 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Impact of metallurgical activities on the content of trace elements in the spatial soil and plant parts of Rubus fruticosus L.†
M. M.
Nujkić
*a,
M. M.
Dimitrijević
a,
S. Č.
Alagić
a,
S. B.
Tošić
b and
J. V.
Petrović
c
aDepartment of Chemical Technology, Technical Faculty Bor, University of Belgrade, V. J. 12, 19210 Bor, Serbia. E-mail: majanujkic@gmail.com; Tel: +381 30 424 555
bDepartment of Chemistry, Faculty of Sciences and Mathematics, University of Nis, Višegradska 33, 18000 Niš, Serbia
cLaboratory for Chemical Testing, Mining and Metallurgy Institute, Zeleni Bulevar 35, 19210 Bor, Serbia
First published on 21st January 2016
Abstract
The concentrations of the trace elements (TEs), Cu, Zn, Pb, As, Cd, Ni, were determined in parts of Rubus fruticosus L. and in topsoil, collected from eight different locations around the copper smelter in Bor, Serbia. Extremely high concentrations of Cu were determined in the soil and in R. fruticosus L., and for arsenic at some locations. The enrichment factors for TEs in soil showed enrichment with Cu, Zn, Pb, and As among which extremely high values were determined for Cu (EFsoil = 8.5–126.1) and As (EFsoil = 6.6–44.4). The enrichment factors for the parts of R. fruticosus L. showed enrichment with all TEs, except for nickel. The most extreme enrichment was found to occur in roots and stems for Cu (EFplant = 56.2 and 51.1) and leaves for Pb (EFplant = 45.68). The mean values of the three ratios of concentrations between plant parts for all TEs indicated pollution via the atmosphere while leaves appeared to be the best indicators for this kind of pollution. Numerous and very strong Pearson's correlations between TEs in the R. fruticosus L. parts confirmed these results. Principal Component Analysis showed that the major pollution source is the copper smelter that contaminates vegetation through soil and air.
Environmental impactRTB Bor is a copper mining complex considered as one of the biggest producers of copper and noble metals in Central Eastern Europe, since 1903. The main causes of soil and vegetation pollution are mining and metallurgy, with accompanying mine pits, landfills for tailing disposal and flotation tailing ponds. Due to the leakage of the latter, large quantities of flotation tailings reached the rivers Bor and Veliki Timok, being transferred by the Danube River up to the Black Sea. Also, dust with a high content of trace elements is emitted from the smelting plant polluting the environment through air deposition. Therefore, this respective region is one of the most affected areas by anthropogenic activities in Europe. |
A Introduction
Soil pollution by toxic elements due to copper mining and smelting is a major problem in many countries around the world1–5 and in Serbia.6–8 The extraction of metals from copper ores through mining and smelting generates high amounts of hazardous waste including mine and flotation tailings, wastewaters, slag, flue gases and dust, which often contain high concentrations of trace elements, TEs (i.e., Cu, Zn, Pb, As, Cd, Ni, etc.).9–12 Emission and wet or dry deposition of TEs into the surrounding environment can severely pollute the topsoil. Large concentrations of several TEs in soil can be toxic to plants, animals and humans. Since TEs tend to accumulate in the environment and plants, they are harmful to living organisms through the food chain, air and water.13,14The town of Bor was built on the perimeter of the Copper Mining and Smelting Complex Bor (RTB Bor). More than 100 years of work on the complex resulted in severe pollution of air, water and soil of the town and its surrounding area. Therefore, Bor is recognized as one of the most polluted towns in Serbia and Europe. Perennial monitoring of the air quality in Bor confirms high concentrations of SO2, Cu, As, and periodically Zn, Cd, and Pb. All these pollutants are present in particulate matter and aero sediments as a result of the smelter operation with old technology. When they are present in high concentrations (even the micronutrients Cu and Zn), all the above mentioned TEs are considered environmentally dangerous as they are toxic, persistent and/or bioaccumulative. However, in spite of the increased concentrations of TEs, numerous plant species in Bor and the surrounding area exhibit adaptation to the existing pollution since they normally grow and reproduce. Even old flotation tailing ponds in the immediate proximity of the smelter chimneys, that are considered as very polluted areas, are populated by vegetation (grass, bushes, and trees).6 One of these plants is R. fruticosus L. or wild blackberry that is adapted to the ecological stress. The wild blackberry is one of the plants that is widely spread in the Bor municipality, and thus is suitable for the biomonitoring of pollution at suitable urban and rural areas when considering wind direction.
Plants are used as passive monitors in areas contaminated with TEs because of their ability to efficiently intercept and accumulate chemicals. Monitoring, assessment and analysis of TEs accumulated in plants and soil is defined as biomonitoring that allows an insight into the mobility and bioavailability of the observed TEs.15 Passive biomonitoring of pollution using plants has been shown to be easy, low-cost and a readily accessible technique for the assessment of environmental pollution.16,17 In order to improve the use of plants as contamination biomonitors, it is necessary to accurately determine the relationship between the level of TEs in the plant tissue and toxicity in the organisms or ecological impacts on the society.18 The soil and plant samples not only can offer information about the sampling time and localization, but also information about the influence of uptake and accumulation of TEs on the plant.19 Plants are in contact with soil and water through roots while through leaves they are in contact with air, which enables simultaneous interactions between three environments. Although it is hard to distinguish between the amount of the elements absorbed from the soil or air, it can be concluded that perennial plants reflect cumulative effects of the polluted soil and air.20 Moreover, this type of assessment provides data about the phytotoxicity of TEs when their critical concentrations in plants are exceeded. Thus, the combination of the data for the total TE concentrations in soils and plants that are growing in the contaminated soil constitutes a basis for identifying the TEs released into the environment and whether or not phytoremediation is necessary.21
The numerous natural populations of R. fruticosus L. can be found all over the world because of their easy and quick reproduction.22 In Serbia, R. fruticosus L. can be found in abandoned areas, contaminated soils and forest fire sites. The blackberry root system penetrates deep into the ground (1 m or more) having higher resistance to drought.23 This fact makes R. fruticosus L. a good candidate for phytoremediation. R. fruticosus L. has a relatively high resistance to contamination and several studies have shown successful growth and development of these plant species in contaminated soil.24–30 Also, in many studies R. fruticosus L. was presented as a bioindicator of emissions from motor vehicles,26 increased concentrations of lead in soil,31 industrial pollution,25,29 pollution in urban areas,24 and TEs of abandoned mine areas and flotation tailings.28,32 However, a few studies have examined the accumulation of TEs in R. fruticosus L.
In this study, we observed that R. fruticosus L. was widely distributed and exhibited no visible signs of toxicity in the contaminated soils around the Bor smelting complex. Therefore, we investigated R. fruticosus L. as a potential biomonitor for TEs. The contents of the latter in this plant and soil samples were measured along transects emanating from the smelter as the primary pollution source.
