Spatial and seasonal variations in the composition of dissolved organic matter in a tropical catchment: the Lower Kinabatangan River, Sabah, Malaysia
Sahana
Harun
 *ab,
Andy
Baker
c,
Chris
Bradley
d and
Gilles
Pinay
e
*ab,
Andy
Baker
c,
Chris
Bradley
d and
Gilles
Pinay
e
aInstitute for Tropical Biology and Conservation, Universiti Malaysia Sabah, Jalan UMS, 88400 Kota Kinabalu, Sabah, Malaysia. E-mail: sahana@ums.edu.my
bWater Research Unit, Universiti Malaysia Sabah, Jalan UMS, 88400 Kota Kinabalu, Sabah, Malaysia
cConnected Waters Initiative Research Centre, UNSW Australia, Sydney, NSW 2052, Australia. E-mail: A.Baker@unsw.edu.au
dSchool of Geography, Earth and Environmental Sciences, The University of Birmingham, Edgbaston, Birmingham, B15 2TT, United Kingdom. E-mail: C.Bradley@bham.ac.uk
eECOBIO, OSUR, University of Rennes 1, campus de Beaulieu, 35042 Rennes Cedex, France. E-mail: Gilles.Pinay@univ-rennes1.fr
First published on 7th December 2015
Abstract
Dissolved organic matter (DOM) was characterised in water samples sampled in the Lower Kinabatangan River Catchment, Sabah, Malaysia between October 2009 and May 2010. This study aims at: (i) distinguishing between the quality of DOM in waters draining palm oil plantations (OP), secondary forests (SF) and coastal swamps (CS) and, (ii) identifying the seasonal variability of DOM quantity and quality. Surface waters were sampled during fieldwork campaigns that spanned the wet and dry seasons. DOM was characterised optically by using the fluorescence Excitation Emission Matrix (EEM), the absorption coefficient at 340 nm and the spectral slope coefficient (S). Parallel Factor Analysis (PARAFAC) was undertaken to assess the DOM composition from EEM spectra and five terrestrial derived components were identified: (C1, C2, C3, C4 and C5). Components C1 and C4 contributed the most to DOM fluorescence in all study areas during both the wet and dry seasons. The results suggest that component C4 could be a significant (and common) PARAFAC signal found in similar catchments. Peak M (C2 and C3) was dominant in all samples collected during wet and dry seasons, which could be anthropogenic in origin given the active land use change in the study area. In conclusion, there were significant seasonal and spatial variations in DOM which demonstrated the effects of land use cover and precipitation amounts in the Kinabatangan catchment.
Environmental impactThe research presented in this paper seeks to characterise dissolved organic matter (DOM) in a tropical catchment in North Borneo that has been affected by significant (and recent), development of oil palm plantations. This paper also examines the variations in DOM according to the land use (specifically secondary forests, oil palm plantations and coastal swamps), and seasonal variations in DOM (during the inter-monsoonal period, wet and dry seasons). We emphasize, in the paper, the potential of fluorescence spectroscopy to determine the environmental impacts of active land use change including deforestation and commercial agricultural activities on water resources. To-date there has been very little work published on catchments that have been affected in this way and particularly with respect to the application of fluorescence spectroscopy. We hope that the research will be of wider interest, and believe that the research is a precursor to solving problems of ecosystem loss for energy production associated with oil palm plantations in tropical regions. |
Introduction
A recent synthesis and re-evaluation of the global carbon cycle suggested that approximately 3 Pg C per year of CO2 is outgassed from global inland waters,1 while the estimated global riverine total carbon flux is 0.80–1.33 Pg C per year.2 Given that it has been estimated that approximately half of the carbon is consumed within river systems before reaching the ocean,3 in-stream and near-stream processing of organic matter is a fundamental component of the carbon cycle. This corroborates the research which found that Amazonian rivers outgassed more than ten times the quantity of carbon exported to the ocean in the form of total organic carbon or dissolved organic carbon (DOC).4 Significantly, the determination of the carbon isotopic composition of DOC suggests that contemporary organic carbon (i.e. carbon <5 years in age) was the dominant source of excess CO2 that drives outgassing in Amazonian first order streams and large rivers.5 Together, these results emphasize the importance of land-derived, biologically available carbon, for heterotrophic microbial processes in river systems.Tropical wetlands provide important ecosystem services including flood mitigation, coastal and wildlife protection, carbon sequestration and respiration.6 Tropical wetland ecosystems include a variety of landforms such as lowland floodplains, forested peatlands, swamps and mangroves.7 The latter are particularly important carbon sinks which have been reported to store ∼49–98% ecosystem carbon in the organic soils.8 Tropical wetlands also experience periodic (prolonged) inundation,9 reflecting marked seasonal variations in precipitation,10 while evapotranspiration rates are high.11 Tropical wetlands have been associated with the release of an estimated ∼60% of total (global) water, sediment and organic carbon input to the ocean.12 However, these wetlands are seriously threatened by environmental deterioration as many catchments have experienced rapid conversion of land to agriculture13–15 with a concomitant reduction in wetland extent.
Among other agricultural threats, conversion of tropical forest to oil palm (Elaeis guineensis) cultivation is a major concern given the recent growth of the palm oil industry.16 Oil palm plantations are now estimated to extend over >13.5 million ha of the tropics17 and have contributed to the drainage of floodplain wetlands, and the loss of primary and secondary forest.18 At present, the majority of oil palm plantations are confined to South East Asia, as Malaysia and Indonesia produce ∼80% of the world's palm oil. However, substantial areas of the Congo and Amazon Basin are suitable for oil palm plantation, and further plantation developments are likely in these areas. This situation emphasizes the urgent need to understand the environmental implications of oil palm development. This is particularly important as the full implications of recent and, in some places accelerating, changes in oil palm cover have yet to be considered in detail and these land use changes are likely to affect the quantity and quality of dissolved organic matter (DOM) and DOC.19,20
Recent advances in fluorescence spectroscopy have considerable potential as we seek to address this research gap, as they have significantly enhanced our ability to characterize DOM.21 DOM fractions possess fluorescent properties enabling DOM monitoring in soils,22 rivers,23,24 lakes,25 estuarine and coastal environments.26,27 Reassessment of fluorescence excitation emission matrix (EEM) spectra using Parallel Factor Analysis (PARAFAC) has been invaluable in characterising and quantifying the changes in DOM fluorescence. By decomposing an EEM dataset into several, mathematically independent components parameterized by concentrations (loadings) and excitation and emission spectra, different DOM fractions have been traced through the natural environment.28 For example, in southern Ontario, Canada, DOM production and transformation processes were successfully studied in areas of different use.24 Specifically in a tropical catchment, DOM export was found to be greater during the April flush (inter-monsoonal period), and it has been suggested that tropical rivers are likely to export more labile DOM during periods of high precipitation.29 This is supported by a study in the sub-tropical Jiulong River catchment, China, where increased DOM concentrations were observed after storms, as a result of terrestrial DOM export to the river; with a decrease in the protein-like fraction of DOM over the same period.30 In sub-tropical Uruguay also, DOM characteristics have been found to vary temporally in catchments with intensive farming practices which was positively related to microbial processing.19 These results have implications for downstream and marine ecosystems, however, the importance of this research has yet to be more widely established.
The potential utility of fluorescence spectroscopy, specifically in SE Asia, was demonstrated in a preliminary study that sought to characterize spatial trends in DOM in the Lower Kinabatangan River Sabah, Malaysian Borneo.31 River flow and photodegradation were found to have a significant effect on DOM properties, however, the extent to which DOM varies seasonally was not considered. This provides the motivation for this paper particularly as, in common with other catchments in this region, there has been a rapid recent increase in the areal extent of oil palm plantations. This, and the conservation of riparian secondary forest and coastal wetlands within the catchment, provides an opportunity to determine the degree to which DOM quantity and quality is affected: first by land-use change, and second by the seasonal flood pulse.32 Accordingly, the objectives of this study were to: (i) characterize DOM quality in waters associated with palm oil plantations, secondary forests and coastal wetlands using fluorescence spectroscopy and PARAFAC;33,34 and (ii) determine the seasonal variability of DOM quantity and quality, and its attribution to each land cover type.
