Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Prolonged exposure to arsenic in UK private water supplies: toenail, hair and drinking water concentrations†
D. R. S.
Middleton
abc,
M. J.
Watts
*b,
E. M.
Hamilton
b,
T.
Fletcher
c,
G. S.
Leonardi
c,
R. M.
Close
c,
K. S.
Exley
c,
H.
Crabbe
c and
D. A.
Polya
a
aSchool of Earth, Atmospheric and Environmental Sciences & William Research Centre for Molecular Environmental Science, University of Manchester, Oxford Rd, Manchester, M13 9PL, UK
bInorganic Geochemistry, Centre for Environmental Geochemistry, British Geological Survey, Nicker Hill, Keyworth, Nottinghamshire NG12 5GG, UK. E-mail: mwatts@bgs.ac.uk
cCentre for Radiation, Chemicals and Environmental Hazards (CRCE), Public Health England, Chilton, Didcot, Oxfordshire OX11 0RQ, UK
First published on 19th April 2016
Abstract
Chronic exposure to arsenic (As) in drinking water is an established cause of cancer and other adverse health effects. Arsenic concentrations >10 μg L−1 were previously measured in 5% of private water supplies (PWS) in Cornwall, UK. The present study investigated prolongued exposure to As by measuring biomarkers in hair and toenail samples from 212 volunteers and repeated measurements of As in drinking water from 127 households served by PWS. Strong positive Pearson correlations (rp = 0.95) indicated stability of water As concentrations over the time period investigated (up to 31 months). Drinking water As concentrations were positively correlated with toenail (rp = 0.53) and hair (rp = 0.38) As concentrations – indicative of prolonged exposure. Analysis of washing procedure solutions provided strong evidence of the effective removal of exogenous As from toenail samples. Significantly higher As concentrations were measured in hair samples from males and smokers and As concentrations in toenails were negatively associated with age. A positive association between seafood consumption and toenail As and a negative association between home-grown vegetable consumption and hair As was observed for volunteers exposed to <1 As μg L−1 in drinking water. These findings have important implications regarding the interpretation of toenail and hair biomarkers. Substantial variation in biomarker As concentrations remained unaccounted for, with soil and dust exposure as possible explanations.
Environmental impactArsenic is an established carcinogen, chronic exposure to which has been linked to several cancers (lung, bladder, skin) as well as non-cancerous (cardiovascular disease, diabetes mellitus) health effects. This work consists of a human biomonitoring study (a collaboration between the University of Manchester, British Geological Survey and Public Health England) of 212 volunteers from 127 households with private water supplies from across Cornwall, UK. It is the largest scale exposure biomonitoring study conducted for As and drinking water in the UK to-date and investigates an exposure source for As that, until recently, had not been investigated in depth in the region. The sampling protocol consists of an initial and follow-up water collection spanning a period of either 8 or 31 months which, together with long-term biomarkers such as toenails and hair, allows for the assessment of prolonged arsenic exposure. Furthermore, the methods employed in this paper allow for an assessment of the efficacy of toenail washing procedures given the recognition of the susceptibility of this biomonitoring matrix to external contamination. The demonstration of effective contamination removal from samples in this study will be of great benefit to the wider field. |
1. Introduction
Chronic exposure to arsenic (As) in contaminated drinking water is an established cause of lung, skin, bladder and kidney cancer1 as well as other adverse health effects, posing a global health concern. Five major As endemic regions of the world provide the strongest evidence of this association: north-west and south-east Taiwan;2 northern Chile;3 Argentina;4,5 Bangladesh6 and West Bengal.7 Although the aforementioned areas are more severely affected, As contaminated municipal and private water supplies (PWS) have been reported in countries across all inhabited continents.8 Notable European examples include Hungary,9 Romania,9 Slovakia9 and Serbia.10A survey11 of PWS in Cornwall, south-west England, reported concentrations exceeding the 10 As μg L−1 UK prescribed concentration or value12 (PCV) and WHO guidance value13 in 5% of drinking water samples collected (n = 497). In a follow-up biomonitoring study,14 a subset of the same cohort, drinking water As concentrations were positively correlated with urinary As concentrations after the exclusion of arsenobetaine (AB) and adjustment for hydration (osmolality adjustment). These urinary As concentrations reflected exposure in the preceding 2–4 days.15 Information on the longevity and temporal variation of exposure in this study group was still outstanding. Two methods that can assess exposure over extended timescales are repeat monitoring of drinking water As concentrations and monitoring of biological matrices, such as toenails and hair, that reflect a longer exposure window than urine. Both approaches were employed in the present study.
There are currently 2460 registered single domestic dwellings served by PWS in Cornwall,16 with the true number likely to be much greater. No published data on the temporal variation of As concentrations in UK PWS were previously available, but studies elsewhere reported mixed findings. In Nevada, USA, although concentration changes (mean = −3 As μg L−1) were measured in some supplies,17 with greater changes associated with higher As concentrations, no clear temporal trends were observed between wet and dry seasons. In a related study,18 strong Spearman correlations (rs) (rs = 0.95) were reported between As concentrations in the same wells over a period of 11–20 years, with both studies concluding that, for the region, limited measurements are sufficient for predicting exposures over such timescales. Similarly, in Michigan, USA, strong Pearson correlations (rp) (rp = 0.88) were reported19 between As measurements taken an average of 14 months apart. Concentrations were affected by point-of-use (POU) treatment systems, highlighting the necessity of collecting treatment usage data. Conversely, a study conducted in Washington, USA20 reported changes as high as 19-fold in As concentrations measured in the same supply 12 months apart, suggesting that temporal stability of As concentrations varied by region due to geological and geochemical variables, if not inconsistencies in sampling methodologies.19
The use of toenail and hair biomonitoring for As exposure offers the assessment of a longer exposure window than that reflected by urine sampling. The affinity of As for sulfhydryl groups in the keratin of nails and hair, the isolation of these matrices from other metabolic processes following their formation and the time taken for them to ‘grow out,’ makes them attractive for measuring biomarkers of past As exposure.21 Nails and hair have the added value of a non-invasive collection protocol and few sample transport/storage requirements. Positive correlations between drinking water and biomarker As concentrations have been reported in numerous studies for both toenails22–25 and hair.21,26 Increased risk of various cancers, including cutaneous melanoma27 and small and squamous-cell carcinoma of the lung,28 have also been positively associated with toenail As concentrations.
Despite the advantages of toenail and hair biomonitoring, caveats apply when using these matrices to assess exposure. Factors unrelated to exposure have been reported to influence As concentrations in hair and nails: namely, the inter-individual variability of growth rates of the biomonitoring matrices, demographic and behavioural factors such as age, gender and smoking,23 their susceptibility to external contamination29,30 and the consumption of dietary items such as fruit juices,31 beer,32 wine32 and dark-meat fish.33
Average growth rates for fingernails are 0.1 mm per day whereas toenails are estimated to grow by 0.03–0.5 mm per day, meaning that fingernails and toenails reflect exposure windows dating back approximately 6 and 12–18 months, respectively.34 Hair reflects a period of just a few months, with reported scalp hair growth rates ranging from 0.2 to 1.12 mm per day.35 Growth rates for both matrices have been demonstrated to vary with demographic factors e.g. age and gender,29,34–36 with obvious implications for interpreting exposure assessments conducted on diverse populations.
