 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Transparency of chemical risk assessment data under REACH†
Ellen
Ingre-Khans
 *a,
Marlene
Ågerstrand
*a,
Marlene
Ågerstrand
 a,
Anna
Beronius
b and
Christina
Rudén
a
a,
Anna
Beronius
b and
Christina
Rudén
a
aDepartment of Environmental Science and Analytical Chemistry, Stockholm University, 106 91 Stockholm, Sweden. E-mail: ellen.ingre-khans@aces.su.se; Tel: +46 72 148 92 78
bInstitute of Environmental Medicine, Karolinska Institutet, 171 77 Stockholm, Sweden
First published on 7th November 2016
Abstract
The REACH regulation requires EU manufacturers and importers of substances to register information on the hazard and risk of their substances with the European Chemicals Agency (ECHA). Risk management of the substances is based on the provided information. It is known that conclusions on hazard and risk are influenced by expert judgements as well as potential conflict of interests. Thus, it is important that hazard and risk assessments are transparent and can be evaluated by a third party. The aim of this study is to scrutinize the transparency, i.e. the accessibility and comprehensibility, of information on substances registered under REACH. Data on repeated dose toxicity and hazard assessment conclusions were extracted for 60 substances from the REACH registration database available on the ECHA website. The data were compiled in a database for systematically evaluating the transparency of information related to the conclusions on hazard or risk. In addition, chemical safety reports (CSR) were requested from ECHA for five substances. The transparency of information on the hazard and risk of substances was found to be limited for several reasons. First, certain information was removed due to confidentiality and certain fields were not published because they could contain confidential information although the information had not been claimed confidential. Also, the extent to which registrants reported information varied, and the presentation of some data and certain terminology required further clarification. In addition, the data source for the majority of the key and supporting studies could not be identified due to confidentiality. Since registrants are only required to summarise studies, it cannot be verified whether all relevant information from non-public industry reports have been reported. Lastly, certain information related to the hazard and risk assessment were only reported in the CSR which is only available upon request; a time-consuming and work-intensive process. As information on registered chemicals is currently provided to the public, it is difficult to follow steps that are undertaken in the hazard and risk assessment. This limits the possibility for a third party to evaluate the assessment.
Environmental impactThe regulation of hazardous chemicals is important to protect human health and the environment. European manufacturers and importers are obliged to register and assess the hazards and risks of their substances under the European chemicals legislation REACH. Since conclusions on hazard and risk are influenced by expert judgements and potential conflict of interests, these assessments need to be transparent to enable a third party to scrutinise the conclusions. We looked at the transparency, i.e. accessibility and comprehensibility, of chemical data registered under REACH and found that several factors limited the transparency of the hazard and risk assessments of the substances. |
1. Introduction
The European chemicals legislation REACH (regulation (EC) no 1907/2006 concerning Registration, Evaluation, Authorisation and restriction of CHemicals) requires industry to register and provide information on substances they produce or import into the EU at or above one tonne per year.1 The information is compiled and submitted as a registration dossier to the European Chemicals Agency (ECHA) in the software programme IUCLID.2 One objective of REACH is to make information on the hazards and risks of chemicals available to the public3 specified in REACH article 77(e) and recital 117. Information on substances becomes publicly available either by dissemination on the ECHA website through the dissemination portal4 (referred to as the REACH registration database in this paper) or by ECHA granting access to certain documents upon request. Before information from the registration dossiers is published on the website, the dossiers undergo an automatic filtering step in which information from the dossier that is not intended to be published or considered confidential is removed.5 The filtering step is based on articles 118 and 119 in REACH which specify information that is never to be published due to confidentiality, information that is always considered public and information that is published unless claimed confidential.The REACH registration database provides a wealth of information on chemicals put on the European market. However, concerns have been raised regarding the extent to which information is reported as well as the quality of the information provided by industry.6–13 In a study performed by the German Federal Institute for Risk Assessment (BfR), in which the availability of data for high tonnage volume substances were screened, over half of the registered substances were found to lack information required by REACH for one or more of the endpoints that were evaluated in the project.7 In a five year report on REACH published in 2016, ECHA emphasised the insufficient data quality for “a significant proportion” of the registration dossiers.8
The physicochemical and (eco)toxicological information on substances and the subsequent hazard and risk assessment submitted to ECHA by registrants form the basis for how chemicals covered by REACH are regulated and managed within the EU. Studies have shown that hazard and risk assessments of chemicals are influenced by scientific uncertainty and rely on expert judgment in selecting, interpreting and evaluating data12,14–18 and can be subject to intentional as well as unintentional bias due to conflict of interests.19–22 Hence, it is important that underlying assumptions and reasoning in hazard and risk assessments as well as the data on which the assessments are based are explicitly stated and transparent to stakeholders, such as policy makers, academic researchers and NGOs.23,24
The aim of this study was to assess the transparency of information on chemical substances submitted by industry to ECHA under REACH focusing on disclosure and clarity of information. This was done by looking at (1) dissemination of information in the REACH registration database on the ECHA website for data registered on the endpoint repeated dose toxicity (RDT) as well as hazard assessment conclusions for a subset of 60 substances and (2) accessibility of chemical safety reports (CSR) that are available on request. A CSR is required for substances manufactured or imported at or above 10 tonnes per year and documents the chemical safety assessment. The overall purpose of the study was to contribute to improving accessibility and comprehensibility of hazard and risk assessment of chemicals since this enables other stakeholders to take part of how registrants come to their conclusion and evaluate the accuracy of the assessment. The accuracy of the gathered information was not investigated in this study.
