaf/air Kleaf/air |
|---|---|---|---|---|---|
| PCB 3 | 0.045 ± 0.022 | −0.43 [−0.51 to −0.36] | 146 ± 64 | 3.5 [3.3–3.7] | 5.6 [5.3–5.7] |
| PCB 4 | 0.015 ± 0.012 | −0.79 [−1.05 to −0.62] | 40 ± 24 | 3.6 [3.2–3.8] | 5.0 [4.6–5.2] |
| PCB 28 | 0.029 ± 0.017 | −0.66 [−0.78 to −0.56] | (1.6 ± 0.7) × 103 | 4.7 [4.4–4.8] | 6.6 [6.3–6.8] |
| PCB 52 | 0.021 ± 0.012 | −1.0 [−1.0 to −0.9] | (1.4 ± 0.7) × 103 | 4.6 [4.3–4.8] | 6.6 [6.3–6.7] |
| PCB 101 | 0.023 ± 0.014 | −1.1 [−1.2 to −1.0] | (5.7 ± 2.8) × 103 | 5.1 [4.8–5.2] | 7.2 [6.9–7.3] |
| PCB 118 | 0.028 ± 0.017 | −0.89 [−1.02 to −0.79] | (2.2 ± 1.1) × 104 | 5.4 [5.1–5.6] | 7.8 [7.5–7.9] |
| PCB 138 | 0.022 ± 0.012 | −1.0 [−1.2 to −0.9] | (1.7 ± 0.8) × 104 | 5.7 [5.4–5.8] | 7.6 [7.3–7.8] |
| PCB 180 | 0.024 ± 0.016 | −1.1 [−1.3 to −1.0] | (8.1 ± 4.1) × 104 | 5.8 [5.5–6.0] | 8.3 [8.0–8.5] |
Discussion
PDMS loading
The final ratio of loading solution to water was two times higher in our experiments than in Birch et al.14 Some of the variability in the PCB concentrations in the loaded PDMS may have been caused by using isooctane as solvent for the standards, as upon the addition of water to the loading solution some of the isooctane was forced into the PDMS. Furthermore, the addition of water caused the PDMS to float on the loading solution, thereby exposing some disks differently than others. We therefore advise the use of more polar solvents such as methanol or acetone for the stock solutions of the chemicals and adding lower amounts of water, as suggested by Booij et al.21Leaf/PDMS partitioning
The time required to approach equilibrium between the PDMS and the leaves was similar for the mono- to hepta-chlorinated congeners and corresponded well with those from a comparable setup for the uptake of PAHs in isolated cuticles.12 That study showed that up to 4 days were needed to reach equilibrium between the cuticle and the PDMS for all but the highest molecular weight PAH congeners investigated in their study. Slower equilibration kinetics for the high molecular weight PAHs were attributed by the authors to higher molar volumes of those compounds. It has recently been shown that there can be considerable differences in the mass transfer kinetics in these types of studies, possibly caused by a difference in pressure exerted on the system by the use of certain clamps or magnets to keep the setup together.22 While unlikely to affect the leaf/PDMS partition ratios, it is possible that the mass transfer kinetics into the leaves were affected by the pressure on the soft leaf tissue which could alter the structure of the leaves. No damage was observed on the leaves in this study in which we used glass sheets to distribute the pressure from the clamps more evenly across the leaves.While the variability between replicate measurements in this study is much larger than that observed in Mayer et al.15 and Trapp et al.,16 it is comparable with that in the study of Kim et al. for isolated cuticles.12 A quantitative comparison with this study is not possible however as the variability can only be estimated from Fig. 2 in their study.12
The observation that the uptake conforms to first-order kinetics and had similar rate constants for the different PCB congeners (Table 1) could be an indication that equilibrium with the whole leaf was not reached with this experimental setup. Plant uptake models based on a two-compartment approach have shown that there is a compartment in some leaves that can respond rapidly to changes in atmospheric concentrations and a second compartment which reacts more slowly.23,24 Our first-order kinetic uptake model assumes implicitly that there is only one compartment, and it fits the data well. We see no evidence of two-compartment uptake in our kinetic curves and all the fits have high R2 values. Based on the literature data cited above,23,24 it is possible that we measure kinetics of uptake into the fast responding compartment only.