The susceptibility of nails and hair to external contamination is well documented, with a range of washing procedures having been implemented.29,37 The degree of sample contamination likely depends on personal hygiene, hobbies, other behavioural variables and the relative ubiquity of the chemical element of interest. Fingernails are reportedly more prone to contamination than toenails38 but this does not likely apply to communities who are often barefoot or wear open toed footwear. Contamination of hair and nails from cosmetic products such as shampoos, hair colourings and nail polish is another important consideration. A study39 of the trace element composition of nail polish estimated that the As contribution from polish, if present, can range from 16 to 633%.
Whilst studies now routinely report the washing of nail and hair samples prior to analysis, few have quantified the degree of exogenous As versus As in toenails, or confirmed the removal of exogenous As from samples. One investigation40 of exposure to As in soils, also conducted in Cornwall, retained toenail washing solutions for As determination. Both the final rinse fractions and a pooled solution of all preceding fractions were retained to quantify exogenous As contamination and confirm its removal from samples. The As content of final rinse fractions accounted for 0.2 to 1.6% of the total As measured in toenails.40 This provided strong evidence of the efficacy of the washing procedure but, with a sample of 17 volunteers, the performance of this method remained to be validated on a greater scale.
The present study aimed to assess exposure to inorganic As via drinking water consumption in a population served by PWS in Cornwall, UK, using hair and toenail biomarkers in addition to initial and follow-up drinking water samples collected up to 31 months apart. Specific objectives were to (i) compare repeat PWS drinking water As concentrations measured either 8 or 31 months apart; (ii) investigate the effects of As concentration, duration between measurements, source type and treatment usage on changes in drinking water As concentrations; (iii) measure the total As concentrations in toenail and hair samples collected from volunteers and assess their relationship with drinking water As concentrations adjusted for other covariables (demographic, behavioural and dietary) and (iv) quantify the potential for external sample contamination to affect As concentrations in toenail and hair samples, including the use of nail polish and hair dye.
2. Experimental
Ethical approval and volunteer communication
Ethical approval was granted by the University of Manchester Research Ethics Committee (Ref 13068) and the NHS Health Research Authority National Research Ethics Service (NRES) (Ref 13/EE/0234). All volunteers provided written informed consent prior to participating. Individual data feedback to participants was provided through a letter containing specific guidance developed by PHE along with BGS and Cornwall County Council. Participants were given advice on any potential health risks and suggested corrective actions if they had one or more exceedances of the water quality standards. All participants were provided with appropriate contact details for any follow-up enquiries.Recruitment and sample collection
Chemical analyses
Toenail samples were dried to constant weight (12 h approx.) in a clean laminar flow hood (Envair, UK) and stored in microcentrifuge tubes in a silica gel desiccator before being weighed (0.1 g or as much as available) into PTFA MARS Xpress vessels (CEM Corporation, UK). Four millilitres of concentrated HNO3 + 1 mL of H2O2 were added and samples were left to rest for 30 minutes until effervescence subsided. Vessels were capped and digested in a microwave assisted reaction system (MARS Xpress, CEM Corporation, UK) on the following heating program: ramped to 100 °C and held for 5 minutes; ramped to 200 °C and held for 30 minutes (100% power: 1200 W). Vessels were left to cool overnight before their contents were transferred into PFA vials with DIW and reduced to a gel at 80 °C on a graphite hot block. One millilitre of 10% v/v HNO3 was added to the vessels, which were then heated for 20 minutes at 50 °C followed by the addition of 4 mL of DIW. Digests were stored in polystyrene ICP-MS tubes.
Hair samples underwent the same cleaning and digestion procedure as toenail samples. Whatman Grade B-2 weighting papers (GE Healthcare Life Sciences, UK) and a Milty Zerostat 3 anti-static gun were used to aid the transfer of hair samples between vessels.
Statistical analyses
Statistical tests and plot production were performed using R version 3.0.0 (base package).43 Pearson correlation coefficients with significance tests (p-values) and 95% confidence intervals (C.I) were used to assess the strength in relationship between: initial versus follow-up drinking water As; well depth versus As concentration difference; rinse versus digest As concentrations and drinking water versus toenail/hair As concentrations. Welch's independent unequal variance tests were used to test for differences in toenail, hair and rinse solution As concentrations between different subsets to account for unequal sample sizes. One-way analysis of variance (ANOVA) was used to test for differences in toenail and hair As concentrations between different age groups. Multiple linear regression models were constructed to assess significant predictors of toenail and hair As in addition to drinking water As. Exploratory analyses revealed positively skewed distributions for drinking water, toenail and hair As concentrations and As concentrations in rinse solutions. To address this, natural log(ln) transformations were applied to these variables prior to Pearson correlations, Welch's tests, ANOVA and multiple regression modelling. For the same reason, geometric means (GM) were calculated instead of arithmetic means. Left censoring was applied to hair As concentrations (n = 8) below the analytical limit of detection (LOD) by replacing values with half of the LOD.3. Results and discussion
Study group
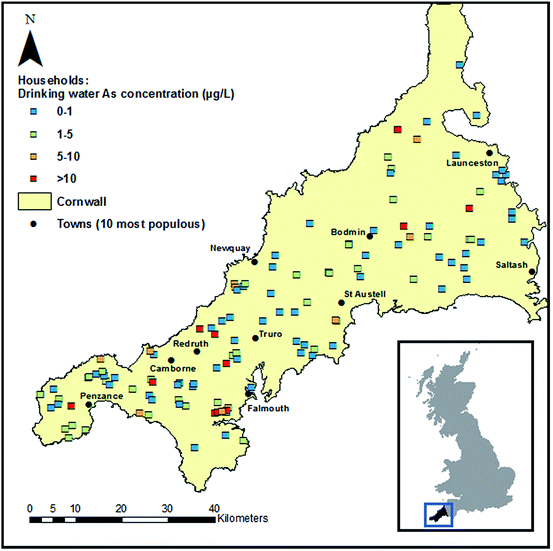
The spatial extent of the study is presented in Fig. 1 and characteristics of households and volunteers are shown in Table 1. Two hundred and twelve volunteers from 129 households reported using their PWS for human consumption and provided either a toenail sample, hair sample or both. This made the present study the largest investigation of long-term exposure to As in drinking water in the UK to-date. Repeated water samples were available for comparison from 127 households, the majority of which were supplied by a borehole. The age distribution of the study group was not representative of the corresponding local rural population, with 63% of volunteers aged over 60. It is noted here that population-based exposure estimates were not the focus of the present paper. Nail polish usage was reported by 17 of the 206 (8%) volunteers who provided toenails, whereas polish was observed on the toenail samples of almost double that number (30, 15%). This underlines the importance of checking nails for visible polish prior to analysis and not relying on questionnaire data alone.| a Subsequent characteristics and percentages in households section refer to this subset. | |
|---|---|
| Households | 129 |
| Initial and follow-up water sample, n (%)a | 127 (98.4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Initial sample year, n (%) | |
| 2011 | 51 (40.2) |
| 2013 | 76 (59.8) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Source type, n (%) | |
| Borehole | 111 (87.4) |
| Well | 11 (8.7) |
| Spring capture | 2 (1.6) |
| Other | 3 (2.4) |
| Borehole depth reported, n (%) | 62 (48.8) |