Methods and analytical procedures
Study area
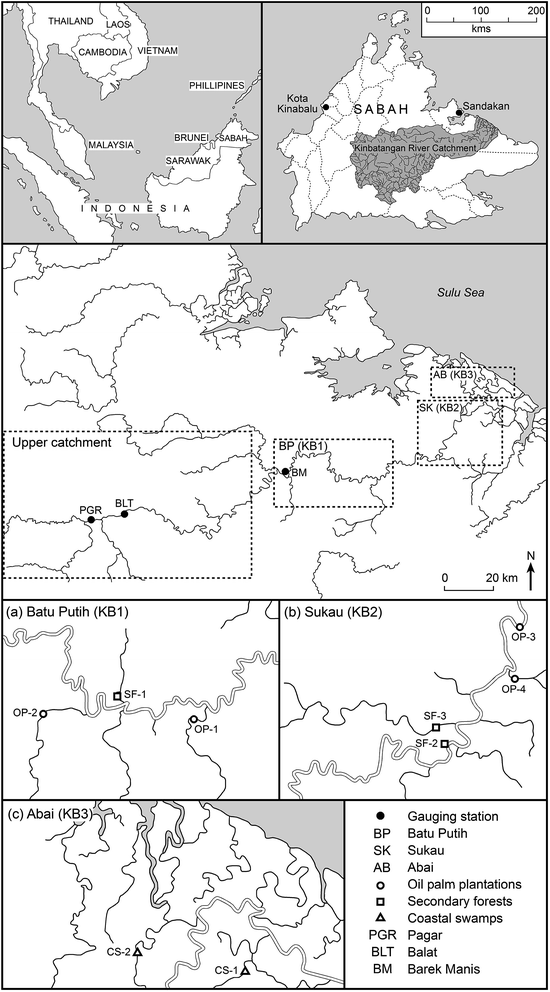
DOM characteristics were determined in selected downstream reaches of the Kinabatangan River and tributaries in Sabah, Malaysia. The Kinabatangan River (560 km in length), is the largest river in Sabah, with a total catchment area of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 km2 (Fig. 1).35 Geologically, the Kinabatangan area is predominantly covered by sandstone and shales, with minor occurrence of cherts and limestones, while the igneous rocks are mainly basalts, serpentinites, gabbros, volcanic breccias and tuffs.36 Four groups of soil parent materials were identified by surveys conducted in the early 1950s: undifferentiated alluvium, peat, sandstone and mudstone and limestone.37,38 Recent alluvium, originating mainly from sedimentary rocks, is found widely on floodplains and in freshwater swamps.38
800 km2 (Fig. 1).35 Geologically, the Kinabatangan area is predominantly covered by sandstone and shales, with minor occurrence of cherts and limestones, while the igneous rocks are mainly basalts, serpentinites, gabbros, volcanic breccias and tuffs.36 Four groups of soil parent materials were identified by surveys conducted in the early 1950s: undifferentiated alluvium, peat, sandstone and mudstone and limestone.37,38 Recent alluvium, originating mainly from sedimentary rocks, is found widely on floodplains and in freshwater swamps.38
The area has a humid tropical climate with mean daily temperatures ranging from 22 °C to 32 °C and a mean annual rainfall of 2500–3000 mm.35,39 Rainfall is greatest between November and March particularly during the northeast (NE) monsoon, and to a lesser degree during the southwest (SW) monsoon.39,40 Transition periods, referred to as the ‘inter-monsoonal periods’, normally occur in April and October and generally correspond to the period of lowest rainfall41 although significant precipitation events may still occur at this time.40,42 Typically, the floodplain and coastal plain are widely inundated during the rainy season but rainfall totals exhibit considerable inter-annual variability.
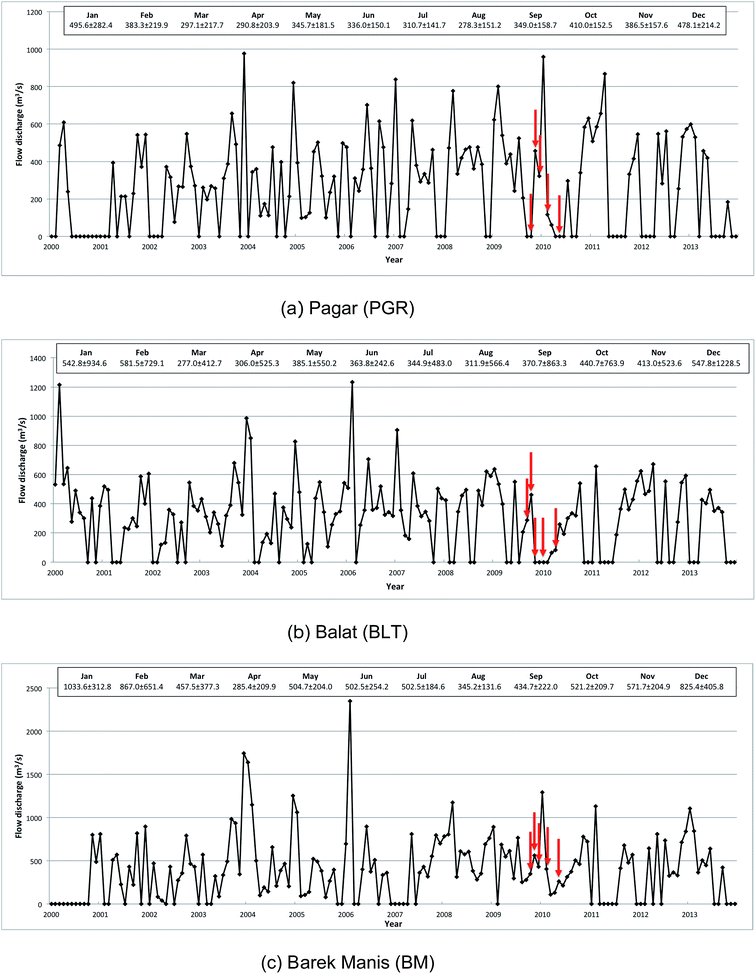
The lower floodplain of the Kinabatangan is >2800 km2 in area (Fig. 1) with two principal land uses: (i) forest (mangroves and peat swamps); (ii) agriculture (primarily oil palm plantations and other crops); with relatively little urban development and only occasional small water bodies.43 Approximately 74% of the Kinabatangan catchment is tropical forest, including floodplains with open reed swamp, and lowland dipterocarp forest in areas that are inundated frequently.39,43 The mean river flow in the upper catchment, recorded at Pagar (PGR) and Balat (BLT) (Fig. 2) varied from ∼14.0 to 1944 m3 s−1 (26–1944 m3 s−1) between 1979 and 2013 (peak daily discharge was recorded in January 1986 at BLT; the lowest flow was observed at PGR in June 1998). Only limited sediment data are available, but a survey at Sukau (at points upstream of coastal swamps) (Fig. 1) in 2005 and 2006 indicated that maximum sediment concentrations were 96 mg l−1 equating to Class IIB of the Malaysian Interim National Water Quality Standard (INWQS).43,44 This appears to reflect commercial logging in the catchment since the 1980s and the development of oil palm plantations which currently extend over ∼4200 km2 which represents approximately 25% of the basin.43
Sampling and analyses
Water was characterized throughout the lower catchment through the manual collection of 510 water samples during five sampling periods in 2009–2010. One period corresponded to the inter-monsoonal period (IM), October 2009; three corresponded to the wet season (WS), November, December 2009 and February 2010; and one the dry season (DS), May 2010. Fieldwork design was constrained by difficulties of access; however, water was sampled along a freshwater – estuarine gradient to determine the seasonal trends in DOM in the Lower Kinabatangan floodplain including across the freshwater–marine interface between the Kinabatangan River and the Sulu Sea. The nearest gauging station was at Barek Manis (BM), situated ∼11 km from sample point SF-1, at which point the upstream catchment is 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 km2 (Fig. 1). Monthly mean discharges during the fieldwork campaign are presented in Table 1.