The studies by Wild et al.25 and subsequently Li and Chen26 and Li and colleagues27 have shown that phenanthrene can be transferred across the cuticle and into the epidermis within 24–48 hours which is within the timeframe of our experiment. In a recent study in our lab,22 the use of the method presented in Mayer et al.15 and Trapp et al.16 to measure the mass transfer kinetics of PAHs and PCBs through leaves gave an indication that this process is very slow with mass transfer coefficients of 1.65 × 10−6 to 2.17 × 10−6 m h−1 for the transfer of fluorene and phenanthrene respectively through leaves of a Hydrangea species while mass transfer through Rhododendron leaves was too slow to result in quantifiable amounts of analytes penetrating through the leaf and accumulating in the acceptor PDMS on the other side within 48 hours. In combination with this study, it seems that while hydrophobic organic chemicals are quickly taken up by the leaf, their transfer through the entire leaf is slow.
K
leaf/water was converted to cuticle/water partition ratios (Kcuticle/water) by dividing by the volume fraction of the leaves that is cuticle (4.7%), assuming a cuticle thickness of 4.15 μm (average from 3 Rhododendron species).28 In this case it meant applying a correction factor of 21.3 (1/0.047). Values for Kcuticle/water were within a factor 2.5 of Kcuticle/water estimated from regressions found in the literature for a variety of plant species (Fig. 3).12,29 Fitting a regression curve through the entire set of values for log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kcuticle/water (n = 75) against the log
Kcuticle/water (n = 75) against the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow of the respective chemicals provides a good fit (R2 = 0.95) and gives the impression that it is possible to estimate Kcuticle/water from Kow measurements with the regression: Kcuticle/water = 1.25 Kow.
Kow of the respective chemicals provides a good fit (R2 = 0.95) and gives the impression that it is possible to estimate Kcuticle/water from Kow measurements with the regression: Kcuticle/water = 1.25 Kow.
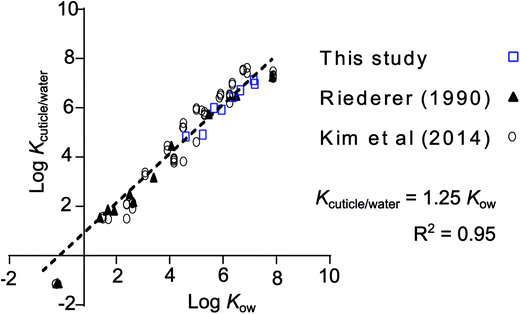 | ||
Fig. 3 Comparison of the data presented in this study normalized to the estimated fraction of cuticle in the leaf and cuticle/water partition ratios from Kim et al.,12 who reported their own measurements, a collection from the literature and measurements from Riederer.29 The regression equation through all data points (n = 75), forced to a slope of 1, is log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kcuticle/water = log Kcuticle/water = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow + 0.0963 (R2 = 0.95), or more conveniently: Kcuticle/water = 1.25 Kow. The data taken from the study by Riederer et al. was for Citrus aurantium and Ficus elastica and that of Kim et al. was for Euonymus japonicus. The literature data collected by Kim et al., which is included in this regression, contains data for 9 different plant species. More info on these data can be found in the supporting material of their study. Kow + 0.0963 (R2 = 0.95), or more conveniently: Kcuticle/water = 1.25 Kow. The data taken from the study by Riederer et al. was for Citrus aurantium and Ficus elastica and that of Kim et al. was for Euonymus japonicus. The literature data collected by Kim et al., which is included in this regression, contains data for 9 different plant species. More info on these data can be found in the supporting material of their study. | ||