2. Method
2.1 Research questions and scope of study
The transparency of data published in the REACH registration database was examined by investigating (a) the possibility to identify and access the data source for studies on RDT that are submitted as part of the registration dossier, (b) the justifications for omitting data on RDT from the REACH information requirements, known as “waiving” and (c) how hazard assessment conclusions were derived and reported. The study was limited to a subset of 60 substances (ESI Table 1†) with a focus on data submitted for the endpoint RDT, which was a standard information requirement for all substances included in the study.2.2 Data selection, collection and compilation
Of the 60 substances included in the study, 51 were selected from a list of 178 European Community (EC) numbers that were categorized depending on data availability. The list was provided by BfR, from their investigation of data availability of dossiers under REACH.11 Equal number of substances, i.e. 17, were randomly selected from each of three categories to have a selection of dossiers with varying degrees of reporting. Another nine substances were selected since they are subject to authorisation and/or restriction processes under REACH and are well investigated substances.4Data up until the 1st of September 2015 were extracted for the 60 substances from the REACH registration database.4 For the majority of the substances, information was extracted from joint dossiers (registrants who manufacture or put the same substance on the market need to share certain information and make a joint submission of the dossier via an appointed lead registrant according to the principle “one substance, one registration”, REACH art. 11). For four of the substances, only data from individually submitted dossiers were available. The extracted information was compiled in a Microsoft Access database, designed for the purpose of this study, to enable a quantitative analysis of the information.
A request for access to full CSRs pursuant to regulation (EC) no 1049/2001 on public access to documents25 was made electronically to ECHA on 11th of September 2014 for nine of the substances.26 The request was withdrawn after correspondence with ECHA and a new request was made on 29th of September 2014.27 The new request included full CSRs for five substances.
3. Results
3.1 Type of data registered for repeated dose toxicity (RDT)
Data needed to fulfil the information requirements under REACH are recorded in the IUCLID standard format “endpoint study record” (ESR) which consists of predefined as well as free text fields.28 For the 60 substances, a total of 499 ESRs were reported for the endpoint RDT (Table 1). The majority of the ESRs (435) were summaries of experimental studies of which 362 ESRs were specified as “experimental study” and 48 as “read-across” (in read-across, the registrant uses information from a similar substance to predict properties or effects for the target substance to fulfil the information requirements29). For the remaining summaries of experimental studies (25 ESRs), no study result type was reported and thus labelled as “not specified” in this study. Studies were reported as key, supporting, weight of evidence and disregarded depending on how they were used in the hazard assessment. For 32 ESRs, the use of the study was not reported and therefore labelled as “not specified” in this study. In addition to the experimental data, “data waiving” was reported for 62 ESRs and for two substances an experimental study was planned (two ESRs).| Study result type | Number of endpoint study records (ESR) (study summaries) | Total ESR (all substances) | |||||
|---|---|---|---|---|---|---|---|
| Substances (excluding DEHP) | DEHP | ||||||
| Inaccessible data source (%) | Accessible data source (%) | Total ESR | Inaccessible data source (%) | Accessible data source (%) | Total ESR | ||
| Experimental | |||||||
| Key | 104 (79) | 28 (21) | 132 | 1 (25) | 3 (75) | 4 | 136 |
| Supporting | 76 (65) | 41 (35) | 117 | 3 (10) | 29 (90) | 32 | 149 |
| WoE | 2 (40) | 3 (60) | 5 | 0 | 0 | 0 | 5 |
| Not specified | 6 (40) | 9 (60) | 15 | 16 (34) | 31 (66) | 47 | 62 |
| Disregarded study | 2 (20) | 8 (80) | 10 | 0 | 0 | 0 | 10 |
| Read-across | |||||||
| Key | 14 (93) | 1 (7) | 15 | 0 | 0 | 0 | 15 |
| Supporting | 26 (87) | 4 (13) | 30 | 0 | 0 | 0 | 30 |
| WoE | 1 (33) | 2 (67) | 3 | 0 | 0 | 0 | 3 |
| Not specified | |||||||
| Supporting | 2 (100) | 0 | 2 | 0 | 1 (100) | 1 | 3 |
| Not specified | 7 (33) | 14 (67) | 21 | 0 | 1 (100) | 1 | 22 |
| Total ESR (experimental data) | 240 (69) | 110 (31) | 350 | 20 (23) | 65 (76) | 85 | 435 |
| Waiving (waiving justifications) | |||||||
| Study scientifically unjustified | 18 | 0 | 18 | ||||
| Exposure considerations | 8 | 0 | 8 | ||||
| Study technically not feasible | 1 | 0 | 1 | ||||
| Other justification | 35 | 0 | 35 | ||||
| Total ESR (waiving) | 62 | ||||||
| Planned | 2 | 0 | 2 | ||||
| Total ESR (for all study result types) | 499 | ||||||
For 41 of the 60 substances, information was provided for all three major exposure routes: oral, inhalation and dermal (ESI Table 2†). For the remaining 19 substances, information (i.e. experimental studies and data waiving) was lacking for one or two of the three exposure routes.