300 km2 (Fig. 1). Monthly mean discharges during the fieldwork campaign are presented in Table 1.
| Average of monthly discharge at Barek Manis (BM) (m3 s−1) | Land cover | pH | Salinity | DOC (mg l−1) | a 340 (m) | S 275–295 (nm) | IC1 | IC2 | IC3 | IC4 | IC5 | I total | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inter-monsoonal (IM) | Oct. 2009, 346.6 | OP | Mean | 6.94 | 0.07 | 15.30 | 42.14 | 0.0128 | 8.17 | 10.93 | 13.95 | 8.92 | 5.72 | 47.69 |
| Std. dev. | 0.52 | 0.03 | 9.50 | 23.25 | 0.0018 | 3.68 | 3.83 | 5.75 | 3.10 | 2.96 | 15.88 | |||
| Variance | 0.27 | 0.00 | 90.24 | 540.63 | 0.0000 | 13.53 | 14.66 | 33.05 | 9.59 | 8.76 | 252.27 | |||
| SF | Mean | 6.89 | 0.04 | 10.20 | 55.75 | 0.0113 | 8.06 | 9.44 | 13.29 | 7.62 | 3.23 | 41.63 | ||
| Std. dev. | 0.25 | 0.01 | 7.64 | 13.17 | 0.0012 | 3.31 | 3.64 | 5.07 | 2.93 | 1.66 | 15.86 | |||
| Variance | 0.06 | 0.00 | 58.35 | 173.43 | 0.0000 | 10.97 | 13.26 | 25.75 | 8.59 | 2.75 | 251.64 | |||
| CS | Mean | 6.20 | 1.33 | 10.36 | 45.49 | 0.0125 | 8.48 | 10.34 | 14.23 | 8.14 | 2.83 | 44.03 | ||
| Std. dev. | 0.76 | 2.15 | 5.03 | 17.49 | 0.0018 | 3.07 | 3.32 | 4.88 | 2.52 | 0.95 | 14.16 | |||
| Variance | 0.57 | 4.62 | 25.27 | 305.76 | 0.0000 | 9.41 | 11.02 | 23.84 | 6.35 | 0.91 | 200.60 | |||
| Wet season (WS) | Nov. 2009, 561.4 | OP | Mean | 6.77 | 0.06 | 11.59 | 62.37 | 0.0104 | 10.46 | 14.94 | 11.70 | 25.63 | 3.39 | 66.11 |
| Std. dev. | 0.66 | 0.02 | 3.58 | 35.46 | 0.0016 | 5.03 | 6.18 | 4.96 | 10.70 | 0.87 | 26.85 | |||
| Variance | 0.44 | 0.00 | 12.82 | 1257.48 | 0.0000 | 25.28 | 38.24 | 24.64 | 114.55 | 0.75 | 721.17 | |||
| Dec. 2009, 432.5 | SF | Mean | 6.93 | 0.04 | 11.17 | 75.88 | 0.0100 | 9.90 | 13.28 | 10.08 | 23.59 | 2.89 | 59.75 | |
| Std. dev. | 0.30 | 0.02 | 5.46 | 65.67 | 0.0019 | 6.55 | 7.69 | 5.95 | 13.77 | 0.75 | 33.96 | |||
| Variance | 0.09 | 0.00 | 29.79 | 4312.30 | 0.0000 | 42.85 | 59.07 | 35.37 | 189.74 | 0.57 | 1153.60 | |||
| Feb. 2010, 404.4 | CS | Mean | 5.95 | 0.08 | 14.70 | 100.49 | 0.0112 | 14.34 | 18.16 | 14.14 | 33.12 | 2.71 | 82.70 | |
| Std. dev. | 0.76 | 0.04 | 4.14 | 62.52 | 0.0023 | 6.15 | 6.50 | 5.45 | 12.16 | 0.85 | 29.65 | |||
| Variance | 0.57 | 0.00 | 17.15 | 3908.20 | 0.0000 | 37.88 | 42.27 | 29.74 | 147.76 | 0.73 | 879.15 | |||
| Dry season (DS) | May 2010, 260.5 | OP | Mean | 6.90 | 0.09 | 7.26 | 20.28 | 0.0125 | 7.13 | 11.21 | 9.11 | 17.61 | 6.27 | 51.33 |
| Std. dev. | 0.75 | 0.03 | 1.61 | 5.80 | 0.0015 | 1.92 | 3.44 | 2.87 | 5.13 | 3.26 | 13.96 | |||
| Variance | 0.56 | 0.00 | 2.58 | 33.59 | 0.0000 | 3.68 | 11.85 | 8.23 | 26.27 | 10.61 | 194.99 | |||
| SF | Mean | 6.87 | 0.07 | 7.42 | 28.04 | 0.0113 | 7.74 | 11.48 | 8.84 | 18.45 | 4.51 | 51.03 | ||
| Std. dev. | 0.24 | 0.04 | 2.40 | 6.40 | 0.0012 | 3.54 | 5.17 | 4.09 | 8.36 | 3.18 | 22.99 | |||
| Variance | 0.06 | 0.00 | 5.77 | 40.93 | 0.0000 | 12.52 | 26.71 | 16.70 | 69.90 | 10.13 | 528.59 | |||
| CS | Mean | 7.11 | 2.39 | 6.21 | 14.03 | 0.0135 | 6.16 | 10.23 | 7.58 | 16.66 | 5.92 | 46.55 | ||
| Std. dev. | 0.30 | 2.09 | 0.95 | 4.59 | 0.0019 | 1.47 | 2.57 | 1.84 | 4.65 | 2.58 | 10.66 | |||
| Variance | 0.09 | 4.38 | 0.91 | 21.02 | 0.0000 | 2.15 | 6.62 | 3.40 | 21.64 | 6.68 | 113.55 |
Water was sampled from streams or creeks situated entirely within: (i) oil palm (Elaies guineensis) plantations: OP-1, OP-2, OP-3 and OP-4 (220 samples); (ii) secondary forests: SF-1, SF-2 and SF-3 (139 samples) and (iii) coastal swamps of Nypa fruticans (nipa palm): CS-1 and CS-2 (151 samples) (Fig. 1). The vegetation characteristics for each land cover type are summarised in Table 2 (after Abram et al.45). At each point, a 200 ml water sample was collected from the middle of the river/stream from a boat at three points in the water profile: the surface, the mid-point and near the riverbed using a WaterMark Horizontal Polycarbonate water sampler. Samples were stored in high-density polyethylene (HDPE) bottles, pre-washed with hydrochloric acid 10% and deionised water. The pH and salinity were determined using a Hanna Water Quality Multiparameter (Model HI 9828) immediately prior to filtering the water samples (within six hours of sample collection) using 47 mm pre-combusted Whatman glassfiber GF/C filter papers (nominal pore size 1.2 μm). Filtered water samples were kept in the dark and stored at 4 °C before shipment to the UK for laboratory analysis, which occurred within seven days of the end of the field-campaign.
| Sampling station | Vegetation characteristics |
|---|---|
| Oil palm plantation | Palm oil classes |
| a SF-1 is oxbow lake. b SF-2 is oxbow lake. | |
| Sg. Pin (OP-1) | (i) Young mature: palms were planted from 3–6 years |
| Sg. Koyah (OP-2) | (ii) Prime mature and full stand: prime yield and planted within the range from 7–24 years |
| Malbumi plantation (OP-3) | (iii) Underproductive at 75%: palm capacity is within 51–75% palms per ha. Older palm with natural mortality start to occur |
| Sg. Resang (OP-4) | (iv) Underproductive at 50%: palm capacity is ranged from 26–50% palms per ha |
| Sampling station | Vegetation characteristics |
|---|---|
| Secondary forest | Forest type |
| Danau Kaboi (SF-1)a | (i) Lowland dry forest: secondary forest, preceding dipterocarp forest with species include Nauclea subdita, Neolamarckia cadamba, Glochidion rubrum |
| Danau Kalinanap (SF-2)b | (ii) Lowland dry dipterocarp forest: preceding logged lowland mixed dipterocarp forest, dominated by Dipterocarp sp. |
| Sg. Menanggol (SF-3) |
| Sampling station | Vegetation characteristics |
|---|---|
| Coastal swamp | Mangrove forest |
| Balat Damit (CS-1) | Nipah palm forest: Nypa fruticans are dominant within the mangrove system. Can be found either in mono-stand or coexist with Rhizophora apiculata |
| Sg. Merah (CS-2) |
Spectral measurements and DOC
Fluorescence analyses of samples were performed at the University of Birmingham, UK, using a Varian Cary Eclipse spectrophotometer. Excitation–emission matrices (EEMs) were generated for each sample over excitation wavelengths 250 to 400 nm at 5 nm intervals and emission wavelengths 280 to 500 nm at 5 nm intervals, with 2 nm bandwidths on excitation and emission modes. The spectrophotometer output was monitored by regular determination of the Raman intensity of ultra pure water in a sealed 10 × 10 mm cuvette at 348 nm excitation and 5 nm bandpass. No significant changes were observed in the EEMs, particularly in samples associated with the secondary forest and coastal swamps (the mean salinity for all samples collected was 1.27‰), although Yang & Hur46 suggested the potential impact of salinity on fluorescence DOM peaks A and M. An inner-filter effect (IFE) correction was applied to the dataset:47| I = I0(10−b(Aex+Aem)) | (1) |
Absorption coefficients at 340 nm and spectral slope over the interval of 275–295 nm (S275–295)48 were determined using a Lightwave (WPA) spectrophotometer in a 10 mm quartz cuvette. Absorption measurements were corrected against Milli-Q water blanks and the slope of the absorption curve was calculated by linear regression of the log-transformed a spectra.