B Materials and methods
Site description
Geographical coordinates of the Bor town in Eastern Serbia are [44°25′N] latitude and [22°06′E] longitude. The Mining and Smelting Complex Bor (RTB Bor), as a predominant industry in the Bor municipality, is located on the northeastern edge of the town. The climate in the Bor area, which is surrounded by the mountains Crni Vrh, Stol, and Veliki and Mali Krš is moderately continental while the height above sea level of the town and the mountain peaks is 378 m and <1000 m, respectively. The mean annual values of the meteorological parameters in 2012 (when samples were collected) were: air temperature 11.34 °C, atmospheric pressure 971.6 mbar, relative humidity 65.75%, precipitation 673.5 mm m−2, wind speed 0.62 m s−1 and no-wind period 57.6%. In this mountainous area, the dominant winds are the west (W), the west-northwest (WNW) and the northwest (NW), as depicted by the rose plot in Fig. 1. Wind direction influences the distribution of pollutants from the industrial facilities to the town Bor and its surrounding areas.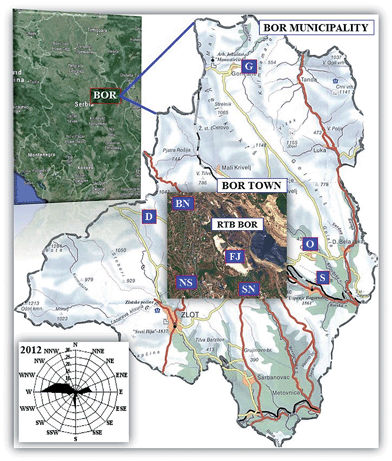 | ||
| Fig. 1 Modified map of the Bor town showing locations of the sampling sites around the Bor basin (RTB Bor). The urban-industrial (UI) zone includes: FJ-flotation tailings (Flotacijsko Jalovište), BN (Bolničko naselje), NS (Naselje Sunce) and SN (Slatinsko naselje), and the out-of-town rural areas: D-Dubašnica, O-Oštrelj and S-Slatina, and G-Gornjane (control site) (“Serbia and Bor”. Map. Google Maps, Google, 28 June 2015. Web. 28 June 2015); inset at the bottom-left corner represents a wind rose plot for the Bor town for the year 2012 (modified and reproduced with permission from Springer-Verlag GmbH, from ref. 8). | ||
In this study, samples of the soil and R. fruticosus L. were collected from eight different sites (Fig. 1). The urban-industrial (UI) zone included four sampling sites: the old flotation tailing pond (FJ, Flotacijsko Jalovište), the hospital settlement (BN, Bolničko naselje, near the city hospital) and the two suburbs namely, the settlement ‘‘Sun’’ (NS, Naselje Sunce) and Slatina settlement (SN, Slatinsko naselje). These sampling sites are located close to the copper smelter, and thus the main source of pollution. Four more sites included three rural settlements [Oštrelj (O), Slatina (S) and Dubašnica (D)] and one control site Gornjane (G) that also represents a rural settlement and an unpolluted area 19 km far from the Bor town. This area is naturally protected from pollution by the mountain Veliki Krš.
Sample collection
The sampling of R. fruticosus L., its parts and the topsoil was performed at each location in late September/October in 2012. Each part of R. fruticosus L. (roots, leaves, stems and fruits) and the soil sample (0–30 cm from the rooting zone) were a composite of 3–5 subsamples. Root samples were thoroughly washed with tap and distilled water. The above-ground parts of R. fruticosus L. were carefully cleaned from dust and visible particles using a brush without further washing with water. All the samples were dried to a constant weight at the room temperature. The dried material was ground in a laboratory mill, passed through a 2 mm sieve, homogenized and stored in paper bags prior to analysis.Sample preparation and analysis
Sample mineralization and analysis were conducted as it was described by Alagić et al.8 According to a microwave-assisted strong acid digestion method of complex matrices recommended by the US Environmental Protection Agency (USEPA method 3052), all samples (1 g of each) were digested by using nitric acid (65% HNO3, Merck, Darmstadt) and hydrogen peroxide (10% H2O2, Merck, Darmstadt) in a microwave digestion system ETHOS 1 (Milestone, Bergamo, Italy). After cooling, the final solutions were filtered, and diluted to a volume of 50 mL using double-distilled water. The solutions were kept in polyethylene bottles at 4 °C before TE analysis by ICP-OES. To avoid any contamination, the bottles were treated with 5% nitric acid and washed with ultra-pure water 0.05 μS cm−1 (MicroMed high purity water system, TKA Wasser aufbere itungs systeme GmbH).The instrumental analysis was performed on an iCAP 6000 inductively coupled plasma optical emission spectrometer (Thermo Scientific, Cambridge, UK) with an Echelle optical design and a charge injection device (CID) solid-state detector. The calibration curves were obtained using a multi-element standard solution of about 20.00 ± 0.10 mg L−1 (Ultra Scientific, USA). All results were calculated on a dry weight basis (mg kg−1 DW).
The soil pH and electrical conductivity (EC), in solutions prepared as solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) distilled water = 1
distilled water = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, were determined using a pH meter (3510 Jenway, UK) and an EC meter (4510 Jenway, UK), respectively. The content of soil organic matter (OM) was determined by the loss-on-ignition (LOI) method at 550 °C.33
2.5, were determined using a pH meter (3510 Jenway, UK) and an EC meter (4510 Jenway, UK), respectively. The content of soil organic matter (OM) was determined by the loss-on-ignition (LOI) method at 550 °C.33
Data processing and statistical analysis
The enrichment factor (EFsoil) represents the accumulation of a potentially toxic element (one of the TEs) in soil due to local industrial activities that can influence its uptake by plants. Hence, the enrichment factor (eqn (1)) represents the ratio of a certain element in the soil relative to the abundance of the same element at the control site:34,35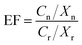 | (1) |
The enrichment factor of R. fruticosus L., EFplant, was calculated in order to assess the degree of anthropogenic influence (eqn (2)), i.e. which elements were relatively enriched in the different samples of the plant:8,34,38
| EFplant = Cp/Xp | (2) |
The ratios of concentrations between plant parts, R, were estimated using eqn (3)–(5), where R > 1 indicates pollution via atmosphere:39
| Rleaf/fruit = Cleaf/Cfruit | (3) |
| Rleaf/stem = Cleaf/Cstem | (4) |
| Rfruit/stem = Cfruit/Cstem | (5) |
The results for the total concentrations of TEs are presented as a mean and standard deviation (Table 1). The mean value of three replications is reported. All the data were analyzed using the statistical package SPSS 17.0 for Windows (SPSS Inc., USA) considering two-tailed statistical significance at a 95% confidence interval. Pearson's correlation coefficients were used to evaluate statistical relationships among soil samples, soil parameters (pH, EC and OM) and plant variables in order to group the similar variables. Principal component analysis (PCA) was performed to transform the original variables into orthogonal components; the Varimax rotation and Kaiser criterion (eigenvalues > 1) were applied and loading coefficients ≥0.100 were used to define the different relationships between TEs in plant parts and soil.