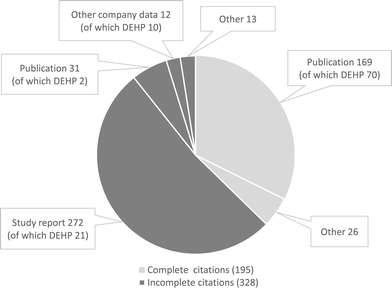 | ||
| Fig. 1 Data source citations. For 60 substances, a total of 523 citations of data sources were recorded of which 63% were not possible to identify, i.e. title, author and/or bibliographic source were not provided (ESI Table 3†). | ||
It was not possible to identify 328 out of 523 (63%) citations, since information on author, title and/or bibliographic source was missing or incomplete (Fig. 1). The majority of the citations, 284 out of the 328 (87%), that were not possible to identify referred to study reports and company data, i.e. data owned by the industry. The remaining 31 (9%) incomplete citations referred to publications. Data sources that were possible to identify mainly referred to publications.
One of the substances, diethylhexyl phthalate (DEHP; EC number 204-211-0), had a substantially higher number of ESRs (85) and data source citations (103) than the other substances included in the study (Fig. 1 and Table 1). Since the majority of the citations for DEHP, 70 out of 103 (68%), referred to publications (Fig. 1), it was possible to identify at least one of the references (if several references were cited for an ESR) for 65 out of 85 (76%) of the ESRs (Table 1). Thus, for DEHP, the reference could be identified and accessed for three out of the four ESRs reported as key studies and for 29 out of 32 (90%) of the ESRs reported as supporting studies.
For the remaining 59 substances included, it was not possible to identify the data source for 240 out of 350 (69%) ESRs (for reported study result types “experimental”, “read-across” and “not specified” in Table 1). For key studies, the data source could not be identified for 118 out of 147 (80%) ESRs since the majority of these referred to study reports or company data. For 14 of the 118 (13%) key studies for which the data source could not be identified, referred to at least one public data source (ESI Table 1†). Hence, it was only possible to identify a cited data source for 29 out of 147 (20%) key studies. Slightly more data sources were possible to identify for supporting studies: 45 out of 147 (31%) ESRs.
Dissemination. The number of citations was not equal to the number of unique studies for several reasons. First, a data source may be cited more than once due to cross-reference to the same study. Second, a study can comprise results on many endpoints which can be recorded under the same or several endpoint sections, or include several experiments.28 Third, for industry reports that have been published, a full reference to the publication should be made in addition to the original study. Since, information on many of the data sources were incomplete, it was not possible to identify the number of unique studies.
The data source for a study summary needs to be reported in the ESR. However, the type of data source reported in the field “reference type”, e.g. study report, publication, and company report, influences what information on the data source will be published on the website.30 The fields for reference type, year and report date are always disseminated for all study summaries regardless of the type of data source. However, author, title and bibliographic source are not automatically disseminated if (1) the IUPAC name of the substance, i.e. the name according to the system developed by the International Union of Pure and Applied Chemistry to unambiguously describe the structure of the substance, is claimed to be confidential, (2) the ESR is claimed as confidential except when the reference type refer to publication, or review article or handbook, (3) the reference type is study report or company data, or (4) if information is given in any of the following fields that are not disseminated on the website: “testing lab”, “report number”, “owner company” or “study number”.30,31 Thus, the origin of study summaries based on industry data are not possible to identify. For the incomplete citations referring to publications, it cannot be confirmed whether the information on the data source was missing due to insufficient reporting on behalf of the registrant or due to filtering according to the dissemination rules. Since the fields “testing lab”, “report number”, “owner company” or “study number” are not disseminated, it cannot be verified whether any information had been provided in these fields and consequently filtered information on author, title and bibliographic source.
Dissemination. Data waiving is reported in IUCLID in two fields: “data waiving” and “data waiving justifications”. The field “data waiving” is a pick list field with four broad categories which is disseminated unless claimed confidential.31,32 In addition, registrants are required to report a justification for waiving data. However, this field is not automatically disseminated on the website.31 According to the filter rule applied to this field, the information is either considered to undermine the commercial interests of the registrant or is not related to the hazard and safe use of the substance.5 Hence, data waiving justifications are not accessible in the REACH registration database unless the registrant has reported the justification in another text field in the ESR that is automatically disseminated.