Dissolved organic carbon (DOC) was determined using a Shimadzu TOC-V-SCH analyser with auto-sampler TOC-ASI-V. Samples were acidified to pH ∼ 2 with HCl and analysed within one month collection. The acidified samples (pH ∼ 2) were sparged for 8 minutes at 75 or 100 ml min−1 with ultra-pure oxygen to remove all inorganic carbon from samples prior to measurement.
PARAFAC modelling
Fluorescence excitation emission matrix (EEM) spectra were reassessed using Parallel Factor Analysis (PARAFAC).33,34 Fluorescence EEMs were combined into a 3-dimensional data array and decomposed to a set of trilinear terms and a residual array: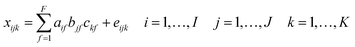 | (2) |
A PARAFAC model with non-negativity constraint on all modes (samples, emission and excitation) was implemented in MATLAB. The data were split into two random halves each comprising 254 EEMs, representing a calibration data array and a validation array. The appropriate number of components (the model rank) was determined by comparing the excitation and emission spectra of components between the calibration and validation data arrays and from split-half analysis, a total of five components were validated. Two categories of independent datasets were successfully validated: first, an inter-seasonal comparison between the wet and dry season, and second a land use comparison: oil palm plantations (OP), secondary forests (SF) and coastal swamps (CS). This compares with an earlier PARAFAC model for the Kinabatangan catchment which had validated three components.31 In the earlier model, however, all sampling stations were situated in the immediate vicinity of oil palm plantations while in the current study, sampling sites distinguished between three land use types (oil palm plantations, secondary forests and coastal swamps). Thus the five components presented in this study could potentially reflect differences in DOM composition according to land use.
The PARAFAC model returns the relative intensities of derived components, and the intensity of the nth component in a given sample remains unknown. Hence In was estimated by determining the fluorescence intensity at the peak excitation and emission maximum of the nth component:49
| In = scoren × Exn(λmax) × Emn(λmax) | (3) |
Statistical analysis
Precipitation data were analysed using a paired-sample t test to determine whether there were significant differences between inter-monsoonal period (IM), wet (WS) and dry seasons (DS). The p-values (p < 0.05) for pairs of IM-WS, IM-DS and WS-DS were 0.950, 0.142 and 0.018, respectively. This analysis was also sought to verify whether the rainfall data used in the study were free from precipitation anomalies, potentially caused by irregular synoptic forcing associated with the El Niño Southern Oscillation (ENSO) and changes in the seasonality of the monsoon in SE Asia.44 Discriminant analysis was applied to characterise DOM according to the land use type and seasonal variations. Calculations of the fluorescence intensities (In) of the individual components indicated that: IC4 > IC2 > IC3 > IC1 > IC5, suggesting that the terrestrially derived peak A had the most abundant spectral characteristics, followed by peak M (IC2 and IC3), peak C (IC1) and peak M (IC5). UV absorbance at 340 nm and fluorescence DOM (FDOM) were normalized to IC4 and fluorescence indices (FI) (ratios as detailed below) were used to determine the pre-dominance of each parameter in each land use type to gain more insight into DOM characterisation: IC4/a340 (peak A/a340), IC2/IC4 (peak M/peak A), IC3/IC4 (peak M/peak A) and IC5/IC4 (peak M/peak A). Both paired-sample t test and discriminant analysis were undertaken using SPSS version 21.0.Results and discussion
The data presented here provide the first evidence of seasonal changes in DOM composition in a catchment affected by the recent development of oil palm plantations. In the following section we compare our results with recent studies of other tropical catchments and consider the wider significance of this work.Characterisation of PARAFAC components
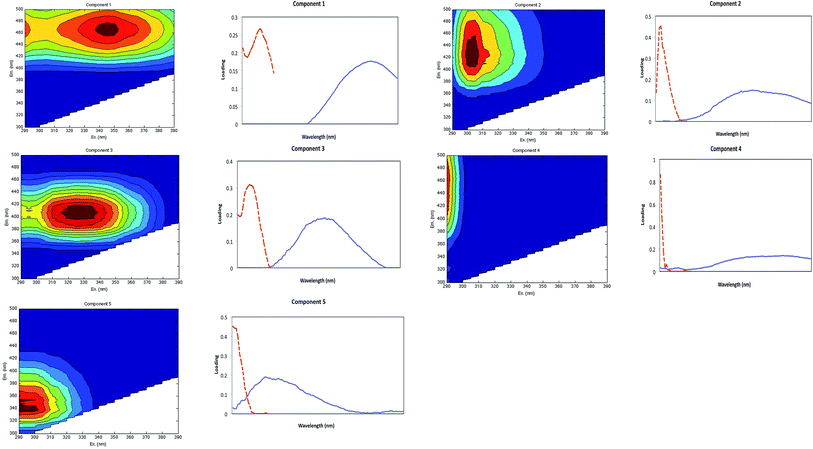
Five fluorescent components were identified by PARAFAC from analysis of the 510 sample dataset (Fig. 3). The excitation and emission pairs of the main peak positions for each component are summarised in Table 3, and the individual components are plotted in Fig. 3. Table 3 also compares the results with components identified in selected studies that have modelled DOM in marine, oceanic and estuarine environments.| Component in this study | Excitation maximum (nm) | Emission maximum (nm) | Coble et al.54 | Description and probable source |
|---|---|---|---|---|
| C1 | 345 | 466 | Peak C 320–360/420–480 | Ubiquitous humic-like substances, widespread, hydrophobic acid fraction (HPOA), Component 1: 350/400–450,50 Component 1: 345/462,52 Component 4: 350/420–480 (ref. 49) |
| C2 | 305 | 426 | Peak M 290–312/370–420 | Terrestrial humic-like substances, widespread, hydrophobic acid fraction (HPOA), suggested as photo-refractory, Component 2: 255/380–460,50 Component 3: 255 (330)/412,60 Component 3: 270 (360)/478,56 Component 3: 250 (355)/461 (ref. 53) |
| C3 | 325 | 408 | ||
| C4 | 290 | 464 | Peak A 260/380–460 | Terrestrial humic-like substances, widespread, hydrophobic acid fraction (HPOA), suggested as photo-refractory, Component 1: 270 (365)/453,60 Component 2: 255/380–460,50 Component 3: 270 (360)/478,56 Component 3: 250 (355)/461 (ref. 53) |
| C5 | 290 | 338 | Peak M 290–312/370–420 | Ditto with description for C2 |
Our PARAFAC model identified five terrestrially-derived substances: component 1 (C1) to component 5 (C5). Our terrestrially-derived components (C1 and C4) have been observed in other tropical and sub-tropical studies: these are ubiquitous, fulvic-acid representing fluorophores that have the longest excitation (and emission) wavelength and broadest excitation (and emission) band. Our components were found to relate specifically to the Component 1 described by Luciani et al.,50 Stedmon and Markager51 and Yamashita et al.,52 to the Component 2 of Fellman et al.,21 Component 3 of Yao et al.53 and to Component 4 of Kowalczuk et al.49 Our earlier DOM characterisation study in the Lower Kinabatangan also reported terrestrial-derived Component 1 (peak A and C).31 Our C1 (identified here) is similar to the humic-like fluorophore in the visible region defined by Coble.54
Our components C2, C3 and C5 have been previously reported as peak M; they have shorter emission wavelengths and were initially attributed to a marine source of DOM.54,55 Subsequently Stedmon et al.56 suggested that this component is found in ‘terrestrially dominated end-member samples’, and Fellman et al.57 described this peak as ultraviolet A (UVA), a low molecular weight component related to microbial activities. While peak M is common in marine environments and is apparently related to biological activity, it is also found in wastewater, in wetlands and agricultural environments. Peak M production could be partly due to the photobleaching of terrestrial FDOM or autochthonous production from microbial processes.58 Our C2 resembles Component 3 found by Murphy et al.,59 and Components 4 and 6 of Stedmon et al.56 and Yamashita et al.52 This component was also reported by Zhang et al.:60 their Component 1; Luciani et al.:50 their Component 2; Stedmon et al.:56 their Component 3; Yao et al.:53 their Component 3; and Stedmon et al.:56 their Component 5.