| Sampling siteb | TE total concentrationa (mg kg−1 DW) | pH | EC (mS cm−1) | OM (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Zn | Pb | As | Cd | Ni | Fe | ||||
| a Data are presented as the mean ± standard deviation for triplicate determinations. Higher values are given in bold type. b Distance from the copper smelter (km). c The Official Gazette of Republic Serbia43 and Kabata-Pendias.42 | ||||||||||
| FJ (0.7) | 2112 ± 80 | 235 ± 7 | 169 ± 7 | 95 ± 3 | 4.3 ± 0.2 | 15.1 ± 1.8 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 020 ± 40 020 ± 40 |
4.73 | 0.26 | 7.2 |
| BN (2.2) | 2210 ± 92 | 191 ± 8 | 260 ± 12 | 148 ± 7 | 6.5 ± 0.3 | 38 ± 2 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 725 ± 675 725 ± 675 |
4.37 | 0.08 | 17.4 |
| NS (2.5) | 950 ± 20 | 307 ± 12 | 98 ± 5 | 9.4 ± 0.4 | 3.5 ± 0.2 | 34 ± 2 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 965 ± 560 965 ± 560 |
6.95 | 0.21 | 17.6 |
| SN (2.3) | 1060 ± 11 | 203 ± 13 | 87 ± 7 | 28 ± 2 | 3.5 ± 0.3 | 20 ± 2 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 605 ± 485 605 ± 485 |
6.73 | 0.16 | 11.2 |
| O (4) | 939 ± 5 | 130.3 ± 0.6 | 78 ± 0.4 | 18.4 ± 0.06 | 2.8 ± 0.02 | 23.6 ± 0.2 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 185 ± 65 185 ± 65 |
7.54 | 0.15 | 11.4 |
| S (6.5) | 1969 ± 18 | 199 ± 2 | 125 ± 1 | 15.4 ± 0.2 | 3.17 ± 0.03 | 14.2 ± 0.2 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 615 ± 170 615 ± 170 |
7.57 | 0.44 | 23.4 |
| D (17) | 144.8 ± 0.5 | 130.2 ± 0.1 | 78.6 ± 0.1 | 34.52 ± 0 | 4.52 ± 0.02 | 53.4 ± 0.05 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 435 ± 250 435 ± 250 |
5.11 | 0.08 | 15.4 |
| G (19) | 9.7 ± 0.2 | 45.3 ± 0.6 | 17.8 ± 0.4 | 1.77 ± 0.04 | 1.23 ± 0.02 | 15 ± 0.2 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 015 ± 100 015 ± 100 |
6.69 | 0.07 | 5.2 |
| MACc | 100 | 300 | 100 | 25 | 3 | 50 | ||||
| Mean background contents of TEs in surface soilc | 14 | 62 | 25 | 4.7 | 1.1 | 18 | ||||
| Ranges of MAC for TEs in agricultural soilsc | 60–150 | 100–300 | 20–300 | 15–20 | 1–5 | 20–60 | ||||
C Results and discussion
Soil
The total concentrations of Cu, Zn, Pb, As, Cd and Ni, pH values, EC and OM determined in soil samples are given in Table 1 (Fe is not reported; it was used only to estimate EFsoil). According to the classification of soil pH recommended by the United States Department of Agriculture,40 our soil samples ranged from extremely acidic (pH < 4.5) to slightly alkaline (pH = 7.4–7.8), while the soil sampled at the control site G may be deemed as neutral (pH = 6.69). Acidic soil is a consequence of the high content of sand fraction with low cation exchange capacity and the presence of pyrite from which cations are leached under the influence of the large amounts of rainfall in the mining and tailing areas.12 The content of OM in the soil ranged from 7.2% to 23.4%. The EC values are relatively low (0.07–0.44 mS cm−1) and comparable with the values determined by Brunetti et al.41 for the polluted soil (0.2–0.7 mS cm−1) and the values for Cu and Pb mine soils determined by Bech et al.1 (0.2 mS cm−1).As seen in Table 1 and when compared with the control site G, much higher concentrations of Cu, Zn, Pb, As and Cd were observed in all soil samples, being highest for Cu. High concentrations of TEs (almost all sites) may be a consequence of the degradation of the parent ore and contamination caused by mining-metallurgical activities that have lasted more than 100 years in this area. The highest total concentrations of Cu, Pb, As and Cd (2210, 260, 148, and 6.5 (mg kg−1)) were recorded at site BN followed by site FJ closest to the Bor copper smelter. The largest concentrations of Cu determined at the site FJ and BN coincide with the hypothesis of Kabata-Pendias,42 that in highly acidic soil, concentrations of Cu increase in the soil solution (Table 1).
From the data in Table 1 it can also be seen that the Cu concentration at site S (6.5 km far from the copper smelter) is approximately the same as that at the sites closest to the smelter, namely FJ and BN. Soil contamination at site S can be explained by the emission of the pollutants downwind from the industrial area (the dominant direction is W and WNW, Fig. 1, wind rose). The highest concentration of Zn (307 mg kg−1) was observed at NS, for Ni (53.4 mg kg−1) it was at D and the highest values for other three elements (Pb, As and Cd) were observed at site BN. However, variation of Cd concentrations along the transect was not observed with nearly equal concentrations of Cd at FJ (0.7 km) and at site D (17 km) which suggests that Cd only partially originates from the Bor industry.
Since the studied sites are inhabited and include both rural and town settings, agricultural activities occur and thus it is helpful to compare the observed concentrations of TEs in soil with allowable values according to Kabata-Pendias42 and Serbian legislation43 (Table 1). The total concentrations of certain elements exceed the MAC at the following sampling locations: Cu at sites FJ, BN, NS, SN, O, S at D; Zn at the site NS; Pb at FJ, BN and S; As at FJ, BN, SN and D; Cd at FJ, BN, NS, SN, S and D; Ni at the site D. Thus, the studied sites are generally highly polluted and require an immediate remediation.