3.2 Hazard assessment conclusions
Registrants need to assess the hazard for particular target groups (workers and the general population) through the major exposure routes (oral, dermal, inhalation and eyes) and for certain types of effects (acute, chronic, local, systemic) referred to as “exposure patterns”.33 In total, 763 hazard assessment conclusions for various exposure patterns were reported for 57 of the 60 substances (ESI Table 4†). For three of the substances, no hazard assessments were provided. The number of exposure patterns reported varied between substances and for some exposure patterns no hazard assessment conclusion was reported. The hazard assessment conclusions were reported as broad, pre-defined categories, such as “DNEL” (derived no-effect level), “DMEL” (derived minimal effect level) “medium hazard” and “hazard unknown (no further information necessary)” (Table 2). In addition to the hazard assessment conclusion, information on the endpoint on which the assessment is based and the route of the original study could be provided. However, information in these fields was mostly provided for the DNEL conclusions only.| Reported categories in the field “Hazard Assessment Conclusions” (HAC) | Total number of HAC reported | Reporting of the field “Most sensitive endpoint” | Reporting of the field “Route of original study” |
|---|---|---|---|
| DMEL | 5 | 4 | 0 |
| DNEL | 292 | 262 | 82 |
| Exposure based waiving | 28 | 0 | 0 |
| Hazard unknown (no further information necessary) | 16 | 0 | 0 |
| Insufficient data available (further information necessary) | 3 | 0 | 0 |
| No-threshold effect and/or no dose-response information available | 109 | 4 | 0 |
| No DNEL required: short term exposure controlled by conditions for long term | 4 | 1 | 0 |
| No hazard identified | 272 | 16 | 0 |
| High hazard (no threshold derived) | 10 | 3 | 0 |
| Medium hazard (no threshold derived) | 8 | 5 | 0 |
| Low hazard (no threshold derived) | 6 | 4 | 0 |
| Not specified | 10 | 10 | 0 |
| Total number of exposure patterns | 763 | 309 | 82 |
In total, 292 DNEL and 5 DMEL conclusions abbreviated as DN(M)ELs were recorded for 52 of the substances (Table 3). The derivation of the DN(M)EL conclusions could be described in further detail by providing the overall assessment factor as well as specific assessment factors applied with justifications, and the dose descriptor starting point value, e.g. the no or lowest observed adverse effect level, NOAEL or LOAEL. The level of detail on the derivation of the DN(M)EL conclusions reported by the registrant varied between dossiers and also between exposure patterns within the dossier. For three DNEL and DMEL conclusions, no DN(M)EL value was reported in the value field. An overall assessment factor was reported for 221 of the 297 (74%) DN(M)EL conclusions, and for 72 (24%) of the conclusions the assessment factor was further specified and justified. The content of free-text fields for justifying specific assessment factors also varied, ranging from one sentence to paragraphs with detailed description of reasoning and/or calculations. For 199 out of 297 (67%) of the DN(M)EL conclusions, the type of dose descriptor starting point (e.g. NOAEL) was given but only 64 out of 297 (22%) specified a value for the dose descriptor starting point. The endpoint on which the hazard assessment was based was reported for 90% of the DN(M)ELs.
| Disseminated IUCLID fields for DNEL and DMEL hazard assessment conclusions | Number of fields reported for DNEL and DMEL conclusions (%) |
|---|---|
| a For example NOAEL. | |
| Most sensitive endpoint | 266 (90) |
| Route of original study | 81 (27) |
| DN(M)EL value | 294 (99) |
| Overall assessment factor | 221 (74) |
| Specific AF and justification | 72 (24) |
| Dose descriptor starting point (after route to route extrapolation)a | 199 (67) |
| Dose descriptor starting point after route to route extrapolation (value) | 64 (22) |
| Justification for route to route extrapolation | 45 (15) |
| DNEL derivation method | 79 (27) |
| Total number of ESR with DNEL and DMEL hazard assessment conclusions | 297 |
Generally, more comprehensive information on the justifications and discussions of the hazard assessment conclusions were provided in the CSRs in which also the study on which the conclusions was based was referred to, if reported by the registrant.
For three substances (EC number 201-553-2, 201-557-4 and 271-094-0), two summaries of toxicological information, labelled “toxicological information 1” and “2” respectively, were provided on the website with sometimes differing hazard assessment conclusions for the same exposure pattern (ESI Table 4†). For one of the substances, one of the summaries was labelled “JS Member”. The label, although not explained on the website, means that the registrant has made an opt-out from the lead dossier.