| Mean borehole depth (m) | 48 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Treatment system, n (%) | |
| Fe/Mn removal | 18 (14.2) |
| pH buffering | 60 (47.2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Storage (e.g. water tank) in system, n (%) | |
| Yes | 62 (48.8) |
| No | 65 (51.2) |
| Volunteers | 212 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Gender, n (%) | |
| Male | 109 (51.4) |
| Female | 103 (48.6) |
| Mean age, years (range) | 62 (18–90) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Age group, n (%) | |
| 18–29 | 6 (2.8) |
| 30–39 | 3 (1.4) |
| 40–49 | 28 (13.2) |
| 50–59 | 42 (19.8) |
| 60–69 | 75 (35.4) |
| 70–79 | 44 (20.8) |
| 80–90 | 14 (6.6) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Smoking status, n (%) | |
| Currently smoking | 13 (6.1) |
| Not currently smoking | 191 (90.1) |
| Not reported | 8 (3.8) |
| Provided toenails, n (%) | 206 (97.2) |
| Provided hair, n (%) | 186 (87.7) |
| Provided both, n (%) | 180 (84.9) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Cosmetic usage, n (%) | |
| Polish usage reported (if toenails provided) | 17 (8.3) |
| Polish observed on toenails | 30 (14.6) |
| Dye usage reported (if hair provided) | 31 (16.7) |
Repeated drinking water measurements
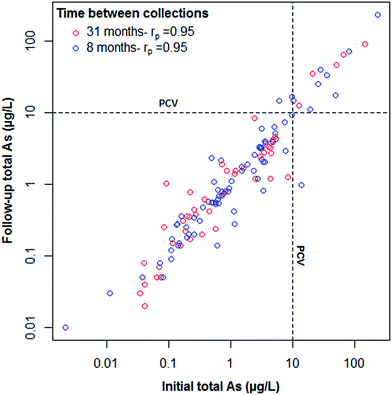
At the initial sampling phase, 125 out of 127 households (98%) had detectable (>0.02 μg L−1) concentrations of total As measured in their drinking water. At follow-up sampling, 126 out of 127 (99%) households had As concentrations >0.02 μg L−1. Only fourteen of the 127 households (11%) exceeded the 10 As μg L−1 UK PCV and WHO guidance value at initial sampling with a maximum As concentration of 231 μg L−1. Two households had borderline results (>9 As μg L−1), one of which exceeded the PCV (17 As μg L−1) at follow-up sampling. One further household, below PCV at initial sampling, exceeded at follow-up (from 6 to 17 As μg L−1). Only one exceedance dropped below PCV at follow-up sampling, from 14 to <1 As μg L−1. Households who had high As concentrations in their PWS were advised to install appropriate remediation. Changes were not attributed to installation of treatment systems. Of three households that reported installation of any kind of treatment system between initial and follow-up measurements, none were among those exceeding the PCV and the impacts on As concentrations were minimal. Of the 14 households above PCV at initial sampling, 11 reported not installing any additional treatment and data were missing for the remaining three. This has important implications regarding risk awareness and the advice given to households above PCV.Overall, As concentrations in PWS were stable over both 8 and 31 month periods. Mean differences in As concentrations, initial and follow-up GM As concentrations and Pearson correlation coefficients between initial and follow-up As concentrations are shown in Table 2. Follow-up As concentrations are plotted against their initial counterparts in Fig. 2. In agreement with previous studies,17,18 strong Pearson correlations were observed between initial and follow-up samples collected both 8 (rp = 0.95) and 31 (rp = 0.95) months apart. A greater mean difference was observed for PWS with >10 As μg L−1 due to the higher concentrations reported in this group. The strongest correlation observed was for the subset of households with both iron (Fe) and manganese (Mn) removal systems and pH buffering systems (rp = 0.998) in addition to a lower mean difference to supplies with neither treatment system. This is not unexpected given that supplies with treatment systems installed are not subject to underlying geochemical variations. Although no household in this study group reported using As-specific treatment systems, Fe/Mn removal units have been reported to reduce As concentrations.11 Of the 62 households where borehole depth information was available, no significant correlation was observed between depth and the difference in As concentration between initial and follow-up sampling. This is consistent with previous studies.17 Source type influence was only assessed between well and borehole sources due to a limited number of other source types. There was no apparent difference in As concentration changes between well or borehole source types or system storage. An observation was made regarding the correct categorisation of source type. One household in the present study reported using a borehole at initial sampling but on receiving initial results (80.5 As μg L−1) it was discovered to be a disused mine adit (categorised as ‘other’ in Table 1). This highlights the importance of homeowners seeking the correct characterisation of their PWS when acquiring a new property.
| Subsets | n | Mean difference (As μg L−1) | Initial total As GM (As μg L−1) | Follow-up total As GM (As μg L−1) | Pearson correlation (rp) |
|---|---|---|---|---|---|
| All households | 127 | −0.7 | 1.0 | 1.0 | 0.95 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Initial sample year | |||||
| 2011 | 51 | −1.1 | 0.8 | 0.9 | 0.95 |
| 2013 | 76 | −0.5 | 1.2 | 1.2 | 0.95 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Initial total As concentration | |||||
| <1 μg L−1 | 67 | 0.1 | 0.2 | 0.3 | 0.87 |
| 1–10 μg L−1 | 46 | −0.1 | 3.2 | 2.7 | 0.68 |
| >10 μg L−1 | 14 | −6.6 | 36.5 | 27.9 | 0.79 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Source type | |||||
| Borehole | 111 | −0.8 | 1.2 | 1.1 | 0.95 |
| Well | 11 | 0.4 | 0.3 | 0.4 | 0.97 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Treatment system | |||||
| Fe/Mn removal only | 12 | −0.2 | 1.7 | 1.6 | 0.95 |
| pH buffering only | 54 | −0.2 | 0.8 | 0.8 | 0.94 |
| Both of above | 6 | −0.3 | 0.5 | 0.5 | 1 (0.998) |
| Neither of above | 55 | −1.8 | 1.2 | 1.3 | 0.94 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Storage (e.g. water tank) in system | |||||
| Yes | 62 | −1.7 | 1.1 | 1.1 | 0.94 |
| No | 65 | 0.3 | 0.9 | 1.0 | 0.95 |
Toenail and hair total As
Due to difficulties with sample collection and handling, many hair samples were of low mass at the point of digestion. This prompted the determination of a minimum mass requirement for toenail and hair samples by digesting triplicate samples of NCS DC 73347 CRM in decreasing mass increments (0.1, 0.08, 0.07, 0.06, 0.05, 0.04, 0.03, 0.02, 0.01, 0.005, 0.002 and 0.001 g). Measured As concentrations were plotted against mass of CRM (Fig. S2a†), in the context of the certified value and upper/lower limits. So too were mean recovery and relative standard deviation (RSD) (Fig. S2b†). On the basis of these results, 0.02 g was chosen as the minimum mass requirement, being the lowest mass at which As concentrations were found to be consistently within upper/lower certified limits of the CRM. This value is not universal and may not apply to other studies but was selected to try and maximise the usage of a compromised sample set. Depending on the amount of As in samples, requirements may be lower or higher. The RSD calculated for triplicates at lower masses may also reflect reduced homogeneity of the CRM.Following the exclusion of samples below the minimum mass, As data were available for the toenails and hair of 200 and 104 volunteers, respectively. All toenail and 96 (92%) hair samples were above the 10 μg kg−1 LOD. Arsenic measured in CRM NCS DC 73347 was 273 ± 10 As μg kg−1 (n = 40), within the certified range of 280 ± 50 As μg kg−1, yielding a mean recovery of 98% with 5% precision. The mean As measured in BAPS 2014 Human Toenail was 93 ± 5 As μg kg−1 (n = 20). The accuracy of BAPS 2014 measurements could not be assessed, but good precision (5% RSD) was maintained. The mean difference between duplicate digests was 1.1% (7 pairs) and 3.4% (6 pairs) for toenail and hair, respectively.
Summary statistics for toenail and hair As concentrations are shown in Table 3 for different demographic and behavioural subsets. The GM toenail As concentration of all 200 volunteers was 151 As μg kg−1 and ranged from 27 to 3354 As μg kg−1. This falls within previously published ranges, with a higher GM and maximum concentration than a study23 conducted in New Hampshire, USA (GM: 90 As μg kg−1; range: 10–810 As μg kg−1), with comparable levels of drinking water exposure (<0.02–66 As μg L−1). A previous study,40 conducted in south west England, reported a range of 858 to 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 981 As μg kg−1 for individuals exposed to high As in soil, with no exposure to As in drinking water. Although conducted in the same geographic region as the present study, Button et al. (2009)40 investigated individuals living in the direct vicinity of a former As mine, possibly explaining the much higher reported concentrations than the present study. Hinwood et al. (2003)26 investigated the toenail As concentrations of volunteers in different exposure categories in rural Australia: high soil (>30 As mg kg−1); high water (>10 As μg L−1) and low exposure (<10 As μg L−1 in drinking water and <30 As mg kg−1 in soil). Overall, much higher toenail As concentrations were reported by Hinwood et al. (2003), across all categories, than those in the present study. For example, the minimum toenail As concentration in the low exposure group was 1350 μg kg−1, of which only eight volunteers exceeded in the present study. Quantification/removal of exogenous As from toenail samples was cited as a limitation by Hinwood et al. (2003) and, therefore, few meaningful conclusions can be drawn from this comparison. Slotnick et al. (2007)44 reported a lower drinking water As GM to the present study (0.59 versus 0.88 As μg L−1) and a lower toenail As GM (70 versus 151 As μg kg−1). Maximum drinking water and toenail as concentrations were also higher in the present study than those reported by Slotnick et al. (2007): 233 versus 99 As μg L−1 and 3353 versus 1260 As μg kg−1, respectively. Other comparable studies include Rivera-Núñez et al. (2011)45 and Yu et al. (2014)24, with drinking water As GMs of 0.74 and 0.28 μg L−1 and toenail As GMs of 90 and 57 μg kg−1, respectively. Widespread As exposure, on the basis of both drinking water and toenail As concentrations, was low in the present study compared to those reported in severely affected areas. Nevertheless, 10 volunteers in the present study exhibited toenail As concentrations above the GM (1010 As μg kg−1) reported by Kile et al. (2005)46 across three villages in Bangladesh – the world's worst affected region – with drinking water As concentrations between 1 and 752 As μg L−1 (GM: 6.2 As μg L−1).
981 As μg kg−1 for individuals exposed to high As in soil, with no exposure to As in drinking water. Although conducted in the same geographic region as the present study, Button et al. (2009)40 investigated individuals living in the direct vicinity of a former As mine, possibly explaining the much higher reported concentrations than the present study. Hinwood et al. (2003)26 investigated the toenail As concentrations of volunteers in different exposure categories in rural Australia: high soil (>30 As mg kg−1); high water (>10 As μg L−1) and low exposure (<10 As μg L−1 in drinking water and <30 As mg kg−1 in soil). Overall, much higher toenail As concentrations were reported by Hinwood et al. (2003), across all categories, than those in the present study. For example, the minimum toenail As concentration in the low exposure group was 1350 μg kg−1, of which only eight volunteers exceeded in the present study. Quantification/removal of exogenous As from toenail samples was cited as a limitation by Hinwood et al. (2003) and, therefore, few meaningful conclusions can be drawn from this comparison. Slotnick et al. (2007)44 reported a lower drinking water As GM to the present study (0.59 versus 0.88 As μg L−1) and a lower toenail As GM (70 versus 151 As μg kg−1). Maximum drinking water and toenail as concentrations were also higher in the present study than those reported by Slotnick et al. (2007): 233 versus 99 As μg L−1 and 3353 versus 1260 As μg kg−1, respectively. Other comparable studies include Rivera-Núñez et al. (2011)45 and Yu et al. (2014)24, with drinking water As GMs of 0.74 and 0.28 μg L−1 and toenail As GMs of 90 and 57 μg kg−1, respectively. Widespread As exposure, on the basis of both drinking water and toenail As concentrations, was low in the present study compared to those reported in severely affected areas. Nevertheless, 10 volunteers in the present study exhibited toenail As concentrations above the GM (1010 As μg kg−1) reported by Kile et al. (2005)46 across three villages in Bangladesh – the world's worst affected region – with drinking water As concentrations between 1 and 752 As μg L−1 (GM: 6.2 As μg L−1).
| n (toenails, hair) | Toenail total As (μg kg−1), GM (range) | p-Value, Welch test (ANOVA for age groups) | Hair total As (μg kg−1), GM (range) | p-Value for Welch's test (ANOVA for age groups) | |
|---|---|---|---|---|---|
| All | 200, 104 | 151 (26.9–3354) | — | 82.6 (<LOD–2908) | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Gender | |||||
| Male | 102, 45 | 155 (26.9–1896) | 0.63 | 150 (28.8–2908) | <0.001 |
| Female | 98, 59 | 146 (39.1–3354) | 52.5 (<LOD–756) | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Age group | |||||
| 18–39 | 6, 3 | 214 (8.1–1497) | 0.28 | 89.9 (56.8–128) | 0.76 (ANOVA) |
| 40–49 | 27, 17 | 204 (57.9–3354) | 121 (10.9–2396) | ||
| 50–59 | 41, 20 | 154 (43–2578) | 79.2 (<LOD–756) | ||
| 60–69 | 74, 32 | 144 (39.1–1896) | 67.4 (18.7–2908) | ||
| 70–79 | 40, 24 | 135 (26.9–1982) | 79.7 (11–742) | ||
| 80–90 | 12, 8 | 111 (39.7–320) | 100 (36.5–670) | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Smoking status | |||||
| Currently smoking | 11,7 | 209 (100–2578) | 0.25 | 324 (28.8–2908) | 0.04 |
| Not currently smoking | 181,93 | 146 (26.9–1982) | 74.6 (<LOD–2396) | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Nail polish usage | |||||
| Reported/observed | 34 | 131 (44.6–1497) | 0.34 | — | — |
| Not reported/observed | 166 | 155 (26.9–3354) | — | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Hair dye usage | |||||
| Reported | 20 | — | — | 41.4 (10.8–756) | 0.003 |
| Not reported | 84 | — | 97.4 (<LOD–2908) | ||
The GM hair concentration measured in the present study was 82 As μg kg−1 (range: <LOD–2906 μg kg−1). The range reported in the only previous study47 of hair As concentrations in Cornwall was 890–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 μg kg−1. Although Peach and Lane (1998)47 identified elevated hair concentrations in local residents, they could only speculate as to the likely exposure routes and, with a small study group of five volunteers and no established washing protocols at the time, few comparisons can be made with their study. It is reported that hair As concentrations between 100 and 500 μg kg−1 are indicative of chronic exposure and concentrations between 1000 and 3000 μg kg−1 are indicative of acute poisoning.48 The As concentrations of 28 volunteers (15%) in the present study were between 100 and 500 μg kg−1 and the concentrations of a further 12 volunteers (6%) were >500 μg kg−1. Of these 40 individuals, 10 were exposed to >10 μg L−1 of As in their drinking water. While it is not possible to conclude that these volunteers are either chronically or acutely exposed, where elevations correspond with drinking water As concentrations above PCV, attention is warranted.
560 μg kg−1. Although Peach and Lane (1998)47 identified elevated hair concentrations in local residents, they could only speculate as to the likely exposure routes and, with a small study group of five volunteers and no established washing protocols at the time, few comparisons can be made with their study. It is reported that hair As concentrations between 100 and 500 μg kg−1 are indicative of chronic exposure and concentrations between 1000 and 3000 μg kg−1 are indicative of acute poisoning.48 The As concentrations of 28 volunteers (15%) in the present study were between 100 and 500 μg kg−1 and the concentrations of a further 12 volunteers (6%) were >500 μg kg−1. Of these 40 individuals, 10 were exposed to >10 μg L−1 of As in their drinking water. While it is not possible to conclude that these volunteers are either chronically or acutely exposed, where elevations correspond with drinking water As concentrations above PCV, attention is warranted.
Welch's tests (Table 3) detected no significant differences in toenail As between any subsets. Significantly lower hair As concentrations were detected for females (p < 0.001) and volunteers who reported using hair dye (p = 0.003). Significantly higher hair As concentrations were detected for smokers (p = 0.04). These findings were compared with a previous study49 investigating demographic and behavioural controls on the composition of hair: Chojnacka et al. (2006) reported 150% more As in the hair of smokers, 210% more As in the hair of males and artificially coloured hair was reported to contain 200% more As than naturally coloured hair.49
Exogenous As quantification
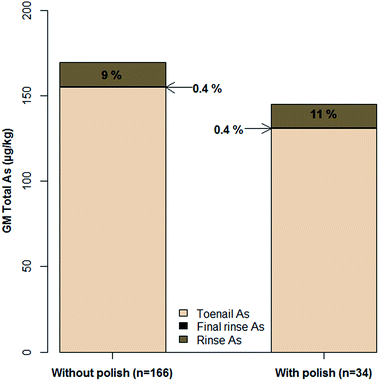
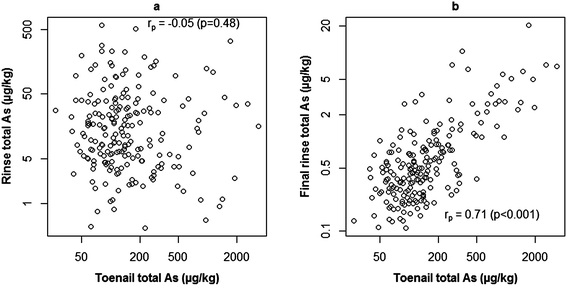
Analysis of rinse solutions from the toenail washing procedure provided a useful insight into exogenous As contamination. The bar plot in Fig. 3 shows the hypothetical contribution of exogenous As to that measured in toenails if they had not been washed. Rinse concentrations were normalised to the mass of toenail washed to allow comparison with digest concentrations. For toenails without polish, the GM As measured in initial rinse fractions was 9% of that measured in digested toenails, whereas the GM final rinse fraction As concentration only accounted for 0.4%. Firstly, this confirmed the necessity of washing toenails, with a maximum percentage contribution of 716% in the case of one volunteer. Secondly, the low contribution from final rinse fractions indicated the effective removal of exogenous As (maximum contribution: 5%). Furthermore, in agreement with previous findings,40,50 the washing procedure appeared to have begun to leach endogenous As from toenails by the final rinsing stage. This is indicated in Fig. 4, where no significant correlation was observed (rp = −0.05; p = 0.43) between initial rinse As concentrations and toenail digest As concentrations (Fig. 4a). Conversely, a significant positive correlation (rp = 0.71; p < 0.001) was observed between final rinse As concentrations and toenail digest As concentrations (Fig. 4b). The relatively small hypothetical contributions (5% maximum) of final rinse As concentrations to those in toenail digests suggests that a small degree of leaching is of no great concern in the present study. It is noted that future efforts could be made to determine an optimum degree of washing for toenail samples and maximise the removal of exogenous As whilst minimising endogenous As leaching. It is likely that the optimum number of rinses would depend on the level of contamination on the nail surface – a difficult metric to quantify.Welch's independent t-tests detected no significant differences in digest As concentrations (p = 0.34), initial rinse As concentrations (p = 0.85), final rinse As concentrations (p = 0.74) or percentage contributions from either initial (p = 0.52) or final (p = 0.35) rinse fractions between samples with and without nail polish. This finding does not dismiss the effects of polish on sample concentrations, as substantial contributions have been demonstrated elsewhere.39 Several factors may have limited findings on this occasion: misreporting of polish usage/failure to identify polish on samples; ineffective polish removal during washing; low sample size of volunteers with polish and a lack of digestion procedure for rinse solutions/the inability to solubilise As present from polish. Contribution from polish has also been demonstrated39 as brand dependent and further work is needed to quantify/mitigate the effects of polish usage on biomonitoring studies using human nails as part of a wider review of the effects of surface contamination.