Assessment of the soil pollution based on EF
EFsoil calculated for the six elements investigated in this work are given in Table 2. All the soil samples are enriched with Cu, among which soil samples from the six sampling sites are extremely enriched (67.6–152.9). EFsoil for Zn indicates moderate enrichment in soils at all sampling sites, while for Pb moderate to significant enrichment is observed. EFsoil for As varies from 6.6–44.4, being significantly to highly enriched. The lowest values of EFsoil are estimated for Ni and Cd, which reflect natural (geological) enrichment of the soil with these elements (EFsoil = 0.5–2). Small deviations from this range for Cd at sites BN and D indicate moderate enrichment of the soil. Based on the results given in Table 2, it can be concluded that soils are enriched the most for Cu and As, in the UI area, i.e. sites FJ, BN and SN, which is a consequence of the emission of the flue gasses and dust from the smelter plant.| Sampling site (km) | TE | |||||
|---|---|---|---|---|---|---|
| Cu | Zn | Pb | As | Cd | Ni | |
| FJ (0.7) | 126.1 | 3.0 | 5.5 | 31.1 | 2.0 | 0.6 |
| BN (2.2) | 121.1 | 2.2 | 7.7 | 44.4 | 2.8 | 1.4 |
| NS (2.5) | 67.6 | 4.7 | 3.8 | 3.7 | 1.9 | 1.6 |
| SN (2.3) | 72.5 | 3.0 | 3.2 | 10.5 | 1.9 | 0.9 |
| O (4) | 70.2 | 2.1 | 3.2 | 7.5 | 1.6 | 1.2 |
| S (6.5) | 152.9 | 3.3 | 5.3 | 6.6 | 1.9 | 0.7 |
| D (17) | 8.5 | 1.6 | 2.5 | 11.1 | 2.1 | 2.0 |
Plant
The concentrations of TEs in all parts of R. fruticosus L. versus sampling site are presented in Fig. 2. Except Ni, the TE concentrations of all parts of R. fruticosus L. at all sites were significantly higher compared to the control site G. It has been established that the uptake and root-to-shoot translocation of elements depend mainly on the concentration of the elements in the soil, soil pH and plant type (ecotype and age).44 Not surprisingly R. fruticosus L. accumulated Cu the most since Cu is the major element present in the soil of Bor municipality due to the pyrometallurgical processing and geology. The highest concentrations of Cu were determined in the stem 986 mg kg−1, the root 961 mg kg−1 and the leaf 521 mg kg−1 at the site closest to the copper smelter. Also, high concentrations of Cu were determined outside the town at rural site S (692 mg kg−1 root, 507 mg kg−1 leaf and 419 mg kg−1 stem), which is in the direction of one of the most dominant western winds (Fig. 1, wind rose plot). Even though the concentrations of Cu in the soil were approximately the same as those at sites FJ and S, the concentrations of Cu in R. fruticosus L. at site FJ were much higher than that at site S. This can be assigned to the highly acidic soil at site FJ unlike the weakly alkaline area S (soil pH values are provided in Table 1) since Cu is strongly adsorbed onto the soil particles at high pH values and is less bioavailable for the plants compared under low pH conditions.3,4,12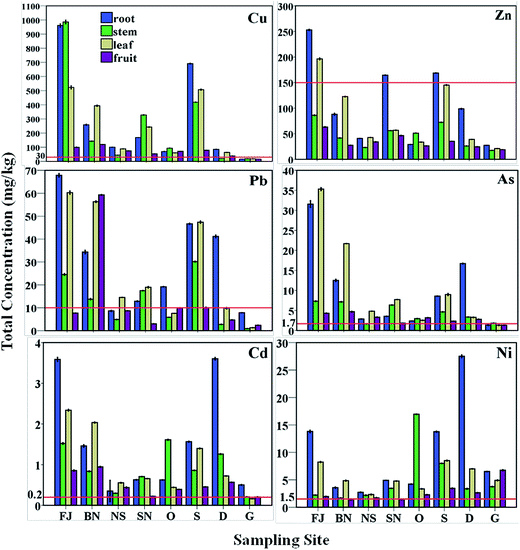 | ||
| Fig. 2 Comparison of the TE concentrations in all parts of R. fruticosus L. at various sampling sites. | ||
The highest concentrations of Cu at site BN were found in the leaves (391 mg kg−1) and the roots (259 mg kg−1), while at site SN they were 328 mg kg−1 in the stems and 243 mg kg−1 in the leaves. The highest concentrations of Zn in the roots were recorded at site FJ, and thus this essential element has no major impact on R. fruticosus L. as a pollutant. The highest concentration of Pb in the roots of R. fruticosus L. was recorded at site FJ (68 mg kg−1). The highest value of Pb in the fruits (59.3 mg kg−1) was observed at site BN. High concentrations of the toxic element As were determined in the roots (32 mg kg kg−1) and leaves (35.3 mg kg kg−1) at site FJ, while increased concentrations of the same element were observed at sites BN and D. Higher concentrations of Cd were observed at site D (3.6 mg kg−1, root) and FJ (3.58 mg kg−1, root), while concentrations of Ni were highest at sites D (27.5 mg kg−1, root), O (16.96 mg kg−1, stem), FJ (13.8 mg kg−1, root) and S (13.8 mg kg−1, root). Therefore, site D 17 km far from the town is shown to be a polluted area, although it is considered as an ecological oasis in the Bor municipality.
In all components of R. fruticosus L. (all sites) concentrations of Cu exceeded the phytotoxic values (20–100 mg kg−1), while As exceeded the phytotoxic values (5–20 mg kg−1) in the roots and the stems according to Kabata-Pendias.42 Concentrations of Pb, Cd and Ni in almost all parts of R. fruticosus L. were above the normal range, thereby reflecting that the developed plant accumulated increased concentrations of the toxic elements. Zn concentrations in all parts of R. fruticosus L. were in the normal range, except in the roots and leaves at site FJ, and in the roots at sites SN and S. Hence, we can conclude that R. fruticosus L. grows in the polluted soil without visible symptoms of toxicity and is able to withstand the high concentrations of TEs as a tolerant plant for the studied TEs.24,42
Enrichment of plant parts (EFplant)
Confirmation of the geological origin of Ni is obtained from the EFplant values calculated for each plant part (Table 3). For instance, the only two values that are somewhat higher than 2 are obtained for the roots at site D (EFplant = 4.22) and for the stems at site O (EFplant = 4.51). All other values for EFplant are very low. This may suggest that wild blackberry assimilated Ni through roots mainly from the minerals present in the soil according to their individual needs.8| Sampling site (km) | EFplant | Cu | Zn | Pb | As | Cd | Ni |
|---|---|---|---|---|---|---|---|
| FJ (0.7) | r/r | 56.2 | 9.2 | 8.6 | 22.9 | 7.1 | 2.1 |
| s/s | 51.1 | 4.9 | 27.0 | 4.0 | 7.2 | 0.6 | |
| l/l | 27.7 | 9.3 | 45.7 | 26.7 | 14.2 | 1.7 | |
| f/f | 5.8 | 3.4 | 3.2 | 3.3 | 4.1 | 0.3 | |
| BN (2.2) | r/r | 15.2 | 3.3 | 4.4 | 8.9 | 2.9 | 1.8 |
| s/s | 7.4 | 2.4 | 15.2 | 3.9 | 4.0 | 0.5 | |
| l/l | 20.8 | 5.8 | 42.6 | 16.4 | 12.4 | 1.0 | |
| f/f | 6.9 | 1.5 | 24.7 | 3.6 | 4.5 | 0.2 | |
| NS (2.5) | r/r | 5.8 | 1.5 | 1.1 | 2.1 | 1.0 | 0.4 |
| s/s | 2.3 | 1.3 | 5.4 | 1.9 | 1.4 | 0.6 | |
| l/l | 4.7 | 2.0 | 11.0 | 3.6 | 3.4 | 0.5 | |
| f/f | 4.3 | 1.8 | 3.6 | 2.5 | 2.1 | 0.3 | |
| SN (2.3) | r/r | 9.9 | 6.0 | 1.6 | 2.5 | 1.3 | 0.7 |
| s/s | 17.0 | 3.2 | 19.2 | 3.5 | 3.4 | 0.9 | |
| l/l | 12.9 | 2.7 | 14.4 | 5.8 | 4.0 | 1.0 | |
| f/f | 3.1 | 2.5 | 1.3 | 1.4 | 1.1 | 0.2 | |
| O (4) | r/r | 4.1 | 1.1 | 2.4 | 1.7 | 1.2 | 0.6 |
| s/s | 4.8 | 2.9 | 6.4 | 1.6 | 7.6 | 4.5 | |
| l/l | 3.2 | 1.6 | 5.7 | 1.9 | 2.7 | 0.7 | |
| f/f | 4.1 | 1.4 | 4.2 | 2.4 | 1.9 | 0.3 | |
| S (6.5) | r/r | 40.5 | 6.2 | 5.9 | 6.2 | 3.1 | 2.1 |
| s/s | 21.7 | 4.1 | 33.2 | 2.5 | 4.1 | 2.1 | |
| l/l | 26.9 | 6.8 | 35.9 | 6.7 | 8.5 | 1.7 | |
| f/f | 4.6 | 1.8 | 4.2 | 1.8 | 2.2 | 0.5 | |
| D (17) | r/r | 5.0 | 3.6 | 5.2 | 11.9 | 7.1 | 4.2 |
| s/s | 1.1 | 1.5 | 3.0 | 1.8 | 6.0 | 0.9 | |
| l/l | 3.4 | 1.8 | 7.4 | 2.5 | 4.4 | 1.4 | |
| f/f | 2.2 | 1.3 | 1.9 | 2.1 | 2.7 | 0.4 |
The enrichment of plant parts with Zn, Pb, As and Cd occurred relatively in all parts of the plant. Moreover, the most polluted locations are sites FJ, BN, SN and S. The highest EFplant for Pb are calculated for leaves, 45.68 (FJ), stems, 33.18 (S) and fruits, 24.70 (BN), while that for As occurred in the roots, 22.86 (FJ). Finally, the EFplant calculated for Cu showed the highest enrichment in all parts of the wild blackberry. EFplant reached extremely high levels of Cu at all sites. The highest EFplant observed are 56.2 (FJ) for the roots, 51.1 (FJ) for the stems, 27.7 (FJ) for the leaves, and 6.9 (BN) for the fruits. Based on the criterion EFplant > 2, it can be concluded that all elements, except Ni, originate from the anthropogenic source.