3.3 Access to CSRs
The first request for CSRs comprised nine substances. This request was withdrawn after consultation with ECHA since it proved difficult for ECHA to disclose the documents within the legislative time frame due to the length of the documents and the obligation to consult with the registrants.26 The regulation (EC) no 1049/2001, which regulates the accessibility of documents held by EU institutions to the public, sets a time frame of 15 working days for processing requests, by either granting access to the documents or informing the applicant why the request is being partially or totally refused, art. 7(1).25 In cases of extensive requests (several or very long documents) institutions may negotiate the requests to seek a “fair solution”, art. 6(3), as well as extend the time limit with an additional 15 days, art. 7(3). Consequently, the second request was restricted to five substances (Table 4).27 ECHA identified 28 CSRs for the five substances consisting of 100 pages each on average. In order to shorten the time frame for processing the request and limiting the administrative work required, the scope of the request was refined to CSRs provided by the lead registrants (in total five CSRs). Nevertheless, it was foreseen by ECHA that the request could not be processed within the time limit due to the expected work load. Therefore, ECHA proposed to disclose the five CSRs within 60 days.| Redactions of CSRs | EC number | Information blanked out (for one or several of the substances) | Legal framework supporting the redaction | ||||
|---|---|---|---|---|---|---|---|
| 204-825-9 | 229-962-1 | 249-951-5 | 270-700-0 | 271-094-0 | |||
| Composition of the substance | x | x | x | x | The degree of purity, composition of the substance, constituents, impurities and additives and their concentration range respectively. | Protection of commercial interests (article 4(2) first indent of regulation (EC) no 1049/2001). | |
| Quantities | x | x | Precise tonnage and information through which the precise tonnage can be back-calculated (e.g. PEC values and risk characterisation ratios). | Protection of commercial interests (article 4(2) first indent of regulation (EC) No 1049/2001 and article 118(2) of the REACH regulation). | |||
| Manufacturing process | x | x | Details specific to the manufacturing process. | Protection of commercial interests including intellectual property (article 4(2) first indent of regulation (EC) no 1049/2001). | |||
| Certain information in specific endpoint sections | x | The discussion of the results of some of the studies. | Protection of commercial interests including intellectual property (article 4(2) first indent of regulation (EC) no 1049/2001). | ||||
| Certain information related to the exposure scenario for manufacture | x | Information on the production conditions and safety measures (e.g. product characteristics, frequency and duration of use, conditions and measures related to the evaluation of personal protection, hygiene and health). | Protection of commercial interests including intellectual property (article 4(2) first indent of regulation (EC) no 1049/2001). | ||||
| References to the studies | x | x | x | x | x | Names of authors of unpublished studies and other details (e.g. names of testing laboratories and owner companies). | Protection of personal data (article 4(1)(b) of regulation (EC) no 1049/2001) or protection of commercial interest (article 4(2) first indent of regulation (EC) no 1049/2001). |
Access to non-confidential versions of the five CSRs was provided after 57 working days. The CSRs were electronically disclosed with a letter that explained certain redactions of the documents due to confidentiality (Table 4).27 Information in the CSR that was approved as confidential had been redacted manually with black boxes. Information on authors of unpublished studies were blanked out in all documents. In one or several of the documents, information related to the composition of the substance, tonnage production, manufacturing process and details in specific endpoint sections as well as exposure scenarios regarding manufacture was redacted.
4. Discussion
ECHA recognises transparency as one of the Agency's core values and is committed to continuously enhance transparency by improving dissemination of information on substances.36,37 As a result, ECHA launched in June 2016 new dissemination pages to present information on substances in the REACH registration database in a simpler and more accessible way to improve the usability of the information, particularly for non-scientific users.38,39 One of the new changes in the dissemination pages addresses one of the aspects highlighted in this study. However, despite this change it remains difficult to follow certain steps that registrants undertake in assessing the hazard and risk of substances as information is currently provided to the public. The transparency of the hazard and risk assessment of substances under REACH was shown to be influenced by (1) confidentiality, (2) reporting by registrants, (3) lack of clarity of disseminated information, (4) verification of information and (5) accessibility to information.4.1 Confidentiality
ECHA states in their approach to transparency that it needs to be balanced with other aspects, such as the laws protecting confidential business information, intellectual property rights and personal data.37 However, confidentiality restricts the access to information that is crucial for understanding the conclusions drawn in the hazard and risk assessment. In one of the CSRs the discussion of the results of some studies as well as data waiving justifications were claimed confidential and thus the text was partially or fully blanked out. This means that it is only ECHA and member states competent authorities that have full access to the information and thus can evaluate and scrutinise justifications and conclusions provided in the assessments.Confidentiality also restricts the possibility to identify the data source of the studies used in the hazard and risk assessment. For the majority of the key studies included in this study, it was not possible to identify the data source since most of the key studies were industry studies for which only information on reference type (i.e. study report) and year of publication was disseminated. Details on the data source of industry studies are not disseminated due to protection of personal data (names of authors, affiliation/and or laboratory) and commercial interests (the title may contain the IUCLID name which can be confidential). In general, industry studies are used and preferred over other types of data when conducting regulatory risk assessment since they are performed according to standardised test methods and GLP and therefore considered to be reliable.40 Thus, if the results from this investigation are representative, then much of the in vivo toxicological data used as key and ESI† in the hazard and risk assessments under REACH are not possible to identify and scrutinize.
The filter rules that are applied to dossiers before they are published on the website also remove information that may be confidential without taking into consideration whether the information in fact is confidential. For example, the justification fields for data waiving and hazard assessment conclusions are not automatically disseminated on the website31 because the information is either considered to undermine the commercial interests of the registrant or not related to the hazard and safe use of the substance according to the filter rule.5 ECHA stated, in a response to an enquiry made by the organisation ClientEarth, that data waiving justification fields were not disseminated since they could contain confidential information.9 Since the hazard assessment conclusion justification is related to the hazard and safe use of the substance, it is likely that this field is not disseminated for the same reason as for data waiving justifications although these justifications are reported in the CSRs. Since the CSRs are manually reviewed, only information that has been claimed and approved as confidential during the consultation with the registrant is blanked out. Thus, information that is not confidential but important for understanding the steps in the hazard and risk assessment are not accessible to the public through the ECHA website but only by requesting access to the CSRs.
This filter rule has until recently also applied to the endpoint summaries which links the DN(M)EL derivation to the toxicological study on which the calculation is based. So far, the study can only be indirectly identified through other fields describing the derivation of the DN(M)EL. The difficulty to identify the study on which the DN(M)EL is based has also been highlighted in previous studies.12,18 However, from June 2016, endpoint summaries will be disseminated for new and updated dossiers as part of ECHA's work on improving transparency. This will hopefully enable the identification of the study used for deriving the DN(M)EL. Nevertheless, what fields will be disseminated from the endpoint summaries still remain to be seen. However, the fields for DNEL justifications and conclusions will as previously not be published30 and access to these fields is also important to fully understand the assessment made.
Although confidentiality may be warranted in certain situations, any non-disclosure of information needs to be weighed against the citizen's right to know, the consequences of keeping information confidential and thus the benefits of transparency to the society as a whole. What is considered to be confidential has varied over time as well as between legislations and Member States and therefore is subject to renegotiation. In cases where transparency is limited due to confidentiality, it is important that the confidentiality claims can be scrutinised. On the new dissemination pages, ECHA indicates what information has been approved as confidential and consequently is removed. Transparency would be further enhanced if the justification for claiming the information as confidential as well as the ECHA decision approving the claim also were to be published just like other ECHA decisions are published on their website.
4.2 Reporting of information
Information can only be disseminated as long as it has been reported by the registrant. The extent of reporting varied between dossiers as well as within certain sections of the dossier. For three substances, no hazard assessment conclusions were reported although required for substances produced at or above 10 tonnes per year. The number of hazard assessment conclusions reported as well as the information provided for DN(M)ELs (e.g. assessment factors and justifications) also varied. For some substances, the registrants did not specify how the information was used in the assessment, i.e. whether studies were used as key or supporting studies or altogether disregarded, and certain bibliographic references were only partly reported. Incomplete reporting inevitably affects the possibility to understand and evaluate the hazard and risk assessment made by the registrant and thus is a prerequisite for transparency. In certain cases, it was difficult to assess whether information was missing due to incomplete reporting or due to the filter rules applied in the dissemination process.The problem with reporting has been brought up in an appeal before the Board of Appeal (BoA). A registrant contested ECHA's decision to consider another registrant's registration dossier as complete although it lacked basic data on physicochemical and toxicological data according to the standard information requirements.41 Since the automated IT system in the completeness check only verifies that information has been provided in the fields and the subsequent compliance check only covers a minimum of five percent of the dossiers registered in each tonnage band, this means that dossiers can be approved without fulfilling all legal requirements. The BoA noted in their decision that the current completeness check with the automated IT system is not sufficient to fulfil ECHA's obligation to verify that dossiers are complete.41 ECHA has announced that they will from now on manually verify the completeness of dossiers in addition to the automated completeness check.8
4.3 Clarity
An important aspect of transparency relates to the clarity and comprehensibility of the published information.42 In the new dissemination pages, ECHA has worked on presenting information on substances in a simpler and more comprehensible way particularly for non-scientific users in an “info card” and “brief profile”. However, the raw data from the dossiers are still partly displayed in a way that makes it difficult to follow the reasoning in the hazard and risk assessment and understand the specifics. The data waiving and hazard assessment conclusions were provided as broad pre-defined categories of which most of the categories are not self-explanatory and sometimes difficult to find the explanation for in the ECHA guidance documents. Clarity would be improved by providing pop-up information windows on the dissemination pages for the raw data source for explaining the meaning of various fields as well as where in the legislation and the guidance more information can be found. Such pop-up windows are currently provided in the info card and brief profile. Although, explaining the application of broad categories will not provide the reason as to why a certain category is applicable in each particular case. This can only be resolved by disseminating the actual justification which is currently removed from the website for confidentiality reasons during the dissemination process.ECHA's decision to publish endpoint summaries will hopefully clarify why no data were submitted for some of the exposure routes and why certain exposure patterns were not considered to be relevant for the assessment. However, it will not explain the multiple summaries of toxicological information with sometimes different hazard assessment conclusions for the same exposure pattern which were published for three of the substances included in the study.
4.4 Verification of information
Accurate reporting of physicochemical properties and results from toxicity studies is important in order to assess the hazards and risks of a substance and the possibility to verify data is central to instill stakeholder trust. In REACH art. 3(29), registrants are not required to provide full study reports, but rather a summary of the study. Thus, information in summaries based on industry reports42 cannot be verified since such studies are intellectual property rights of the company. Moreover, sufficient information to enable an independent assessment of the study is only required for robust study summaries such as key studies, REACH art. 3(28). Consequently, the system places high trust in data that cannot be verified and could potentially be biased due to conflict of interest. In a study which looked at the registration dossiers of five endocrine disrupting substances, ClientEarth found that important information in the original studies had not been included in the study summary.9 Although ECHA can request information required under REACH, art. 36, it would be resource and time-consuming for the agency to validate and verify all submitted data.4.5 Accessibility to information
The disclosure of information on substances through the REACH registration database greatly increases the availability and accessibility of chemical data to the public. However, some information from the registration dossier is not accessible via the website, but only through the CSR. This includes information that is not confidential but filtered in the dissemination process, as well as the exposure assessment (although information on exposure scenarios will be published from June 2016) and risk characterisation required for substances fulfilling certain hazard categories under REACH art. 14(4).The current system for retrieving CSRs from ECHA is work-intensive and time-consuming which restricts the amount of information that the public can request and how readily it can be provided. Therefore, it is advised to find a solution that will provide the same information in a less work-intensive and timely manner, for example by requiring registrants to submit a non-confidential version of the CSR.