Comparison of the fluorescence intensities, In, indicated that terrestrially-derived peak IC4 (peak A) had the most abundant spectral characteristics. The peak component has been described as ubiquitous, photo-labile, terrestrially-derived OM which originates from agricultural activities52 but it could also represent a photodegradation processing pathway.61 Natural forest cover in the Lower Kinabatangan river catchment has declined from ∼91% in the 1970s to ∼47% in 1995;62 and at present ∼25% of the catchment is largely cultivated with oil palm plantations,39 which could explain the spectral characteristics and abundance of component IC4.
The PARAFAC components summarised in this paper are similar to those outlined in other studies of tropical catchments31,50,52 indicating that common attributes can be identified. However, the DOM characteristics described in most previous studies are of DOM that has a very different origin (including subtropical wetlands50 and enclosed coastal water bodies52) to that found in our study in NE Sabah.31 Consequently the results and the implications for both the Kinabatangan catchment, and tropical regions generally, should be interpreted with caution, as there might be site-specific contributions of natural organic matter from other land use and vegetation types might be only applicable in a local context.23 It might also be possible for the fluorescence characteristics to appear ‘identical’ in different catchments, albeit associated with a different DOM composition.63
Seasonal and land use variations
Discriminant analyses of the DOM dataset and land use type yielded two discriminant functions as summarised in Table 4 and Fig. 4. The ratios of (i) IC3/IC4 and (ii) IC2/IC4 were found to always correlate positively with IC5/IC4. They were classified in discriminant function 1 (DF1) and explained 79.2% of the variance. These results suggest that DF1 corresponds to fluorescence properties arising through photodegradation thus representing a DOM processing signature. Moreover, samples from coastal swamps (CS) were found to comprise DOM which was less processed (i.e. the DOM was fresher or younger), while DOM in waters sampled from the oil palm plantations (OP) showed evidence of greater processing, particularly in those samples collected from canals with stagnant water.| Fluorescence indices | Discriminant function (DF) | |
|---|---|---|
| 1 | 2 | |
| Land use | ||
| IC3/IC4 (peak M/peak A) | 0.913* | −0.015 |
| IC2/IC4 (peak M/peak A) | 0.618* | 0.095 |
| IC5/IC4 (peak M/peak A) | 0.405* | −0.054 |
| IC1/IC4 (peak C/peak A) | −0.285* | −0.043 |
| IC4/a340 (peak A/a340) | −0.374 | 0.746* |
| Spectral slope | 0.144 | −0.626* |
| 45.9% of original group cases correctly classified | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Seasonal | 1 | 2 |
| IC5/IC4 (peak M/peak A) | 0.678* | 0.003 |
| IC4/a340 (peak A/a340) | −0.672* | 0.133 |
| IC2/IC4 (peak M/peak A) | 0.538* | 0.503 |
| IC3/IC4 (peak M/peak A) | 0.406 | 0.586* |
| IC1/IC4 (peak C/peak A) | −0.108 | −0.384* |
| Spectral slope | 0.205 | 0.253* |
| 63.9% of original group cases correctly classified | ||
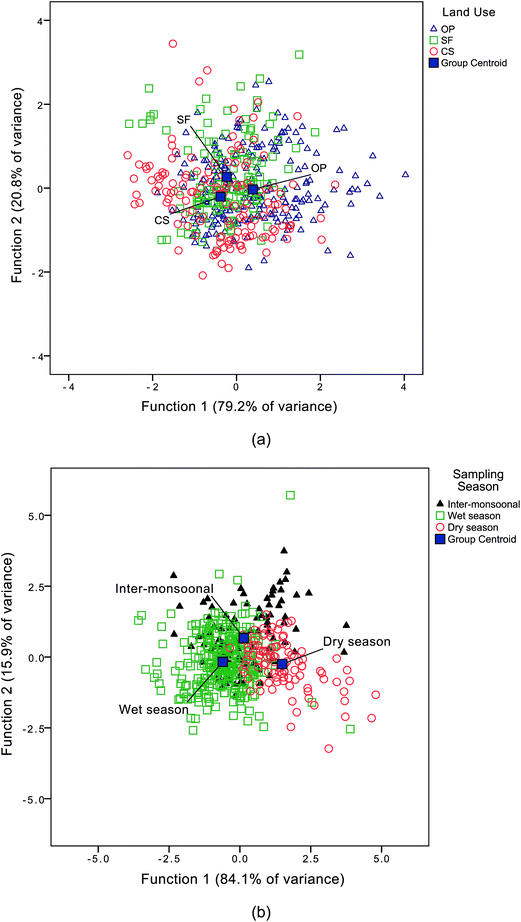 | ||
| Fig. 4 Group separation from the discriminant analysis according to: (a) types of land use; (b) seasonal variations. | ||
Seasonal trends in DOM characteristics were also evident in the discriminant analysis: DF1 suggests that the ratio of IC5/IC4 correlated positively with IC2/IC4 and explained 84.1% of the variance (Table 4). IC4/a340 was dominant in water sampled during the wet season and (Fig. 4), suggesting that DOM was fresher during the wet season (WS) compared with the dry season (DS) when DOM was more processed. While no seasonal variations in EEMs were observed by Baker & Spencer64 in their study in a temperate maritime catchment with anthropogenic DOM inputs in the Tyne, UK, other studies highlighted seasonal variability in EEMs. For example, Zhao et al.65 observed seasonal variations in EEMs from semi-arid lakes in NE China. Seasonal patterns of DOM distribution also have been found in subtropical Florida Bay, USA where relative abundance of humic-like (Ex/Em = <260, 345/462) and protein-like component (Ex/Em = 275/326) was higher during the early wet season (June to August).66
The ratios IC2/IC4 and IC5/IC4 were high in samples from the oil palm plantations (OP) during the dry season, suggesting that the DOM was more processed in the OP samples and could have been affected by microbial activities and/or photo-degradation during this period. Preliminary δ13C and molar C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N values of both DOM and particulate organic matter (POM) in an Australian tropical rainforest catchment suggested that exports of microbially processed organic matter were higher from upper soil horizons during the dry season.67 Subsequently, Lee-Cruz et al.68 investigated soil bacterial communities in logged forest and oil palm plantations in Sabah and found a high abundance of Actinomycetales, which are dominant in cultivated areas.69 Their study indicated that oil palm plantation soils have a higher bacterial diversity and turnover and are more heterogeneous. A study in Jambi, Indonesia revealed a high abundance of the genus Burkholderia, Cupriavidus and Acinetobacter in bacteria isolates from oil palm plantation aquatic sediments.70Burkholderia and Cupriavidus are nitrogen-fixing71,72 plant growth promoting bacteria72 while Acinetobacter, which has been reported ubiquitous in soil and surface waters,73 is a nonmotile, agent for biodegradation, leaching and removal of organic and inorganic waste.74 An earlier water quality study in the Sukau area of the Kinabatangan catchment (Fig. 1) during a weak La Niña event (2005 to 2006) indicated that the Biochemical Oxygen Demand (BOD) of a stream in an oil palm plantation ranged from 1.3 to 2.1 mg l−1.44 Dry season water samples from downstream reaching Sg. Langat in Selangor, Malaysia, which was also located within oil palm plantations, had mean BOD values ranging from 2.1 to 2.6 mg l−1.75 Therefore, we hypothesize that peak M we found in the Lower Kinabatangan River catchment, which varied seasonally and according to land use, could be derived from microbial and/or photo-degradation processes.