Ratios of TE concentrations between plant parts
The wild blackberry is a perennial, shrubby and thorny plant that, as it is previously mentioned, grows at different locations including contaminated ones. Therefore, it was interesting to investigate its ability for biomonitoring the atmospheric pollution since it grows at different sites that lack evergreen trees that are well-known as excellent indicators of pollution.39,45 As described in the experimental part of this work, the above-ground parts of the blackberry were not washed to assess the ability of the plant parts (stems, leaves, and fruits) to reflect the direct effect of atmospheric pollution.39Table 4 shows the median of the tree ratios (R) for the six TEs at all sampling sites. Rl/f has values >1, except for the control site (G) and are the highest at sites FJ, BN, SN and S as expected. Sites FJ and BN are the closest to the smelter chimneys and are exposed the most during a year (mostly in the windless periods, ∼60%) to atmospheric pollution, while sites SN and S are in the path of the dominant winds (W, WNW). Values for Rl/s are >1 at almost all sites, while somewhat lower values are observed for Rf/s. In general, the accumulation pattern of the TEs in the wild blackberry is: stem < fruit < leaf. Values for the three ratios imply that the above-ground parts of the blackberry can be used for pollution biomonitoring. The leaves of the blackberry exhibited the highest ability as indicators of atmospheric pollution. The reason for this is the fact that blackberry has leaves with a feather like nervature and lot of bumps, which are jagged around the rim, and on the opposite side are covered with hairs. This means that leaves have a high surface area that enables good absorption and adsorption of TE. It should be pointed out that blackberry fruits are directly consumed as food while the leaves are used for tea. Thus, it is very important to be careful in their consumption even in the areas that are relatively far from the pollution source.| Ratio (R) | Sampling site (km) | |||||||
|---|---|---|---|---|---|---|---|---|
| FJ (0.7) | BN (2.2) | NS (2.5) | SN (2.3) | O (4) | S (6.5) | D (17) | G (19) | |
| a l – leaf, f – fruit, s – stem. | ||||||||
| R l/f | 5.19 | 3.15 | 1.37 | 3.76 | 1.04 | 4.08 | 1.73 | 0.88 |
| R l/s | 2.56 | 3.01 | 2.11 | 1.06 | 0.66 | 1.56 | 1.96 | 1.07 |
| R f/s | 0.53 | 1.40 | 1.53 | 0.363 | 0.74 | 0.41 | 1.10 | 1.36 |
Soil correlation analysis
Correlations between TE concentrations in soil and pH, EC, OM and the distance from the copper smelter were examined. Robust and intermediate inter/element correlations were evident for all elements except Ni (p < 0.05, p < 0.01, Table 5); for example between Cu and Pb (0.86), As and Pb (0.92), Cd and Pb (0.88), and Cd and As (0.86). The significant correlations between Cu, Zn, Pb, As and Cd in highly contaminated soils indicate a similar origin of these five elements (anthropogenic source of pollution), while negligible correlations of Ni suggest that its source is primarily of geological origin.7,8,45| Cu | Zn | Pb | As | Cd | Ni | pH | OM | EC | D | |
|---|---|---|---|---|---|---|---|---|---|---|
| a Correlation is significant at the 0.01 level (2-tailed). b Correlation is significant at the 0.05 level (2-tailed). c D – distance from smelter. | ||||||||||
| Cu | 1.00 | |||||||||
| Zn | 0.565a | 1.00 | ||||||||
| Pb | 0.859a | 0.466b | 1.00 | |||||||
| As | 0.679a | 0.215 | 0.921a | 1.00 | ||||||
| Cd | 0.590a | 0.438b | 0.885a | 0.859a | 1.00 | |||||
| Ni | −0.280 | 0.061 | 0.165 | 0.217 | 0.567a | 1.00 | ||||
| pH | −0.284 | −0.063 | −0.636a | −0.839a | −0.754a | −0.454b | 1.00 | |||
| OM | 0.377 | 0.447b | 0.369 | 0.040 | 0.399 | 0.325 | 0.160 | 1.00 | ||
| EC | 0.554a | 0.491b | 0.122 | −0.183 | −0.121 | −0.526a | 0.400 | 0.485b | 1.00 | |
| D | −0.768a | −0.771a | −0.615a | −0.435b | −0.450b | 0.218 | 0.049 | −0.203 | −0.379 | 1.00 |
Cu, Zn and Ni are significantly correlated with EC reflecting the presence of their ionic forms in the soil being bioavailable for the uptake by plants. On the other hand, concentrations of Pb, As, Cd and Ni are inversely dependent on the soil pH. Unfavorable pH leads to lower uptake through the roots and consequently lower concentrations of TEs accumulated in R. fruticosus L. The same was observed for Ni, Pb and As by Kabata-Pendias and Mukherjee46 and Martínez-Sánchez et al.47
The negative dependence on the distance for Cu, Zn, Pb, As and Cd identifies the copper smelter as a major source of pollution. This has been reported previously for Cu.36,42,48
Relationships between plant and soil
The matrix of Pearson correlation coefficients for TE concentrations in different blackberry parts are summarized in Table 6 and for their dependence on soil, pH, EC, OM and D in Table S1.†| CuR | ZnR | PbR | AsR | CdR | NiR | CuS | ZnS | PbS | AsS | CdS | NiS | CuL | ZnL | PbL | AsL | CdL | NiL | CuF | ZnF | PbF | AsF | CdF | NiF | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Correlation is significant at the 0.01 level (2-tailed). b Correlation is significant at the 0.05 level (2-tailed). c XR, XS, XL, XF, and XZ represent the levels of Cu, Zn, Pb, As, Cd and Ni in roots (R), stems (S), leaves (L), fruits (F) and soil (Z), respectively. | ||||||||||||||||||||||||
| CuR | 1 | |||||||||||||||||||||||
| ZnR | 0.872a | 1 | ||||||||||||||||||||||
| PbR | 0.858a | 0.779a | 1 | |||||||||||||||||||||
| AsR | 0.751a | 0.746a | 0.916a | 1 | ||||||||||||||||||||
| CdR | 0.551a | 0.612a | 0.865a | 0.907a | 1 | |||||||||||||||||||