Drinking water and biomarker relationships
Due to the difference in duration between initial and follow-up drinking water samples, follow-up water samples (all of which were collected during the same sampling campaign as the hair and toenail collections) were used as explanatory variables of biomarker As concentrations for consistency. In agreement with previous findings,21,23,24,26 significant positive correlations were observed between drinking water and toenail (Fig. 5a, rp = 0.53; p < 0.001; 95% C.I: 0.43, 0.63) and drinking water and hair (Fig. 5b, rp = 0.38; p < 0.001; 95% C.I: 0.20, 0.53) As concentrations. This confirmed previous findings14 of human exposure to As from PWS but over a longer timescale. When grouped by drinking water As concentration (Table 4), strong significant correlations were only observed where drinking water As was >10 μg L−1 for both toenails and hair. Fig. 5a shows that, for volunteers exposed to drinking water with <10 As μg L−1, a considerable number toenail samples contained notable As concentrations. Given the encouraging results from the assessment of the washing procedure, sample contamination was unlikely to account for these results. Cornwall is a region of elevated environmental As51 and, as noted previously by Button et al. (2009), alternative exposure routes, such as the ingestion of As-bearing soil and dust, are possible explanations for elevated toenail As where drinking water As is low.40 The investigation of additional exposure routes in the present study population will form the basis of further research.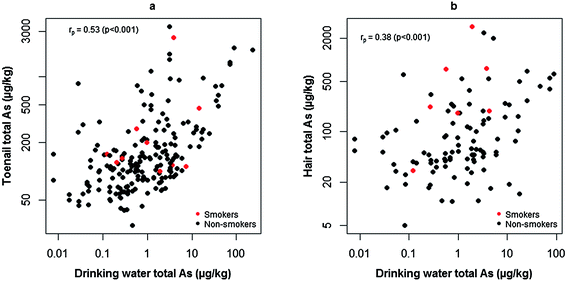 | ||
| Fig. 5 Significantly positive Pearson correlations (rp) between toenail (a) and hair (b) biomarker As concentrations and those measured in drinking water. | ||
| Pearson's rp (p-value, [95% C.I]) | ||||
|---|---|---|---|---|
| Drinking water As <1 μg L−1 | Drinking water As 1–10 μg L−1 | Drinking water As >10 μg L−1 | Full range | |
| Toenail total As | 0.15 (p = 0.13, [−0.04, 0.33]) (n = 107) | 0.12 (p = 0.32, [−0.12, 0.34]) (n = 73) | 0.86 (p < 0.001, [0.66, 0.94]) (n = 19) | 0.53 (p < 0.001, [0.43, 0.63]) (n = 199) |
| Hair total As | 0.11 (p = 0.45, [−0.18, 0.38]) (n = 48) | 0.15 (p = 0.34, [−0.16, 0.43]) (n = 43) | 0.62 (p = 0.02, [0.10, 0.87]) (n = 13) | 0.38 (p < 0.001, [0.20, 0.53]) (n = 104) |
Fig. 5b depicts similar results for hair to those observed for toenails, albeit with a weaker correlation. Due to problems encountered with sample handling and the difficulty determining the mass of hair washed, assessing the performance of washing was not possible for hair samples. Sample contamination cannot be ruled out as a possible explanation for this weaker correlation. Based on the results from Welch's t-tests, cigarette smoking might have accounted for elevated As in the hair of some individuals. Tobacco smoke has been demonstrated52 to cause elevated As in hair samples from non-occupationally exposed smokers and passive smokers. This pattern was not evident for toenail As concentrations, suggesting external contamination of hair from tobacco smoke among smokers as a possible explanation. Although statistically significant, caution is advised when interpreting these results due to the small number of smokers in the present study group.
Demographic, behavioural and dietary covariables
Multiple linear regression was used to determine significant predictors of toenail and hair As concentrations in addition to drinking water As. These included demographic, behavioural and dietary covariables. Data were stratified into two groups: volunteers with drinking water containing <1 As μg L−1 (low) and ≥1 As μg L−1 (high). This was to maintain consistency with previous studies45 that reported a greater predominance of additional, notably dietary, sources of As intake when drinking water concentrations were <1 μg L−1. This stratification resulted in four initial models for toenail (Model 1a, 1b) and hair (Model 2a, 2b) As concentrations as a function of demographic and behavioural variables only.Coefficients for each model are shown in Table 5. There were no significant demographic/behavioural predictors of toenail As in the low drinking water As group (Model 1a) but both increasing drinking water As and age resulted in a significant increase in toenail As when As in drinking water was >1 μg L−1. The effect of age on toenail As concentration has been reported by previous studies23 but in the opposite direction to the effect found in the present study. The mechanism of this relationship has not been elucidated. For example, Kile et al. (2005) note that toenail growth decreases with age. This may result in a higher concentration of As relative to a lower mass of nail. The high proportion of volunteers in older age groups in the present study may have limited the detection of a positive relationship on this occasion.
| Model | Terms | β coefficient (significance) |
|---|---|---|
| 1a. ln(toenail As), drinking water <1 As μg L−1 | Intercept | 5.309 (***) |
| ln(drinking water As) | 0.072 | |
| Age (continuous) | −0.01 (.) | |
| Gender (male) | 0.137 | |
| Nail polish usage (true) | −0.268 | |
| Adjusted R2 = 0.07 | Smoking status (smoker) | 0.31 |
| 1b. ln(toenail As), drinking water ≥1 As μg L−1 | Intercept | 5.916 (***) |
| ln(drinking water As) | 0.469 (***) | |
| Age (continuous) | −0.018 (**) | |
| Gender (male) | −0.101 | |
| Nail polish usage (true) | −0.157 | |
| Adjusted R2 = 0.29 | Smoking status (smoker) | 0.005 |
| 2a. ln(hair As), drinking water <1 As μg L−1 | Intercept | 2.646 (**) |
| ln(drinking water As) | 0.08 | |
| Age (continuous) | 0.017 | |
| Gender (male) | 0.826 (**) | |
| Dye usage (true) | −0.159 | |
| Adjusted R2 = 0.24 | Smoking status (smoker) | 0.77 |
| 2b. ln(hair As), drinking water ≥1 As μg L−1 | Intercept | 5.349 (***) |
| ln(drinking water As) | 0.433 (***) | |
| Age (continuous) | −0.025 (*) | |
| Gender (male) | 0.810 (**) | |
| Dye usage (true) | −0.76 (.) | |
| Adjusted R2 = 0.42 | Smoking status (smoker) | 2.08 (**) |
| 3. ln(toenail As), drinking water < 1 As μg L−1 | Intercept | 4.662 (***) |
| ln(drinking water As) | 0.089 (.) | |
| Adjusted R2 = 0.04 | Seafood (continuous) | 0.081 (*) |
| 4. ln(hair As), drinking water < 1 As μg L−1 | Intercept | 4.392 (***) |
| ln(drinking water As) | 0.213 (*) | |
| Gender (male) | 0.905 (***) | |
| Home-grown vegetables (never) | −0.975 (**) | |
| Home-grown vegetables (potted only) | 0.546 | |
| Adjusted R2 = 0.33 | Home-grown vegetables (seasonally) | −0.343 |
Male gender had a significant positive effect on hair As in the low drinking water group. Drinking water As, age, gender (male), dye usage and smoking were all significantly positively associated hair As in the high drinking water group. Findings of the model for hair As in the high drinking water group complimented those of Welch's tests, namely the significantly lower As concentrations in hair collected from females and those who reported using dye. The association with dye usage strengthened with the omission of the gender term. Furthermore, with all but one volunteer reporting dye usage being female and 29% of hair providing volunteers being females that did not report dye usage, the apparent effect of dye implied by Welch's test was an indirect effect of gender. This would be consistent with previous findings49,53 already discussed regarding lower As in the hair of females. Wolfsperger et al. (1994) attributed the higher As in male hair samples to smoking and a higher intake of seafood and wine than females.53
To test the influence of food and drink items known to contain As, dietary terms were added to the abovementioned models. None of the dietary variables tested had a significant effect on either toenail or hair As concentrations in the high drinking water group. In the low drinking water group, more servings of seafood per week resulted in a significant increase in toenail As concentration. Specific varieties of seafood were not significant. The model (Model 3) was re-performed with the omission of non-significant covariables and the results are presented in Table 5. A negative association was observed between hair As concentrations and never eating home-grown vegetables. The results of this model (Model 4), with non-significant covariables omitted, are presented in Table 5.
The positive association between seafood consumption and toenail As concentrations and the negative association between home-grown veg consumption and hair As concentrations are of plausible validity. Although seafood derived arsenic species such as arsenobetaine are primarily excreted via urine,54 seafood also contains arsenosugars and arsenolipids which are metabolised into methylarsonate and dimethylarsinate, both of which have been measured in small quantities in human toenails.40 In the present study, drinking water exposure was the primary focus of the investigation, hence, speciation analysis was not performed. On the basis of these findings, future studies considering dietary sources in low drinking water exposure groups should consider speciation analysis to ensure meaningful results. The negative effect of not eating home-grown vegetables on hair As concentration is consistent with reported high soil As concentrations in the study region51 and, although values in local vegetables themselves have been found at relatively low concentrations,55 the ingestion of soil particles adhered to vegetables is a possible exposure pathway.
4. Conclusions
This study is the largest investigation of long-term exposure to As in drinking water in the UK to-date and confirms the presence of prolonged exposure to inorganic As from drinking water of householders with PWS in Cornwall, UK. The temporal stability of As concentrations in PWS suggests that, for this particular region, measurements of As taken in the present are strong predictors of past levels of exposure dating back at least 31 months. Arsenic concentrations measured in toenails and hair were useful in assessing prolonged exposure to As from PWS, in agreement with numerous previous studies. Analysis of washing solutions built on the findings of Button et al. (2009)40 in that the washing procedure employed here was effective in removing exogenous contamination from a large sample set. Both toenail and hair biomarkers were susceptible to the influence of covariables on As concentrations. Although useful in assessing prolonged exposures to As from drinking water, other factors, such as diet, predominate where As concentrations in drinking water are low e.g. <1 μg L−1. A large degree of variation in toenail and hair biomarkers was still unaccounted for in this study, with exposure to soil and dust highly possible explanations in a region of well-documented elevated environmental As. Investigation into the significance of other exposure routes will be the focus of future research.Acknowledgements
The authors are grateful for the contributions of Andrew Dunne and Dr Andrew Marriott during the field work campaign, Dr Louise Ander for help with constructing the field questionnaire database, Amanda Gardner for laboratory work and the efforts of Amy Rimell and Dr Mike Studden in logistical and management operations. Funding for this research was provided by the Natural Environment Research Council (NERC) via a University of Manchester/British Geological Survey (BGS) University Funding Initiative (BUFI) PhD studentship (Contract No. GA/125/017, BUFI Ref: S204.2) and the Centre for Environmental Geochemistry, BGS. The participation of the 215 volunteers in the wider study is also gratefully acknowledged. More information can be found at: http://www.bgs.ac.uk/sciencefacilities/laboratories/geochemistry/igf/Biomonitoring/arsenicSW.html.Notes and references
- IARC., IARC Monogr. Eval. Carcinog. Risks Hum., 2012, 100C, 41–85.
- C. J. Chen, Y. C. Chuang, T. M. Lin and H. Y. Wu, Cancer Res., 1985, 45, 5895–5899 CAS.
- G. Marshall, C. Ferreccio, Y. Yuan, M. N. Bates, C. Steinmaus, S. Selvin, J. Liaw and A. H. Smith, J. Natl. Cancer Inst., 2007, 99, 920–928 CrossRef CAS PubMed.
- J. O'Reilly, M. Watts, R. Shaw, A. Marcilla and N. Ward, Environ. Geochem. Health, 2010, 32, 491–515 CrossRef PubMed.
- M. Watts, J. O'Reilly, A. Marcilla, R. Shaw and N. Ward, Environ. Geochem. Health, 2010, 32, 479–490 CrossRef CAS PubMed.
- D. Chakraborti, M. M. Rahman, B. Das, M. Murrill, S. Dey, S. Chandra Mukherjee, R. K. Dhar, B. K. Biswas, U. K. Chowdhury and S. Roy, Water Res., 2010, 44, 5789–5802 CrossRef CAS PubMed.
- D. Chakraborti, B. Das, M. M. Rahman, U. K. Chowdhury, B. Biswas, A. Goswami, B. Nayak, A. Pal, M. K. Sengupta and S. Ahamed, Mol. Nutr. Food Res., 2009, 53, 542–551 CAS.
- NRC, Arsenic in drinking water, National Research Council. Subcommittee on Arsenic in Drinking Water, National Academies Press, 1999 Search PubMed.
- A. L. Lindberg, W. Goessler, E. Gurzau, K. Koppova, P. Rudnai, R. Kumar, T. Fletcher, G. Leonardi, K. Slotova and E. Gheorghiu, J. Environ. Monit., 2006, 8, 203–208 RSC.
- D. Jovanovic, B. Jakovljević, Z. Rašić-Milutinović, K. Paunović, G. Peković and T. Knezević, Environ. Res., 2011, 111, 315–318 CrossRef CAS PubMed.
- E. L. Ander, M. J. Watts, P. L. Smedley, E. M. Hamilton, R. Close, H. Crabbe, T. Fletcher, A. Rimell, M. Studden and G. Leonardi, Variability in the chemistry of private drinking water supplies and the impact of domestic treatment systems on water quality, Environmental Geochemistry and Health, 2016 DOI:10.1007/s10653-016-9798-0.
- Private Water Supplies Regulations 2009, Applying in England and coming into force on 1st of January 2010, http://www.legislation.gov.uk/uksi/2009/3101/introduction/made.
- WHO, Arsenic in drinking-water – Background document for development of WHO Guidelines for Drinking-water Quality, World Health Organisation, WHO/SDE/WSH/03.04/75/Rev/1, 2011 Search PubMed.
- D. R. S. Middleton, M. J. Watts, E. M. Hamilton, E. L. Ander, R. M. Close, K. S. Exley, H. Crabbe, G. S. Leonardi, T. Fletcher and D. A. Polya, Urinary arsenic profiles reveal substantial exposures to inorganic arsenic from private drinking water supplies in Cornwall, UK, Sci. Rep., 2016 Search PubMed , in press.
- J. P. Buchet, R. Lauwerys and H. Roels, Int. Arch. Occup. Environ. Health, 1981, 48, 71–79 CrossRef CAS PubMed.
- DWI, Drinking water 2014, Private water supplies in England, A report by the Chief Inspector of Drinking Water, July 2015, 2015.
- J. G. Thundiyil, Y. Yuan, A. H. Smith and C. Steinmaus, Environ. Res., 2007, 104, 367–373 CrossRef CAS PubMed.
- C. M. Steinmaus, Y. Yuan and A. H. Smith, Environ. Res., 2005, 99, 164–168 CrossRef CAS PubMed.
- M. J. Slotnick, J. R. Meliker and J. O. Nriagu, Sci. Total Environ., 2006, 369, 42–50 CrossRef CAS PubMed.
- F. Frost, D. Franke, K. Pierson, L. Woodruff, B. Raasina, R. Davis and J. Davies, Environ. Geochem. Health, 1993, 15, 209–214 CrossRef CAS PubMed.
- A. G. Gault, H. A. L. Rowland, J. M. Charnock, R. A. Wogelius, I. Gomez-Morilla, S. Vong, M. Leng, S. Samreth, M. L. Sampson and D. A. Polya, Sci. Total Environ., 2008, 393, 168–176 CrossRef CAS PubMed.
- M. R. Karagas, J. S. Morris, J. E. Weiss, V. Spate, C. Baskett and E. R. Greenberg, Cancer Epidemiol., Biomarkers Prev., 1996, 5, 849–852 CAS.
- M. R. Karagas, T. D. Tosteson, J. Blum, B. Klaue, J. E. Weiss, V. Stannard, V. Spate and J. S. Morris, Am. J. Epidemiol., 2000, 152, 84–90 CrossRef CAS PubMed.
- M. Y. Zhijie, T. J. Dummer, A. Adams, J. D. Murimboh and L. Parker, J. Exposure Sci. Environ. Epidemiol., 2014, 24, 135–144 CrossRef PubMed.
- M. R. Karagas, C. X. Le, S. Morris, J. Blum, X. Lu, V. Spate, M. Carey, V. Stannard, B. Klaue and T. Tosteson, International Journal of Occupational Medicine and Environmental Health, 2001, 14, 171–175 CAS.
- A. L. Hinwood, M. R. Sim, D. Jolley, N. de Klerk, E. B. Bastone, J. Gerostamoulos and O. H. Drummer, Environ. Health Perspect., 2003, 111, 187 CrossRef CAS PubMed.
- L. E. Beane Freeman, L. K. Dennis, C. F. Lynch, P. S. Thorne and C. L. Just, Am. J. Epidemiol., 2004, 160, 679–687 CrossRef PubMed.
- J. E. Heck, A. S. Andrew, T. Onega, J. R. Rigas, B. P. Jackson, M. R. Karagas and E. J. Duell, Environ. Health Perspect., 2009, 117, 1718 CrossRef CAS PubMed.
- M. J. Slotnick and J. O. Nriagu, Environ. Res., 2006, 102, 125–139 CrossRef CAS PubMed.
- D. K. Harkins and A. S. Susten, Environ. Health Perspect., 2003, 111, 576 CrossRef CAS PubMed.
- J. Roberge, A. T. Abalos, J. M. Skinner, M. Kopplin and R. B. Harris, Am. J. Environ. Sci., 2009, 5, 688–694 CrossRef CAS.
- J. Xue, V. Zartarian, S.-W. Wang, S. V. Liu and P. Georgopoulos, Environ. Health Perspect., 2010, 118, 345 CAS.
- K. L. Cottingham, R. Karimi, J. F. Gruber, M. S. Zens, V. Sayarath, C. L. Folt, T. Punshon, J. Morris and M. R. Karagas, Nutr. J., 2013, 12, 1 CrossRef PubMed.
- P. Fleckman, in Nails: Therapy, Diagnosis, Surgery, ed. R. K. Scher and C. R. Daniel, WB Saunders Co, Philadelphia, 1997, ch. 4, p. 37 Search PubMed.
- M. R. Harkey, Forensic Sci. Int., 1993, 63, 9–18 CrossRef CAS PubMed.
- N. Orentreich, J. Markofsky and J. H. Vogelman, J. Invest. Dermatol., 1979, 73, 126–130 CrossRef CAS PubMed.
- I. M. Kempson and W. M. Skinner, Biol. Trace Elem. Res., 2012, 150, 10–14 CrossRef PubMed.
- K. Orloff, K. Mistry and S. Metcalf, J. Toxicol. Environ. Health, Part B, 2009, 12, 509–524 CAS.
- P. Favaro, P. Bode and E. De Nadai Fernandes, J. Radioanal. Nucl. Chem., 2005, 264, 61–65 CrossRef.
- M. Button, G. R. Jenkin, C. F. Harrington and M. J. Watts, J. Environ. Monit., 2009, 11, 610–617 RSC.
- M. Esteban, B. K. Schindler, J. A. Jiménez-Guerrero, H. M. Koch, J. Angerer, T. C. Rivas, M. Rosado, S. Gómez, L. Casteleyn and M. Kolossa-Gehring, Environ. Res., 2014, 141, 24–30 CrossRef PubMed.
- E. M. Hamilton, T. S. Barlow, C. J. Gowing and M. J. Watts, Microchem. J., 2015, 123, 131–138 CrossRef CAS.
- R Core Team, R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria, 2015, http://www.R-project.org Search PubMed.
- M. J. Slotnick, J. R. Meliker, G. A. AvRuskin, D. Ghosh and J. O. Nriagu, J. Toxicol. Environ. Health, Part A, 2007, 70, 148–158 CrossRef CAS PubMed.
- Z. Rivera-Núñez, J. R. Meliker, J. D. Meeker, M. J. Slotnick and J. O. Nriagu, J. Exposure Sci. Environ. Epidemiol., 2011, 22, 182–190 CrossRef PubMed.
- M. L. Kile, E. A. Houseman, E. Rodrigues, T. J. Smith, Q. Quamruzzaman, M. Rahman, G. Mahiuddin, L. Su and D. C. Christiani, Cancer Epidemiol., Biomarkers Prev., 2005, 14, 2419–2426 CAS.
- D. F. Peach and D. W. Lane, Environ. Geochem. Health, 1998, 20, 231–237 CrossRef CAS.
- R. N. Ratnaike, Postgrad. Med. J., 2003, 79, 391–396 CrossRef CAS PubMed.
- K. Chojnacka, H. Górecka and H. Górecki, Environ. Toxicol. Pharmacol., 2006, 22, 52–57 CrossRef CAS PubMed.
- B. K. Mandal, Y. Ogra and K. T. Suzuki, Toxicol. Appl. Pharmacol., 2003, 189, 73–83 CrossRef CAS PubMed.
- P. Mitchell and D. Barr, Environ. Geochem. Health, 1995, 17, 57–82 CrossRef CAS PubMed.
- A. Saad and M. A. Hassanien, Environ. Int., 2001, 27, 471–478 CrossRef CAS PubMed.
- M. Wolfsperger, G. Hauser, W. Göβler and C. Schlagenhaufen, Sci. Total Environ., 1994, 156, 235–242 CrossRef CAS PubMed.
- A. Navas-Acien, K. A. Francesconi, E. K. Silbergeld and E. Guallar, Environ. Res., 2011, 111, 110–118 CrossRef CAS PubMed.
- G. Norton, C. Deacon, A. Mestrot, J. Feldmann, P. Jenkins, C. Baskaran and A. A. Meharg, Environ. Sci. Technol., 2013, 6164–6172 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6em00072j |
| This journal is © The Royal Society of Chemistry 2016 |