N values of both DOM and particulate organic matter (POM) in an Australian tropical rainforest catchment suggested that exports of microbially processed organic matter were higher from upper soil horizons during the dry season.67 Subsequently, Lee-Cruz et al.68 investigated soil bacterial communities in logged forest and oil palm plantations in Sabah and found a high abundance of Actinomycetales, which are dominant in cultivated areas.69 Their study indicated that oil palm plantation soils have a higher bacterial diversity and turnover and are more heterogeneous. A study in Jambi, Indonesia revealed a high abundance of the genus Burkholderia, Cupriavidus and Acinetobacter in bacteria isolates from oil palm plantation aquatic sediments.70Burkholderia and Cupriavidus are nitrogen-fixing71,72 plant growth promoting bacteria72 while Acinetobacter, which has been reported ubiquitous in soil and surface waters,73 is a nonmotile, agent for biodegradation, leaching and removal of organic and inorganic waste.74 An earlier water quality study in the Sukau area of the Kinabatangan catchment (Fig. 1) during a weak La Niña event (2005 to 2006) indicated that the Biochemical Oxygen Demand (BOD) of a stream in an oil palm plantation ranged from 1.3 to 2.1 mg l−1.44 Dry season water samples from downstream reaching Sg. Langat in Selangor, Malaysia, which was also located within oil palm plantations, had mean BOD values ranging from 2.1 to 2.6 mg l−1.75 Therefore, we hypothesize that peak M we found in the Lower Kinabatangan River catchment, which varied seasonally and according to land use, could be derived from microbial and/or photo-degradation processes.
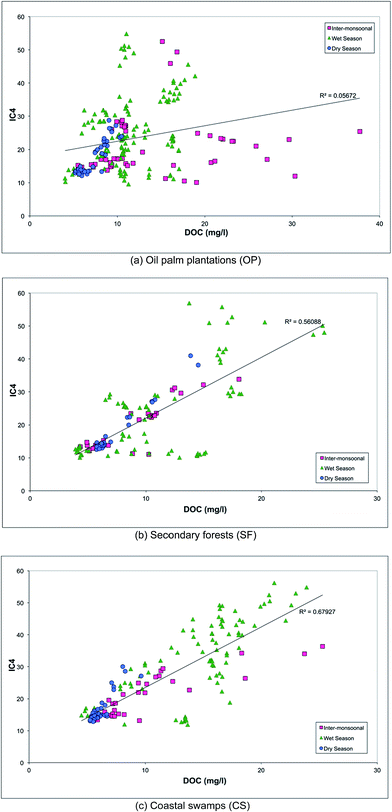
The variation in DOM according to the season and land cover is illustrated in Fig. 5 by plotting DOC against PARAFAC component IC4 for each land use type. Tabulated DOC concentrations varied from 9.88 to 12.85 mg l−1 (Table 1). Samples from secondary forests (SF) and coastal swamps (CS) showed a strong positive correlation between DOC and PARAFAC component C4 (r2 of 0.6 and 0.7 respectively). It also showed that DOM composition in both SF and CS was moderately constrained by monsoon and flow, compared to samples from the oil palm plantations (OP), which were highly constrained in particular during the inter-monsoonal period and wet season. This is consistent with the discriminant analysis (Fig. 4), which indicated that the ratio of IC4 to a340 was dominant in samples collected in SF during the wet season, while the spectral slope (275–295 nm) was found to be dominant in CS during the inter-monsoonal period (October 2009). There were positive correlations between UV absorbance a340 nm and PARAFAC component C4 (peak A) with a regression value of 0.5 for all types of land cover (Fig. 6). UV absorbance at ∼340 nm and spectral slope have been showed to be indicative of DOM molecular weight,48,76 and to correlate positively with the lignin concentration.29 The lignin concentration in aquatic ecosystems was strongly influenced by seasonal hydrology, river catchment discharge, flooding events and types of vegetation and land use.77 A quantitative aquatic carbon budget for the Langat River in Malaysia indicated that although C3 plant-derived matter was the primary source of carbon in wetland areas, sewage treatment and landfill sites in the lower catchment provided significant additional inputs of organic carbon.78 Nedwell et al.79 demonstrated that carbon mineralisation in a subtropical mangrove swamp in Jamaica was higher compared to other areas, indicating abundant OM availability. Mangrove forests also typically have rich tannins, which is likely to be the main source of protein-like fluorescence.80 They are also associated with decreasing bacterial counts81 and hydrophobic acids,82 which could explain observations of low molecular weight DOM in CS samples in the Lower Kinabatangan River catchment during the inter-monsoonal period.
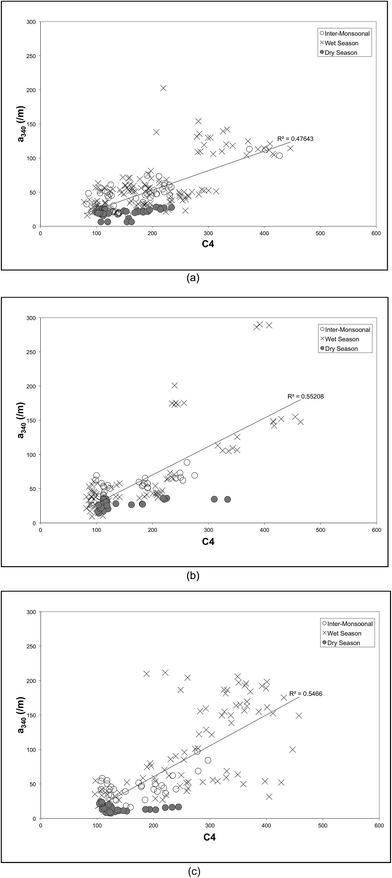 | ||
| Fig. 6 Correlation between PARAFAC component C4 and UV absorbance at 340 nm according to the sampling period: (a) oil palm plantations (OP); (b) secondary forests (SF); (c) coastal swamps (CS). | ||
Our results indicated that the ratio IC4 (peak A) to a340 was high and the spectral slope (S275–295) was low in waters sampled from secondary forests during the wet season. This could be associated with DOM inputs that were fresher and of higher molecular weight. There was also evidence of DOM degradation (bio- and photo-degradation) in river reaches downstream, including the estuary. The consistent high DOC concentrations that we observed in our study are indicative of high concentrations of humic materials in the waters sampled. Previous work has demonstrated that secondary forests have the potential to absorb and store a large proportion of carbon and nutrients lost as a result of changes in land use and particularly deforestation.83–85 Secondary forests can be effective nutrient sinks enabling the rapid accumulation of nutrients over time. With respect to organic matter production, secondary forests can return significant OM in litter fall although they store fewer nutrients in their litter.86,87 This results in high nutrient cycling rates but may also potentially contribute to nutrient loss.86
Conclusion
We conclude that the characteristics of DOM in the Lower Kinabatangan River, Malaysia are dominated by the fluorescence peaks A (IC4) and M (IC2, IC3 and IC5). These peaks indicate the importance of microbial and photo-degradation processes, particularly during the dry season, which break down the aromatic carbon molecules that account for DOM fluorescence. Discriminant analysis of the PARAFAC dataset indicated that OP samples could be distinguished by plotting peak M (IC2, IC3 and IC5) against a340, confirming the importance of microbial activity and photo-degradation processes in streams associated with oil palm plantations. The ratio IC4/a340 and spectral slope successfully distinguished secondary forests, followed by oil palm plantations and coastal swamps, suggesting that DOM with a higher MW is found in SF. This also suggests variations in the quality of DOC production in different land use types, modified by the monsoonal cycle. This is supported by the PARAFAC model presented here which yielded three peak M components. Hence the paper suggests that analysis of EEMs, supported by PARAFAC, is a useful tool to determine and characterise the humic and fulvic substances in aquatic ecosystems, which correlate strongly with DOC.Acknowledgements
We thank the Malaysia Ministry of Higher Education (MoHE), Universiti Malaysia Sabah, Department of Irrigation and Drainage (DID) Sabah, Malaysian Meteorological Department for providing fund, hydrological and meteorological data for this study. Thanks are also extended to Sabah Forestry Department and Sabah Wildlife Department for permitting this research to be undertaken in Kinabatangan, Sabah, Malaysia. We are grateful to: Ms Anne Ankcorn for drawing Fig. 1; Mr Zainal Abidin Jaafar, Mr Budin Ransangan and Ms Asnih Etin, for their great help with the field sampling. Finally, we are thankful to the editor and two anonymous reviewers for their valuable comments and suggestions.References
- G. Abril, J. M. Martinez, L. F. Artigas, P. Moreira-Turcq, M. F. Benedetti, L. Vidal, T. Meziane, J. H. Kim, M. C. Bernardes, N. Savoye, J. Deborde, E. L. Souza, P. Alberic, M. F. L. de Souza and F. Roland, Nature, 2015, 505(7483), 395–398 CrossRef PubMed.