| NiR | 0.242 | 0.352 | 0.597a | 0.552a | 0.839a | 1 | ||||||||||||||||||
| CuS | 0.931a | 0.915a | 0.760a | 0.756a | 0.516a | 0.150 | 1 | |||||||||||||||||
| ZnS | 0.875a | 0.846a | 0.713a | 0.561a | 0.370 | 0.082 | 0.891a | 1 | ||||||||||||||||
| PbS | 0.870a | 0.843a | 0.651a | 0.443b | 0.271 | 0.067 | 0.788a | 0.898a | 1 | |||||||||||||||
| AsS | 0.628a | 0.775a | 0.615a | 0.616a | 0.419b | 0.026 | 0.689a | 0.711a | 0.707a | 1 | ||||||||||||||
| CdS | 0.399 | 0.392 | 0.647a | 0.582a | 0.609a | 0.411b | 0.444b | 0.586a | 0.258 | 0.392 | 1 | |||||||||||||
| NiS | −0.146 | −0.305 | −0.134 | −0.360 | −0.281 | −0.140 | −0.164 | 0.182 | −0.052 | −0.290 | 0.483b | 1 | ||||||||||||
| CuL | 0.903a | 0.829a | 0.757a | 0.610a | 0.395 | 0.074 | 0.802a | 0.839a | 0.943a | 0.806a | 0.274 | −0.217 | 1 | |||||||||||
| ZnL | 0.955a | 0.828a | 0.855a | 0.762a | 0.531a | 0.149 | 0.866a | 0.826a | 0.860a | 0.763a | 0.372 | −0.237 | 0.964a | 1 | ||||||||||
| PbL | 0.837a | 0.720a | 0.765a | 0.676a | 0.436b | 0.034 | 0.725a | 0.721a | 0.808a | 0.818a | 0.289 | −0.296 | 0.955a | 0.960a | 1 | |||||||||
| AsL | 0.798a | 0.738a | 0.753a | 0.836a | 0.547a | 0.034 | 0.832a | 0.675a | 0.606a | 0.812a | 0.390 | −0.382 | 0.794a | 0.889a | 0.878a | 1 | ||||||||
| CdL | 0.816a | 0.720a | 0.829a | 0.801a | 0.577a | 0.135 | 0.736a | 0.683a | 0.704a | 0.830a | 0.403 | −0.329 | 0.892a | 0.943a | 0.974a | 0.936a | 1 | |||||||
| NiL | 0.761a | 0.769a | 0.850a | 0.697a | 0.755a | 0.708a | 0.646a | 0.613a | 0.669a | 0.482b | 0.384 | −0.172 | 0.675a | 0.695a | 0.568a | 0.473b | 0.577a | 1 | ||||||
| CuF | 0.558a | 0.389 | 0.506b | 0.451b | 0.180 | −0.258 | 0.468b | 0.554a | 0.558a | 0.660a | 0.347 | −0.065 | 0.719a | 0.732a | 0.844a | 0.739a | 0.830a | 0.111 | 1 | |||||
| ZnF | 0.770a | 0.873a | 0.559a | 0.637a | 0.388 | 0.033 | 0.916a | 0.791a | 0.690a | 0.655a | 0.341 | −0.260 | 0.668a | 0.706a | 0.591a | 0.743a | 0.603a | 0.405b | 0.443b | 1 | ||||
| PbF | 0.025 | −0.088 | 0.138 | 0.118 | −0.021 | −0.301 | −0.102 | −0.012 | 0.105 | 0.473b | 0.009 | −0.190 | 0.347 | 0.315 | 0.553a | 0.400 | 0.551a | −0.108 | 0.712a | −0.173 | 1 | |||
| AsF | 0.395 | 0.234 | 0.530a | 0.602a | 0.400 | −0.054 | 0.345 | 0.313 | 0.208 | 0.506b | 0.471b | −0.136 | 0.452b | 0.568a | 0.667a | 0.723a | 0.756a | 0.035 | 0.877a | 0.327 | 0.673a | 1 | ||
| CdF | 0.526a | 0.394 | 0.720a | 0.765a | 0.614a | 0.191 | 0.427b | 0.350 | 0.323 | 0.623a | 0.443b | −0.314 | 0.595a | 0.705a | 0.791a | 0.806a | 0.883a | 0.345 | 0.807a | 0.316 | 0.696a | 0.922a | 1 | |
| NiF | −0.191 | −0.335 | −0.241 | −0.290 | −0.180 | 0.088 | −0.253 | −0.368 | −0.297 | −0.525a | −0.417b | 0.051 | −0.332 | −0.304 | −0.405b | −0.396 | −0.434b | 0.126 | −0.677a | −0.485b | −0.349 | −0.649a | −0.478b | 1 |
Since significant correlations between TEs and pH were not observed, it is concluded that the soil pH did not affect the uptake of Cu, Zn and Ni by R. fruticosus L. By contrast, concentrations of Pb, As and Cd in all parts of R. fruticosus L. and soil pH values showed significant negative correlations (p < 0.05, p < 0.01), which likely reflect the competition related to the different absorption mechanisms in plants.49 All elements in the stems, leaves and fruits (no correlation with roots), except Ni, exhibited negative significant correlations with the distance from the smelter. TE contents and soil conductivity were positively correlated for Cu, Zn and Pb in the roots, stems and leaves (p < 0.01). Thus EC affects the uptake efficiency of these elements through the roots, which is in agreement with high concentrations found in the roots of R. fruticosus L. However, no significant correlation was found between concentrations of TEs in R. fruticosus L. and the OM content in soil.
Strong correlations between Cu, Pb, As, Cd, and Ni concentrations in the roots and the soil reflect an efficient uptake of these elements while those in the stem and the soil represent their efficient translocation or atmospheric deposition (p < 0.05, p < 0.01). The negative correlations of Ni in the soil, Cu in the root, and Cu, Zn and Pb in the stem (Table S1†) suggest different mechanisms for the uptake compared with other elements. The content of Ni in all parts of R. fruticosus L. has no significant correlations with the elements in the soil. By contrast the Ni content in the fruits of R. fruticosus L. is robustly and negatively correlated with all other elements in the soil. Therefore, it can be concluded that the content of Ni in R. fruticosus L. partially originates from the atmospheric deposition and predominantly from the mineralization of the parent rock in the observed area.3,50 Atmospheric deposition may increase the level of TEs in plants, and the same applies to fruits but in a lower amount due to shorter exposure.51–53
In general, concentrations of TEs (except Ni) in the leaves and fruits have significant correlations (p < 0.01) with the content in the soil, compared with correlations of soil with the roots and stems (Table S1†). The positive significant correlations of TEs in different parts of R. fruticosus L., excluding Ni (in all parts of R. fruticosus L.) and Pb (in the fruits) (Table 6) are in agreement with the correlations determined between TEs in soil and different parts of R. fruticosus L.
Additionally, R. fruticosus L. has a large biomass and rapid growth, and represents the native plant species that are apparently well adapted to the ecological stress. Consequently, this plant is helpful in the remediation of any metalliferous areas.
Principal component analysis
Principle Component Analysis (PCA) was performed using factor extraction with an eigenvalue larger than 1 (Kaiser criterion) after varimax rotation. The factor loadings obtained for various TEs in R. fruticosus L. and the soil are presented in Table S2.† The loadings that exceed 0.70 (typically regarded as excellent54) are marked in bold type in Table S2.† The results show that five components (PCA 1–PCA 5) are with eigenvalues >1 which explains 94.5% of total variance in the dataset, with individual contributions of 55.2%, 16.4%, 11.3%, 6.0% and 5.6%, respectively.The first component is characterized by distinct high loadings for Cu and Zn in all parts of R. fruticosus L. and soil, Pb in the stem and leaf, and Ni in the soil. These results indicated the existence of a strong relationship between TE concentrations in R. fruticosus L. and the soil, confirming correlations shown in Tables 6 and S1.† The PCA 1 results are clearer if we consider that the annual emissions from the metallurgical processes contain around 700 t As, 217 t Pb, 1075 t Zn and other TE on the average.55 The flotation tailing pond is added to at a rate of 1.1 to 45.3 kg s−1 and represents an additional pollution source.55 Dust particles rich in TEs and carried by dominant wind are deposited on the soil, thus contaminating both soil and the existing vegetation. These elements dominate component 1, which suggests that these variables have a similar source. From the results for soil and plant parts along with the PCA results it can be concluded that the pollution comes from the anthropogenic activities. However, Ni is excluded from this grouping since its factor loadings in soil is −0.730 which supports its geological origin.
The second and the third components (PCA 2 and PCA 3) with 16.4% and 11.3% of variance, respectively, are characterized by significant positive values for Pb, As, and Cd in soil, Cd in the leaves, Cu, Pb, As, and Cd in the fruits (PCA 2) and As, Cd and Ni in the roots (PCA 3). Therefore, the other two factors reflect emissions from the smelter and the copper mine through atmospheric deposition of TE on the soil and aboveground parts of R. fruticosus L. This grouping of elements indicates that all parts of R. fruticosus L. and even soil serve as accumulators of the examined elements from the polluted atmosphere. PCA is considered useful if the cumulative percentage of variance approaches 80%.56
The first three components explained 83% of the total variance and the visualization of relations among the contents of TE in soil and parts of R. fruticosus L. is illustrated in the three-dimensional (3D) space (Fig. 3). It is evident that the Ni contents of the plant components and soil are grouped at the left-hand corner of the cube, which is consistent with contributions for this metal from a non-anthropogenic source.
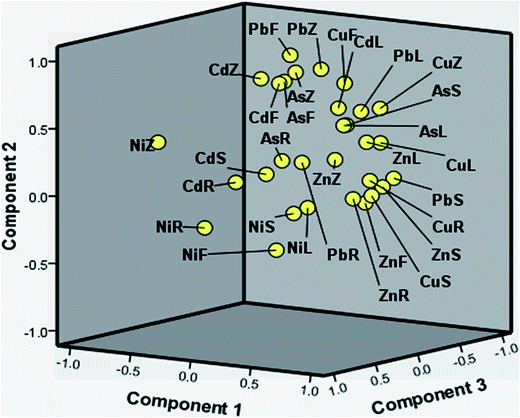 | ||
| Fig. 3 PCA results in the 3D space showing loadings of the first three principal components (R – root, S – stem, L – leaf, F – fruit, Z – soil). | ||
D Conclusions
In this study, the total concentrations of the six TEs (Cu, Zn, Pb, As, Cd and Ni) in R. fruticosus L. (wild blackberry) and contaminated soils around the Bor basin are determined. Since the soils are generally highly polluted by TEs (the most with Cu and As), the wild blackberry and its parts are under constant stress of the highly increased concentrations of TE at all sites studied. Therefore, R. fruticosus L. represents a plant that is tolerant to the TEs studied because of its ability to withstand TE concentrations that exceed phytotoxic and normal values. Therefore, it may be classified as a metallophyte. This is also supported by the fact that R. fruticosus L. successfully grows and reproduces in the pollution zone studied without visible symptoms of toxication.EFsoil showed that the highest enrichment was by Cu and As. Significant negative correlations between the concentrations of Cu, Zn, Pb, As and Cd and the distance from the copper smelter as a major source of pollution confirm that pollution decreased with increasing distance from the smelter. Element enrichment factors for R. fruticosus L. parts showed significant values at all sites and for all examined TE, except Ni. In general, the highest enrichment was calculated for the roots and leaves, and with Cu among all TEs. The three R ratios, Pearson's correlation and Principal Component Analysis results confirm that the concentration of TEs in plant parts was affected by airborne pollution originating from the copper smelter, whereas geological factors primarily contributed to the Ni concentration. Leaves of the wild blackberry appeared to be the best indicator for atmospheric pollution due to its specific anatomy. Because of this and its high abundance, blackberry can be successfully used as an effective biomonitor of pollution. On the other hand, it should be pointed out that consumption of the leaves and fruits as food is not recommended in the industrially polluted areas because the content of Cu and As exceeded phytotoxic values while the observed concentrations of the other TEs were comparable to normal.
Acknowledgements
The authors are grateful to the Ministry of Education, Science and Technological Development of Serbia for financial support (Project No. 172031).References
- J. Bech, N. Roca, J. Barceló, P. Duran, P. Tume and C. Poschenrieder, J. Geochem. Explor., 2012, 113, 94–99 CrossRef CAS.
- D. Lei and C. Duan, J. Environ. Sci., 2008, 20, 1202–1209 CrossRef CAS.
- S. M. Ghaderian and A. A. Ghotbi Ravandi, J. Geochem. Explor., 2012, 123, 25–32 CrossRef CAS.
- J. M. Gomez-Ros, G. Garcia and J. M. Peñas, Ecol. Eng., 2013, 57, 393–402 CrossRef.
- F. Nannoni, G. Protano and F. Riccobono, Geoderma, 2011, 161, 63–73 CrossRef CAS.
- M. M. Antonijević, M. D. Dimitrijević, S. M. Milić and M. M. Nujkić, J. Environ. Monit., 2012, 14, 866–877 RSC.
- S. M. Serbula, T. S. Kalinovic, A. A. Ilic, J. V. Kalinovic and M. M. Steharnik, Aerosol Air Qual. Res., 2013, 13, 563–573 CAS.
- S. Alagić, S. Tošić, M. Dimitrijević, M. Antonijević and M. Nujkić, Environ. Sci. Pollut. Res., 2015, 22, 7155–7175 CrossRef PubMed.
- B. Balabanova, T. Stafilov, R. Šajn and K. Bačeva, Int. J. Environ. Sci. Technol., 2013, 11, 517–528 CrossRef.
- H.-Y. Xiao, S.-Y. Jiang, D.-S. Wu and W.-B. Zhou, Soil Sediment Contam., 2011, 20, 592–604 CrossRef CAS.
- M. M. Antonijević, M. D. Dimitrijević, Z. O. Stevanović, S. M. Serbula and G. D. Bogdanovic, J. Hazard. Mater., 2008, 158, 23–34 CrossRef PubMed.
- C. Candeias, R. Melo, P. F. Ávila, E. Ferreira da Silva, A. R. Salgueiro and J. P. Teixeira, Appl. Geochem., 2014, 44, 12–26 CrossRef CAS.
- M. Maric, M. Antonijevic and S. Alagic, Environ. Sci. Pollut. Res., 2013, 20, 1181–1188 CrossRef CAS PubMed.
- M. N. Rashed, J. Hazard. Mater., 2010, 178, 739–746 CrossRef CAS PubMed.
- J. Chen, J. Yuan, S. Wu, B. Lin and Z. Yang, J. Environ. Monit., 2012, 14, 2663–2672 RSC.
- S. Norouzi, H. Khademi, A. F. Cano and J. A. Acosta, Ecol. Indic., 2015, 57, 64–73 CrossRef CAS.
- V. S. Lin, Environ. Sci.: Processes Impacts, 2015, 17, 1137–1140 CAS.
- M. L. Gall, A. G. B. Poore and E. L. Johnston, J. Environ. Monit., 2012, 14, 830–838 RSC.
- J. Mertens, S. Luyssaert and K. Verheyen, Environ. Pollut., 2005, 138, 1–4 CrossRef CAS PubMed.
- M. Tomašević, M. Aničić, Lj. Jovanović, A. Perić-Grujić and M. Ristić, Ecol. Indic., 2011, 11, 1689–1695 CrossRef.
- E. Marguí, I. Queralt, M. L. Carvalho and M. Hidalgo, Environ. Pollut., 2007, 145, 179–184 CrossRef PubMed.
- L. V. Clark, K. J. Evans and M. Jasieniuk, Biological Invasions, 2012, 15, 1331–1342 CrossRef.
- R. Blagojević and V. Božić, Technology of production of blackberries. (Serbian text) http://www.fb.org.rs/upload/content/Tehnologija_proizvodnje_kupine.pdf, Serbia, 2012.
- J. Yoon, X. Cao, Q. Zhou and L. Q. Ma, Sci. Total Environ., 2006, 368, 456–464 CrossRef CAS PubMed.
- A. P. G. C. Marques, H. Moreira, A. O. S. S. Rangel and P. M. L. Castro, J. Hazard. Mater., 2009, 165, 174–179 CrossRef CAS PubMed.
- C. Weeks, M. Croasdale, M. Osborne, L. Hewitt, P. F. Miller, P. Robb, M. J. Baxter, P. D. Warriss and T. G. Knowles, Food Addit. Contam., 2006, 23, 140–147 CrossRef CAS PubMed.
- H. Moreira, A. P. G. C. Marques, A. O. S. S. Rangel and P. M. L. Castro, Water, Air, Soil Pollut., 2011, 221, 377–389 CrossRef CAS.
- F. Baroni, A. Boscagli, L. A. Di Lella, G. Protano and F. Riccobono, J. Geochem. Explor., 2004, 81, 1–14 CrossRef CAS.
- N. Massa, F. Andreucci, M. Poli, M. Aceto, R. Barbato and G. Berta, Ecotoxicol. Environ. Saf., 2010, 73, 1988–1997 CrossRef CAS PubMed.
- S. Č. Alagić, S. S. Šerbula, S. B. Tošić, A. N. Pavlović and J. V. Petrović, Arch. Environ. Contam. Toxicol., 2013, 65, 671–682 CrossRef PubMed.
- A. P. Murphy, M. Coudert and J. Barker, J. Environ. Monit., 2000, 2, 621–627 RSC.
- M. M. Reglero, L. Monsalve-González, M. Taggart and R. Mateo, Sci. Total Environ., 2008, 406, 287–297 CrossRef CAS PubMed.
- C. Jolivet, D. Arrouays and M. Bernoux, Commun. Soil Sci. Plant Anal., 1998, 29, 2227–2233 CrossRef CAS.
- M. D. Mingorance, B. Valdés and S. R. Oliva, Environ. Int., 2007, 33, 514–520 CrossRef CAS PubMed.
- J. Wu, Y. Teng, S. Lu, Y. Wang and X. Jiao, PLoS One, 2014, 9, e112917 Search PubMed.
- M. D. Dimitrijević, M. M. Nujkić, S. C. Alagić, S. M. Milić and S. B. Tošić, Int. J. Environ. Sci. Technol., 2016, 13, 615–630 CrossRef.
- R. A. Sutherland, Environ. Geol., 2000, 39, 611–627 CrossRef CAS.
- G. C. Kisku, S. C. Barman and S. K. Bhargava, Water, Air, Soil Pollut., 2000, 120, 121–137 CrossRef CAS.
- S. R. Oliva and M. D. Mingorance, Chemosphere, 2006, 65, 177–182 CrossRef CAS PubMed.
- R. Burt, U.S. Department of Agriculture, Natural Resources Conservation Service. www.nrcs.usda.gov, 2014.
- G. Brunetti, P. Soler-Rovira, K. Farrag and N. Senesi, Plant Soil, 2008, 318, 285–298 CrossRef.
- A. Kabata-Pendias, Trace Elements in Soils and Plants, CRC Press, Boca Raton, 4th edn, 2011 Search PubMed.
- The Official Gazette of Republic Serbia, No. 23/94, 11/92 and 32/2002, Regulation about allowable quantities of hazardous and harmful substances in the soil and methods for their investigation (in Serbian).
- R. C. González and M. C. González-Chávez, Environ. Pollut., 2006, 144, 84–92 CrossRef PubMed.
- T. S. Kalinović, S. M. Serbula, A. A. Radojević, J. V. Kalinović, M. M. Steharnik and J. V. Petrović, Geoderma, 2016, 262, 266–275 CrossRef.
- A. Kabata-Pendias and A. B. Mukherjee, Trace Elements from Soil to Human, Springer-Verlag, Berlin, 2007 Search PubMed.
- M. J. Martínez-Sánchez, S. Martínez-López, M. L. García-Lorenzo, L. B. Martínez-Martínez and C. Pérez-Sirvent, Geoderma, 2011, 160, 535–541 CrossRef.
- T. Stafilov, R. Sajn, Z. Pancevski, B. Boev, M. V. Frontasyeva and L. P. Strelkova, J. Hazard. Mater., 2010, 175, 896–914 CrossRef CAS PubMed.
- C. Xiong, Y. Zhang, X. Xu, Y. Lu, B. Ouyang, Z. Ye and H. Li, Sci. Hortic., 2013, 164, 295–302 CrossRef CAS.
- Heavy Metals in Soils: Trace Metals and Metalloids in Soils and their Bioavailability, Environmental Pollution, ed. B. J. Alloway, vol. 22, Springer Netherlands, 2013 Search PubMed.
- G. M. Bermudez, R. Jasan, R. Plá and M. L. Pignata, J. Hazard. Mater., 2012, 213–214, 447–456 CrossRef CAS PubMed.
- R. K. Sharma, M. Agrawal and F. M. Marshall, Environ. Pollut., 2008, 154, 254–263 CrossRef CAS PubMed.
- J. Pandey and U. Pandey, Environ. Monit. Assess., 2009, 148, 61–74 CrossRef CAS PubMed.
- J. H. Garcia, W. W. Li, R. Arimoto, R. Okrasinski, J. Greenlee, J. Walton and C. Schloesslin, Sci. Total Environ., 2004, 325, 95–112 CrossRef CAS PubMed.
- M. I. Najdenov, Ph.D. Thesis, University of Belgrade, 2013.
- W.-Q. Li, L. Xiao-Jing, M. A. Khan and B. Gul, Pak. J. Bot., 2008, 40, 1081–1090 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5em00646e |
| This journal is © The Royal Society of Chemistry 2016 |