Aside from Kcuticle/water, there is a wide range of literature available on plant/air partitioning from both field studies and models. Differences between plant species and the applied methods result in a range of 3 orders of magnitude of measured Kplant/air (Fig. 4). Converting our Kleaf/PDMS to Kplant/air using partition ratios from the literature resulted in data points that lie within the range of existing data for clover, plantain, hawk's beard and yarrow measured using a fugacity meter,10 and that differed by roughly an order of magnitude from data derived from deposition measurements,2,5 or empirical regressions.14
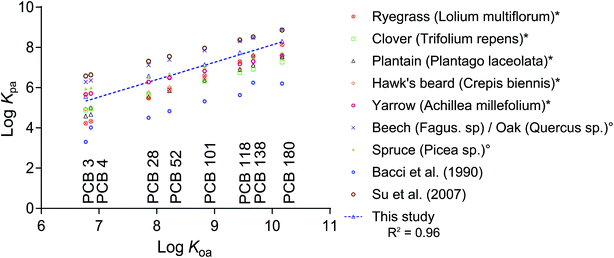 | ||
Fig. 4 Plant/air partition ratios (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kplant/air) from different reports in the literature and this study plotted versus the chemicals' log Kplant/air) from different reports in the literature and this study plotted versus the chemicals' log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOA.19 Data marked with * originate from fugacity meter measurements by Kömp and McLachlan,10 and those marked with ° were derived from deposition fluxes by McLachlan and Horstmann.5,10 Bacci et al.11 reported Kleaf/air for azalea leaves and Su et al.2 derived their Kplant/air from deposition fluxes in a deciduous Canadian forest. All literature data was either measured or derived at a temperature of 25 °C. The regression line for our dataset is: log KOA.19 Data marked with * originate from fugacity meter measurements by Kömp and McLachlan,10 and those marked with ° were derived from deposition fluxes by McLachlan and Horstmann.5,10 Bacci et al.11 reported Kleaf/air for azalea leaves and Su et al.2 derived their Kplant/air from deposition fluxes in a deciduous Canadian forest. All literature data was either measured or derived at a temperature of 25 °C. The regression line for our dataset is: log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kplant/air = 0.8637 Kplant/air = 0.8637![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOA − 0.5108. KOA − 0.5108. | ||
Recently, Vorkamp et al.30 demonstrated that equilibrium sampling of PCBs in indoor air using silicone-coated baking paper is feasible. The Kleaf/PDMS data from the present study can provide conversion factors for equilibrium sampling concentrations from silicone-based air sampling to equilibrium partitioning concentrations in leaves. More specifically, the Kleaf/PDMS data can be used (i) to estimate PCB concentrations in leaves based on equilibrium sampling measurements in air, (ii) for consistency checking between equilibrium sampling in air and leaf monitoring data or (iii) for checking the equilibrium status of PCBs in leaves based on parallel equilibrium sampling in air and measurements of concentrations in leaves. The fugacity capacity of leaves can also be used directly in studies of the uptake of pollutants in plants at contaminated sites31 and in studies that use leaves as passive samplers for indoor air32 or as a screening tool to assess spatial variability of semi-volatile chemicals in air.33
To give lipid-normalized concentrations, the concentrations in the leaves were normalized to the leaves' total solvent-extractable organic matter (determined using the Jensen extraction)17 as an indicator of lipid content. There was poor agreement between these so-derived lipid/PDMS partition ratios (Klipid/PDMS) with measurements of Klipid/PDMS for olive oil and a wide range of animal lipids from the literature (Fig. s2†).34–37 The lower Klipid/PDMS values for the Rhododendron leaves are consistent with a certain fraction of the extractable organic matter not being available for partitioning. For instance, the crystalline nature of some cuticular waxes could make them less available for partitioning.
The solvent-extractable organic matter of leaves normally does not contain the depolymerizable lipids such as cutin (and for some species also cutan), which have been identified as potentially responsible for a major fraction of the sorptive capacities of leaves for HOCs.38 If the depolymerizable lipids reached equilibrium in our experiments and were not extracted with other lipids, then the Klipid/PDMS values that we measure for the leaves would be highly overestimated. However, our measured Klipid/PDMS are below those found in the literature. A possible explanation is that the depolymerizable lipids are part of the slowly responding compartment observed in other studies, and did not reach equilibrium with the PDMS in our experiments. Another possible explanation for the poor agreement could be that the partitioning properties of the extractable organic matter of foliage and the other lipids studied in the literature are fundamentally different from each other.
Our experimental setup provides a straightforward way of measuring the fugacity capacity of leaves, which can then readily be applied to calculate Kleaf/water, Kleaf/air and other partition ratios of interest. While no observation of a two-compartment system was made, it is possible that our setup only reached equilibrium with the cuticle and the inner compartments of the leaf that are easy to access. The impact of the loss of water from the leaves on the fugacity capacity of these leaves for HOCs is most likely very limited. However, future studies should test if these results can be extrapolated to leaves with a higher water content than the Rhododendron leaves, which would require simple modifications of the experimental setup such as keeping moist tissues underneath the setup, or keeping the setup in a closed environment with a beaker of water.
Acknowledgements
This research was funded by the Swedish Research Council Vetenskapsrådet (VR, #2011-3890). We acknowledge Tommy Landberg from Stockholm University for providing us with plants for trial studies, Susanna Lampo from Phenomenex, Denmark for providing us with free samples of roQ QuEChERS and Gunvor Larsson from Bergius Botanic Garden, Stockholm, for help with the identification of the Rhododendron species. We also thank the three anonymous reviewers whose constructive criticism helped improve this paper.References
- F. Matzner, Annual rates of deposition of polycyclic aromatic hydrocarbons in different forest ecosystems, Water, Air, Soil Pollut., 1984, 21(1–4), 425–434 CrossRef.
- Y. Su, F. Wania, T. Harner and Y. D. Lei, Deposition of polybrominated diphenyl ethers, polychlorinated biphenyls, and polycyclic aromatic hydrocarbons to a boreal deciduous forest, Environ. Sci. Technol., 2007, 41(2), 534–540 CrossRef CAS PubMed.
- K. Welsch-Pausch, M. S. McLachlan and G. Umlauf, Determination of the principal pathways of polychlorinated dibenzo-p-dioxins and dibenzofurans to Lolium multiflorum (Welsh Ray Grass), Environ. Sci. Technol., 1995, 29(4), 1090–1098 CrossRef CAS PubMed.
- M. Horstmann and M. S. McLachlan, Atmospheric deposition of semivolatile organic compounds to two forest canopies, Atmos. Environ., 1998, 32(10), 1799–1809 CrossRef CAS.
- M. S. McLachlan and M. Horstmann, Forests as filters of airborne organic pollutants: a model, Environ. Sci. Technol., 1998, 32(3), 413–420 CrossRef CAS.
- M. MacLeod, On the influence of forests on the overall fate of semi-volatile organic contaminants, Stochastic Environmental Research and Risk Assessment, 2003, 17(4), 256–259 CrossRef.
- Y. Su and F. Wania, Does the forest filter effect prevent semivolatile organic compounds from reaching the arctic?, Environ. Sci. Technol., 2005, 39(18), 7185–7193 CrossRef CAS PubMed.
- F. Wania, K. Breivik, N. J. Persson and M. S. McLachlan, CoZMo-POP 2 – A fugacity-based dynamic multi-compartmental mass balance model of the fate of persistent organic pollutants, Environ. Model. Software, 2006, 21(6), 868–884 CrossRef.
- M. MacLeod, H. von Waldow, P. Tay, J. M. Armitage, H. Wöhrnschimmel, W. J. Riley, T. E. McKone and K. Hungerbühler, BETR global – A geographically-explicit global-scale multimedia contaminant fate model, Environ. Pollut., 2011, 159(5), 1442–1445 CrossRef CAS PubMed.
- P. Kömp and M. S. McLachlan, Interspecies variability of the plant/air partitioning of polychlorinated biphenyls, Environ. Sci. Technol., 1997, 31(10), 2944–2948 CrossRef.
- E. Bacci, D. Calamari, C. Gaggi and M. Vighi, Bioconcentration of organic chemical vapors in plant leaves: experimental measurements and correlation, Environ. Sci. Technol., 1990, 24(6), 885–889 CrossRef CAS.
- S.-J. Kim, H. Lee and J.-H. Kwon, Measurement of partition coefficients for selected polycyclic aromatic hydrocarbons between isolated plant cuticles and water, Sci. Total Environ., 2014, 494–495, 113–118 CrossRef CAS PubMed.
- D. Gilbert, G. Witt, F. Smedes and P. Mayer, Polymers as reference partitioning phase: polymer calibration for an analytically operational approach to quantify multimedia phase partitioning, Anal. Chem., 2016, 88(11), 5818–5826 CrossRef CAS PubMed.
- H. Birch, V. Gouliarmou, H.-C. Holten Lützhøft, P. S. Mikkelsen and P. Mayer, Passive dosing to determine the speciation of hydrophobic organic chemicals in aqueous samples, Anal. Chem., 2010, 82(3), 1142–1146 CrossRef CAS PubMed.
- P. Mayer, U. Karlson, P. S. Christensen, A. R. Johnsen and S. Trapp, Quantifying the effect of medium composition on the diffusive mass transfer of hydrophobic organic chemicals through unstirred boundary layers, Environ. Sci. Technol., 2005, 39(16), 6123–6129 CrossRef CAS PubMed.
- S. Trapp, A. Cammarano, E. Capri, F. Reichenberg and P. Mayer, Diffusion of PAH in potato and carrot slices and application for a potato model, Environ. Sci. Technol., 2007, 41(9), 3103–3108 CrossRef CAS PubMed.
- S. Jensen, L. Häggberg, H. Jörundsdóttir and G. Odham, A quantitative lipid extraction method for residue analysis of fish involving nonhalogenated solvents, J. Agric. Food Chem., 2003, 51(19), 5607–5611 CrossRef CAS PubMed.
- N. Chamkasem, S. Lee and T. Harmon, Analysis of 19 PCB congeners in catfish tissue using a modified QuEChERS method with GC-MS/MS, Food Chem., 2016, 192, 900–906 CrossRef CAS PubMed.
- U. Schenker, M. MacLeod, M. Scheringer and K. Hungerbühler, Improving data quality for environmental fate models: a least-squares adjustment procedure for harmonizing physicochemical properties of organic compounds, Environ. Sci. Technol., 2005, 39(21), 8434–8441 CrossRef CAS PubMed.
- F. Smedes, R. W. Geertsma, T. van der Zande and K. Booij, Polymer–water partition coefficients of hydrophobic compounds for passive sampling: application of cosolvent models for validation, Environ. Sci. Technol., 2009, 43(18), 7047–7054 CrossRef CAS PubMed.
- K. Booij, F. Smedes and E. M. Van Weerlee, Spiking of performance reference compounds in low density polyethylene and silicone passive water samplers, Chemosphere, 2002, 46(8), 1157–1161 CrossRef CAS PubMed.
- H. Ahmadi, D. J. Bolinius, A. Jahnke and M. MacLeod, Mass transfer of hydrophobic organic chemicals between silicone sheets and through plant leaves and low-density polyethylene, Chemosphere, 164, 683–690 CrossRef PubMed.
- L. Schreiber and J. Schonherr, Uptake of two chlorinated chemicals in conifer needles: reversibility and compartmental analysis, New Phytol., 1993, 123(3), 547–554 CrossRef CAS.
- M. S. McLachlan, K. Welsch-Pausch and J. Tolls, Field validation of a model of the uptake of gaseous SOC in Lolium multiflorum (Welsh Ray Grass), Environ. Sci. Technol., 1995, 29(8), 1998–2004 CrossRef CAS PubMed.
- E. Wild, J. Dent, G. O. Thomas and K. C. Jones, Visualizing the air-to-leaf transfer and within-leaf movement and distribution of phenanthrene: further studies utilizing two-photon excitation microscopy, Environ. Sci. Technol., 2006, 40(3), 907–916 CrossRef PubMed.
- Q. Li and B. Chen, Organic pollutant clustered in the plant cuticular membranes: visualizing the distribution of phenanthrene in leaf cuticle using two-photon confocal scanning laser microscopy, Environ. Sci. Technol., 2014, 48(9), 4774–4781 CrossRef CAS PubMed.
- Y. Li, Q. Li and B. Chen, Organic pollutant penetration through fruit polyester skin: a modified three-compartment diffusion model, Sci. Rep., 2016, 6, 23554 CrossRef CAS PubMed.
- Y.-F. Cai, S.-F. Li, S.-F. Li, W.-J. Xie and J. Song, How do leaf anatomies and photosynthesis of three Rhododendron species relate to their natural environments, Bot. Stud., 2014, 55(1), 36 CrossRef.
- M. Riederer, Estimating partitioning and transport of organic chemicals in the foliage/atmosphere system: discussion of a fugacity-based model, Environ. Sci. Technol., 1990, 24(6), 829–837 CrossRef CAS.
- K. Vorkamp, L. Odsbjerg, M. Langeland and P. Mayer, Utilizing the partitioning properties of silicone for the passive sampling of polychlorinated biphenyls (PCBs) in indoor air, Chemosphere, 2016, 160, 280–286 CrossRef CAS PubMed.
- O. Mikes, P. Cupr, S. Trapp and J. Klanova, Uptake of polychlorinated biphenyls and organochlorine pesticides from soil and air into radishes (Raphanus sativus), Environ. Pollut., 2009, 157(2), 488–496 CrossRef CAS PubMed.
- T. A. Wetzel and W. J. Doucette, Plant leaves as indoor air passive samplers for volatile organic compounds (VOCs), Chemosphere, 2015, 122, 32–37 CrossRef CAS PubMed.
- S. L. Simonich and R. A. Hites, Organic pollutant accumulation in vegetation, Environ. Sci. Technol., 1995, 29(12), 2905–2914 CrossRef CAS PubMed.
- A. Jahnke, M. S. McLachlan and P. Mayer, Equilibrium sampling: partitioning of organochlorine compounds from lipids into polydimethylsiloxane, Chemosphere, 2008, 73(10), 1575–1581 CrossRef CAS PubMed.
- A. Jahnke, P. Mayer, M. Adolfsson-Erici and M. S. McLachlan, Equilibrium sampling of environmental pollutants in fish: comparison with lipid-normalized concentrations and homogenization effects on chemical activity, Environ. Toxicol. Chem., 2011, 30(7), 1515–1521 CrossRef CAS PubMed.
- L. Jin, C. Gaus, L. van Mourik and B. I. Escher, Applicability of passive sampling to bioanalytical screening of bioaccumulative chemicals in marine wildlife, Environ. Sci. Technol., 2013, 47(14), 7982–7988 CrossRef CAS PubMed.
- I. J. Allan, K. Bæk, T. O. Haugen, K. L. Hawley, A. S. Høgfeldt and A. D. Lillicrap, In vivo passive sampling of nonpolar contaminants in brown trout (Salmo trutta), Environ. Sci. Technol., 2013, 47(20), 11660–11667 CrossRef CAS PubMed.
- B. Chen, Y. Li, Y. Guo, L. Zhu and J. L. Schnoor, Role of the extractable lipids and polymeric lipids in sorption of organic contaminants onto plant cuticles, Environ. Sci. Technol., 2008, 42(5), 1517–1523 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional material as cross-referenced throughout. See DOI: 10.1039/c6em00423g |
| This journal is © The Royal Society of Chemistry 2016 |