- T. H. Huang, Y. H. Fu, P. Y. Pan and C. C. T. Arthur, Current Opinion in Environmental Sustainability, 2012, 4(2), 162–169 CrossRef.
- J. J. Cole, Y. T. Prairie, N. F. Caraco, W. H. McDowell, L. J. Tranvik, R. G. Striegel, C. M. Duarte, P. Kortelainen, J. A. Downing, J. J. Middelburg and J. Melack, Ecosystems, 2007, 10, 171–184 CrossRef CAS.
- J. E. Richey, J. M. Melack, A. K. Aufdenkampe, V. M. Ballester and L. L. Hess, Nature, 2002, 416, 617–620 CrossRef CAS PubMed.
- E. Mayorga, A. K. Aufdenkampe, C. A. Masiello, A. V. Krusche, J. I. Hedges, P. D. Quay, J. E. Richey and T. A. Brown, Nature, 2005, 436, 538–541 CrossRef CAS PubMed.
- W. J. Mitsch, B. Bernal, A. M. Nahlik, Ü. Mander, L. Zhang, C. J. Anderson, S. E. Jorgensen and H. Brix, Landscape Ecology, 2013, 28(4), 583–597 CrossRef.
- I. Aselmann and P. J. Crutzen, J. Atmos. Chem., 1989, 8, 307–358 CrossRef CAS.
- D. C. Donato, J. B. Kauffman, D. Murdiyarso, S. Kurnianto, M. Stidham and M. Kanninen, Nat. Geosci., 2011, 4, 293–297 CrossRef CAS.
- G. Mayora, M. Devercelli and F. Giri, Hydrobiologia, 2013, 717(1), 51–63 CrossRef CAS.
- N. Saigusa, S. Yamamoto, R. Hirata, Y. Ohtani, R. Ide, J. Asanuma, M. Gamo, T. Hirano, H. Kondo and Y. Kosugi, Agr. Forest Meteorol., 2008, 148, 700–713 CrossRef.
- Y. Ogata, T. Ishigaki, Y. Ebie, N. Sutthasil, C. Chiemchaisri and M. Yamada, Waste Manage., 2015, 44(C), 164–171 CrossRef PubMed.
- M. Alkhatib, T. C. Jennerjahn and J. Samiaji, Limnol. Oceanogr., 2007, 52, 2410–2417 CrossRef CAS.
- S. Atapattu and D. Kodituwakku, Agr. Forest Meteorol., 2009, 96(3), 361–373 Search PubMed.
- B. Mattsson, C. Cederberg and L. Blix, J. Cleaner Prod., 2000, 8, 283–292 CrossRef.
- R. Sidle, M. Tani and A. Ziegler, For. Ecol. Manage., 2006, 224(1–2), 1–4 CrossRef.
- C. M. Ji, P. P. Eong, T. B. Ti, C. E. Seng and C. K. Ling, Renewable Sustainable Energy Rev., 2013, 26(C), 717–726 Search PubMed.
- E. B. Fitzherbert, M. J. Stuebig, A. Morel, F. Danielsen, C. A. Bruhl, P. F. Donald and B. Phalan, Trends in Ecology and Evolution, 2008, 23(10), 538–545 CrossRef PubMed.
- L. P. Koh and D. S. Wilcove, Trends in Ecology and Evolution, 2008, 24(2), 67–68 CrossRef PubMed.
- D. Graeber, G. Goyenola, M. Meerhoff, E. Zwirnmann, N. B. Ovesen, M. Glendell, J. Gelbrecht, F. Teixeira de Mello, I. González-Bergonzoni, E. Jeppesen and B. Kronvang, Hydrol. Earth Syst. Sci., 2015, 19, 2377–2394 CrossRef.
- Y. H. Lu, J. E. Bauer, E. A. Canuel, R. M. Chambers, Y. Yamahita, R. Jaffé and A. Barrett, Biogeochemistry, 2014, 119, 275–292 CrossRef CAS.
- J. B. Fellman, M. P. Miller, R. M. Cory, D. V. D'Amore and D. White, Environ. Sci. Technol., 2009, 43(16), 6228–6234 CrossRef CAS PubMed.
- M. Fuentes, G. Gonzalezgaitano and J. Garchiamina, Org. Geochem., 2006, 37(12), 1949–1959 CrossRef CAS.
- U. K. Ahmad, Z. Ujang, Z. Yusop and T. L. Fong, Water Sci. Technol., 2002, 46, 117–125 CAS.
- C. J. Williams, Y. Yamashita, H. F. Wilson, R. Jaffé and M. A. Xenopoulos, Limnol. Oceanogr., 2010, 55(3), 1159–1171 CrossRef CAS.
- M. Miller, D. McKnight, S. Charpra and M. Williams, Limnol. Oceanogr., 2009, 54, 2213–2227 CrossRef.
- C. Stedmon and S. Markager, Limnol. Oceanogr., 2005, 50(2), 686–697 CrossRef CAS.
- Y. Yamashita, R. Jaffé, R. Maie and E. Tanque, Limnol. Oceanogr., 2008, 53, 1900–1908 CrossRef CAS.
- R. M. Cory and D. M. McKnight, Environ. Sci. Technol., 2005, 39(21), 8142–8149 CrossRef CAS PubMed.
- R. Spencer, P. Hernes, R. Rug, A. Baker, R. Dyda, A. Stubbins and J. Six, J. Geophys. Res., 2010, 115, G03013 Search PubMed.
- H. Hong, L. Yang, W. Guo, F. Wang and X. Yu, Biogeochemistry, 2012, 109, 163–174 CrossRef CAS.
- S. Harun, A. Baker, C. Bradley, G. Pinay, I. Boomer and R. L. Hamilton, Hydrol. Res., 2015, 46(3), 411–428 CrossRef CAS.
- W. J. Junk, Environ. Conserv., 2002, 29(4), 414–435 CrossRef.
- R. Bro, Chemom. Intell. Lab. Syst., 1997, 38, 149–171 CrossRef CAS.
- C. A. Stedmon and S. Markager, Estuarine, Coastal Shelf Sci., 2003, 57(5–6), 973–979 CrossRef CAS.
- R. Josephine, R. J. Alfred and I. Rajah, Presented in Part at World Water Day, 2004, pp. 1–10 Search PubMed.
- F. Tongkul, J. Southeast Asian Earth Sci., 1991, 6, 395–405 CrossRef.
- B. D. Acres and C. J. Folland, The Soils of Sabah. Vol. 2, Land Resources Division, Ministry of Overseas Development, Sandakan and Kinabatangan Districts, England, 1975 Search PubMed.
- Town and Regional Planning Department Sabah, in Sabah Coastal Zone Profile, The Integrated Coastal Zone Management Unit, Kota Kinabalu, Sabah, 1998 Search PubMed.
- R. Boonratana, Int. J. Primatol., 2000, 21, 497–517 CrossRef.
- N. M. Gazzaz, M. K. Yusoff, M. F. Ramli, A. Z. Aris and H. Juahir, Mar. Pollut. Bull., 2012, 64(4), 688–698 CrossRef CAS PubMed.
- R. Dambul and P. Jones, Geografia, 2008, 5(1), 1–25 Search PubMed.
- J. Suhaila, S. M. Deni, W. W. Wan Zin and A. A. Jemain, Sains Malays., 2010, 39, 533–542 Search PubMed.
- Department of Environment Malaysia, Study on pollution and water quality improvement for Sg. Kinabatangan Basin. Final Report Volume II: Main Report (Part I). Unpublished Report, Ministry of Natural Resources and Environment Malaysia, 2009.