5. Conclusion
One of the objectives of REACH is to increase transparency by making information on substances accessible to the public, which is realised by disseminating information on the ECHA website. Thus, basic information on the hazards of the substances and common uses is summarised in info cards and brief profiles in an accessible way to the general public. However, it is also of interest to know how registrants reach their conclusions in the hazard and risk assessments. As shown in this study, the information underlying conclusions on hazards and risks for substances registered under REACH is accessible to the public in a semi-transparent way that makes it difficult for a third party to fully evaluate the registrant's assessment. First, the protection of commercial interests by keeping information confidential severely limits the accessibility to important information. Furthermore, non-confidential information is removed in the automatic filtering step in the dissemination process of the dossiers as a precaution because they may contain confidential information. We suggest that ECHA review the filtering step so that only information that is truly claimed or always considered to be confidential is removed. Second, poor reporting by registrants affects the availability of data. Improving the completeness check of dossiers will be an important step towards ensuring that the required data is also provided. Third, the information provided in the dossier is not always clear and could be further explained to improve the comprehensibility of the information. Fourth, the reference for the majority of the studies could not be identified since they referred to industry data that are intellectual property of the company. For this reason, it is not possible to verify if all important information to evaluate the study has been provided since the registrants are only required to submit a summary of the studies. It would be preferable if all data on which assessments were based were available for public scrutiny and evaluation. Fifth, the process of requesting CSRs that contain information that is currently not disseminated on the ECHA website is work-intensive and time-consuming. Thus, it cannot be guaranteed that information that in theory is non-confidential can be accessed. Publishing a non-confidential version of the CSR on the website would make more information on the hazard and risk assessment of the substances easily accessible to the public. Since it is known that hazard and risk assessments are influenced by expert judgments and conflict of interests, it is important to enable other stakeholders to evaluate the scientific robustness of the assessment. Some of the recommended/proposed changes require a change in the regulation, others fall under ECHA's mandate to change.Conflict of interests
Christina Rudén is a member of ECHA's management board, nominated by the European parliament. However, ECHA was not involved in funding this project nor in any parts of the research.Authors' contribution
The study was designed by all authors. The draft was written by Ellen Ingre-Khans. All authors commented on the data, revised the manuscript and approved it for publication.Abbreviation
| BfR | German federal institute for risk assessment (Bundesinstitut für Risikobewertung) |
| BoA | Board of Appeal |
| CSR | Chemical Safety Report |
| DNEL | Derived No Effect Level |
| DMEL | Derived Minimal Effect Level |
| ECHA | European CHemicals Agency |
| EC number | European Community number |
| ESR | Endpoint Study Record |
| GLP | Good Laboratory Practice |
| IUCLID | International Uniform Chemical Information Database |
| LOAEL | Lowest Observed Adverse Effect Level |
| NOAEL | No Observed Adverse Effect Level |
| RDT | Repeated Dose Toxicity |
| REACH | Registration, Evaluation, Authorisation and restriction of CHemicals |
Acknowledgements
This project was funded by Stockholm University faculty grants. The authors would like to thank BfR for providing a data set of dossiers for selection for the project, Marsha Hanson for valuable help in building the Microsoft Access database and the two anonymous reviewers for valuable comments on previous drafts of the manuscript. The authors would also like to thank Steve Hollins for having reviewed and commented on the manuscript in a personal capacity.References
- EC, 2006, Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC.
- European Chemicals Agency (ECHA), Guidance on Registration. Guidance for the implementation of REACH, 2012 Search PubMed.
- EC, 2001, White Paper, Strategy for a future chemicals policy, COM(2001) 88 final.
- REACH Registration database, September 2015, http://echa.europa.eu/web/guest/information-on-chemicals/registered-substances Search PubMed.
- European Chemicals Agency (ECHA), Data submission manual. Part 15-Dissemination, 2012 Search PubMed.
- G. Stieger, M. Scheringer, C. A. Ng and K. Hungerbühler, Chemosphere, 2014, 116, 118–123 CrossRef CAS PubMed.
- Bundesinstitut für Risikobewertung (BfR), REACH Compliance: Data Availability of REACH Registrations. Part 1: Screening of Chemicals >1000 tpa, 2015 Search PubMed.
- European Chemicals Agency (ECHA), Report on the operation of REACH and CLP 2016, 2016 Search PubMed.
- Client Earth, REACH registrations and endocrine disrupting chemicals, 2013 Search PubMed.
- E. Westerholm and L. Schenk, Regul. Toxicol. Pharmacol., 2014, 68, 51–58 CrossRef CAS PubMed.
- Joint NGOs, REACH dissemination of information on chemical substances, CARACAL Agenda item 5.2, June 2016, http://www.eeb.org/?LinkServID=7D4B5C42-BA27-0707-83F32CCA38A892F0&showMeta=0&aa Search PubMed.
- L. Schenk, N. Palmen and D. Theodori, Evaluation of worker inhalation DNELs. Part A: quality assessment of a selection of DNELs, 2014 Search PubMed.
- European Environmental Bureau and Client Earth, Identifying the bottlenecks in REACH implementation. The role of ECHA in REACH's failing implementation, 2012 Search PubMed.
- C. Rudén, PhD thesis, Karolinska Institute, 2002.
- L. Schenk, Int. J. Occup. Environ. Health, 2010, 16, 249–262 CrossRef CAS PubMed.
- A. Beronius, C. Ruden, H. Hakansson and A. Hanberg, Reprod. Toxicol., 2010, 29, 132–146 CrossRef CAS PubMed.
- C. Rudén, Toxicol. Lett., 2006, 167, 201–204 CrossRef PubMed.
- L. Schenk, U. Deng and G. Johanson, Ann. Occup. Hyg., 2015, 59, 416–438 CrossRef PubMed.
- B. Wandall, S. O. Hansson and C. Ruden, Arch. Toxicol., 2007, 81, 605–617 CrossRef CAS.
- T. Wilholt, Stud. Hist. Philos. Sci., 2008, 40, 92–101 CrossRef.
- J. R. Rohr and K. A. McCoy, Conservation Letters, 2010, 3, 143–150 CrossRef.
- F. S. vom Saal and C. Hughes, Environ. Health Perspect., 2005, 113, 926–933 CrossRef CAS.
- B. Wandall, Toxicol. Lett., 2004, 152, 265–272 CrossRef CAS.
- M. Ågerstrand and A. Beronius, Environ. Int., 2016, 92–93, 590–596 CrossRef.
- EC, 2001, Regulation (EC) No 1049/2001 of the European Parliament and of the Council of 30 May 2001 regarding public access to European Parliament, Council and Commission documents.
- European Chemicals Agency (ECHA), Clarification of access to document application, Reference number ADT 40/2014, 2014 Search PubMed.
- European Chemicals Agency (ECHA), Access to documents, Reference number ATD/45/2014. Registration number: D(2014)D5585, 2014 Search PubMed.
- European Chemicals Agency (ECHA), IUCLID 5. End-user Manual, 2013 Search PubMed.
- European Chemicals Agency (ECHA), Practical guide 6-how to report read-across and categories, 2012 Search PubMed.
- European Chemicals Agency (ECHA), Dissemination and confidentiality under REACH regulation, 2016 Search PubMed.
- European Chemicals Agency (ECHA), Data submission manual. Part 15-dissemination: how to determine what will be published on the ECHA website from the registration dossier, Technical annex for IUCLID section 7, 2012 Search PubMed.
- European Chemicals Agency (ECHA), Data submission manual. Part 05-how to complete a technical dossier for registrations and PPORD notifications, 2013 Search PubMed.
- European Chemicals Agency (ECHA), Practical guide 14: how to prepare toxicological summaries in IUCLID and how to derive DNELs, 2012 Search PubMed.
- European Chemicals Agency (ECHA), Guidance on information requirements and chemical safety assessment, 2012, ch. R.8: Characterisation of dose [concentration]-response for human health Search PubMed.
- European Chemicals Agency (ECHA), More information to be published from REACH registrations, May 2016, http://echa.europa.eu/view-article/-/journal_content/title/more-information-to-be-published-from-reach-registrations.
- European Chemicals Agency (ECHA), Transparency, May 2016, http://echa.europa.eu/about-us/the-way-we-work/procedures-and-policies/transparency Search PubMed.
- European Chemicals Agency (ECHA), ECHA's approach to transparency, May 2016, http://echa.europa.eu/documents/10162/13608/mb_61_2014_echa_transparency_en.pdf Search PubMed.
- European Chemicals Agency (ECHA), From an info card to detailed data source – ECHA's plans for chemicals communication, May 2016, http://newsletter.echa.europa.eu/home/-/newsletter/entry/1_14_from-an-info-card-to-detailed-source-data Search PubMed.
- European Chemicals Agency (ECHA), New substance infocards – more useful and transparent information on chemicals, May 2016, http://newsletter.echa.europa.eu/home/-/newsletter/entry/5_15_new-substance-infocards-more-useful-and-transparent-information-on-chemicals Search PubMed.
- H. J. Klimisch, M. Andreae and U. Tillmann, Regul. Toxicol. Pharmacol., 1997, 25, 1–5 CrossRef CAS.
- European Chemicals Agency (ECHA), Decision of the Board of Appeal of the European Chemicals Agency. Case number A-022–2013, 2016 Search PubMed.
- A. K. Schnackenberg and E. C. Tomlinson, J. Manag., 2014 DOI:10.1177/0149206314525202.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6em00389c |
| This journal is © The Royal Society of Chemistry 2016 |