- S. Harun, S. Al-Shami, R. Dambul, M. Mohamed and M. H. Abdullah, Sains Malays., 2015, 44(4), 545–558 CrossRef.
- N. K. Abram, P. Xofis, J. Tzanopoulos, D. C. MacMillan, M. Ancrenaz, R. Chung, L. Peter, R. Ong, I. Lackman, B. Goossens, L. Ambu and A. T. Knight, PLoS One, 2014, 9(6), e95388 Search PubMed.
- L. Yang and J. Hur, Water Res., 2014, 59, 80–89 CrossRef CAS PubMed.
- T. Ohno, Environ. Sci. Technol., 2002, 36(4), 742–746 CrossRef CAS PubMed.
- J. R. Helms, A. Stubbins, J. Ritchie, E. Minor, D. Kieber and K. Mopper, Limnol. Oceanogr., 2008, 3, 955–969 CrossRef.
- P. Kowalczuk, M. J. Durako, H. Young, A. E. Kahn, W. J. Cooper and M. Gonsior, Mar. Chem., 2009, 113(3–4), 182–196 CrossRef CAS.
- X. Luciani, S. Mounier, H. Paraquetti, R. Redon, Y. Lucas, A. Bois, L. Lacerda, M. Raynaud and M. Ripert, Mar. Environ. Res., 2008, 65(2), 148–157 CrossRef CAS PubMed.
- C. Stedmon and S. Markager, Limnol. Oceanogr., 2005, 50, 1415–1426 CrossRef CAS.
- Y. Yamashita, L. J. Scinto, N. Maie and R. Jaffé, Ecosystems, 2010, 13, 1006–1019 CrossRef CAS.
- X. Yao, Y. Zhang, G. Zhu, B. Qin, L. Feng, L. Cai and G. Gao, Chemosphere, 2011, 82(2), 145–155 CrossRef CAS PubMed.
- P. Coble, Mar. Chem., 1996, 51(4), 325–346 CrossRef CAS.
- E. Parlanti, K. Wörz, L. Geoffroy and M. Lamotte, Org. Geochem., 2000, 31, 1765–1781 CrossRef CAS.
- C. A. Stedmon, S. Markager and R. Bro, Mar. Chem., 2003, 82, 239–254 CrossRef CAS.
- J. B. Fellman, E. Hood and R. G. M. Spencer, Limnol. Oceanogr., 2010, 55(6), 2452–2462 CrossRef CAS.
- J. R. Helms, J. Mao, A. Stubbins, K. Schmidt-Rohr, R. G. M. Spencer, P. J. Hernes and K. Mopper, Aquat. Sci., 2014, 76(3), 353–373 CrossRef CAS.
- K. R. Murphy, C. A. Stedmon, T. D. Waite and G. M. Ruiz, Mar. Chem., 2008, 108(1–2), 40–58 CrossRef CAS.
- Y. Zhang, M. A. van Dijk, M. Liu, G. Zhu and B. Qin, Water Res., 2009, 43(18), 4685–4697 CrossRef CAS PubMed.
- C. M. Sharpless and N. V. Blough, Environ. Sci.: Processes Impacts, 2014, 16, 656–671 Search PubMed.
- J. Payne, Sabah Biodiversity Conservation Project, Malaysia: Kinabatangan Multi Disciplinary Study, Ministry of,ourism and Environmental Development, Sabah & Danish Co-operation for Environment and Development (DANCED), 1996 Search PubMed.
- R. Jaffe, K. M. Cawley and Y. Yamashita, Applications of Excitation Emission Matrix Fluorescence with Parallel Factor Analysis (EEM-PARAFAC) in Assessing Environmental Dynamics of Natural Dissolved Organic Matter (DOM) in Aquatic Environments: A Review, in Advances in the Physicochemical Characterization of Dissolved Organic Matter: Impact on Natural and Engineered Systems, ed. F. Rosario-Ortiz, Washington, DC, American Chemical Society, 2014, vol. 1160, pp. 27–73 Search PubMed.
- A. Baker and R. G. M. Spencer, Sci. Total Environ., 2004, 333, 217–232 CrossRef CAS PubMed.
- Y. Zhao, K. Song, Z. Wen, L. Li, S. Zang, T. Shao, S. Li and J. Du, Biogeosciences Discuss, 2015, 12, 5725–5756 CrossRef.
- N. Maie, Y. Yamashita, R. M. Cory, J. N. Boyer and R. Jaffe, Appl. Geochem., 2012, 27, 917–929 CrossRef CAS.
- A. M. Bass, M. I. Bird, M. J. Liddell and P. N. Nelson, Limnol. Oceanogr., 2011, 11, 399–405 Search PubMed.
- L. Lee-Cruz, D. P. Edwards, B. M. Tripathi and J. M. Adams, Appl. Environ. Microbiol., 2013, 79, 7290–7297 CrossRef CAS PubMed.
- P. Hill, V. Kristufek, L. Dijkhuizen, C. Boddy, D. Kroetsch and J. D. van Elsas, Microb. Ecol., 2011, 61, 286–302 CrossRef PubMed.
- M. Wijayanti, A. Meryandini, A. T. Wahyudi and M. Yuhana, Makara Journal of Science, 2014, 18, 71–78 Search PubMed.
- A. M. Hirsch and N. A. Fujishige, Molecular Signals and Receptors: Communication between Nitrogen-fixing Bacteria and their Plant Hosts, in Biocommunication of Plants: Signaling and Communication in Plants, ed. Witzany G. and Baluska F., Springer-Verlag Berlin, Heidelberg, 2012, vol. 14, pp. 255–280 Search PubMed.
- P. Paganin, S. Tabacchioni and L. Chiarini, Cent. Eur. J. Biol., 2011, 6, 997–1005 CAS.
- A. Howard, M. O'Donoghue, A. Feeney and R. D. Sleator, Virulence, 2012, 3, 243–250 CrossRef PubMed.
- D. Abdel-El-Haleem, Afr. J. Biotechnol., 2003, 2(4), 71–74 CrossRef.
- M. Z. Azrina, C. K. Yap, A. Rahim Ismail, A. Ismil and S. G. Tan, Ecotoxicol. Environ. Saf., 2006, 64(3), 337–347 CrossRef CAS PubMed.
- A. Baker, E. Tipping, S. Thacker and D. Gondar, Chemosphere, 2008, 73(11), 1765–1772 CrossRef CAS PubMed.
- C. N. Jex, G. H. Pate, A. J. Blyth, R. G. M. Spencer, P. J. Hernes, S. J. Khan and A. Baker, Quat. Sci. Rev., 2014, 87, 46–59 CrossRef.
- K. Y. Lee, M. I. Syakir, I. D. Clark and J. Veizer, Aquat. Geochem., 2013, 19(5–6), 443–475 CrossRef CAS.
- D. B. Nedwell, T. H. Blackburn and W. J. Wiebe, Mar. Ecol.: Prog. Ser., 1994, 110, 223–231 CrossRef CAS.
- N. Maie, N. M. Scully, O. Pisani and R. Jaffe, Water Res., 2007, 41, 563–570 CrossRef CAS PubMed.
- K. Sahoo and N. K. Dhal, Indian J. Mar. Sci., 2009, 38, 249–256 CAS.
- J. A. Aitkenhead-Peterson, W. H. McDowell and J. C. Neff, Sources, production, and regulation of allochthonous DOM inputs to surface waters, in Aquatic Ecosystems: Interactivity of Dissolved Organic Matter, ed. S. E. G. Findlayand R. L. Sinsabaugh, Elsevier, New York, 2003, pp. 25–70 Search PubMed.
- R. F. Hughes, J. B. Kauffman and V. J. Jaramillo, Ecology, 1999, 80, 1892–1907 Search PubMed.
- J. L. Schedlbauer and K. L. Kavanagh, For. Ecol. Manage., 2008, 255, 1326–1335 CrossRef.
- M. van Breugel, J. Ransijn, D. Craven, F. Bongers and J. S. Hall, For. Ecol. Manage., 2011, 262(8), 1648–1657 CrossRef.
- S. Brown and A. E. Lugo, J. Trop. Ecol., 1990, 6, 1–32 CrossRef.
- C. E. M. Silva, J. F. C. Goncalves and E. G. Alves, Photosynthetica, 2011, 49(2), 246–252 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |