Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Towards industrial products from microalgae†
Jesús
Ruiz
ab,
Giuseppe
Olivieri
b,
Jeroen
de Vree
b,
Rouke
Bosma
b,
Philippe
Willems
c,
J. Hans
Reith
b,
Michel H. M.
Eppink
b,
Dorinde M. M.
Kleinegris
a,
René H.
Wijffels
*bd and
Maria J.
Barbosa
b
aFood and Biobased Research, AlgaePARC, Wageningen UR, PO Box 17, 6700 AA Wageningen, The Netherlands
bWageningen University, Bioprocess Engineering, AlgaePARC, P.O. Box 16, 6700 AA Wageningen, The Netherlands. E-mail: rene.wijffels@wur.nl
cValue for Technology bvba, Acaciastraat 14, B-3071 Erps-Kwerps, Belgium
dNord University, Faculty of Biosciences and Aquaculture, N-8049 Bodø, Norway
First published on 19th August 2016
Abstract
Microalgae show an enormous potential as sustainable feedstock for numerous bioproducts. The current work analyzes the feasibility of business cases for different markets of products from microalgae. We perform a techno-economic evaluation of the whole process chain including cultivation, biorefinery and market exploitation for a 100 hectares facility in six locations. Our projections show a current cost per unit of dry biomass of 3.4 € kg−1 for microalgae cultivation in Spain (excluding biorefining products), with an expected reduction to 0.5 € kg−1 in ten years. A sensitivity analysis reveals the roadmap to achieve this. Production of high-value products (e.g. pigments) would be currently profitable, with a net present value of 657 M€ in 15 years. Markets aimed at food and chemical commodities require further cost reductions for cost competitiveness, reachable in the next decade.
Broader contextOur society needs new sustainable biobased feedstocks to meet population growth and reduce dependence on fossil fuels. Microalgae are considered one of the most promising feedstocks for sustainable production of food, feed, chemicals, materials and fuels. Our mission is to develop a commercial and sustainable production chain for commodity products from microalgae. Estimations of biomass production costs for a 100 ha plant facility have been done. Projections of different scenarios allowed us to compare effect of variables, such as location of the facility, type of cultivation system and operational parameters. As result, this tool shows the most suitable location and system for algae production, as well as the main cost factors. The biomass produced follows to the biorefinery process to be fractionated into the main components: proteins, lipids, carbohydrates and pigments. Several chains have been specifically designed for different market scenarios. The overall turnover coming from the exploitation of the different biomass fractions depends on the end product. A market analysis has been conducted looking to different scenarios according to the biomass value pyramid: biofuels, chemicals, food, feed, specialties in food, cosmetics and a combination of these. Projections show that production of high-value products from microalgae could be profitable nowadays and commodities will become profitable within 10 years. |
Introduction
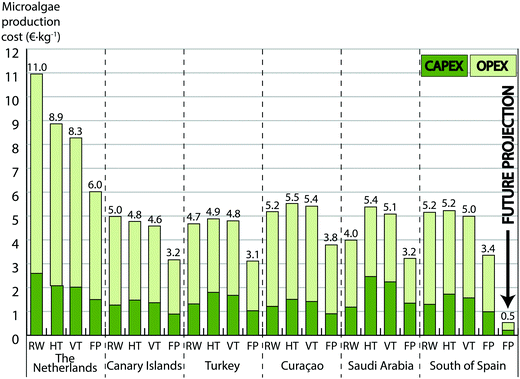
Microalgae are considered a promising sustainable feedstock for food and feed products, materials, chemicals, fuels and various high-value products.1,2 Algae do not require arable land or freshwater supply and can be harvested nearly all-year-round,3 which makes them attractive for commercial exploitation. Thus microalgae can be an alternative to current unsustainable overexploitation of natural resources, with options to become a solution to the environmental dilemma of food and energy. However, the reality is not so categorical and in practice there are some hurdles limiting their expansion and establishment. Commercialization of different functional components requires selective biorefining of biomass with a cascade approach that still remains a challenge.4 Besides, technology readiness on cultivation and development of commercialization have been debated recently.5 Currently, microalgae production aims for niche markets with almost absent competition, which results in inflated product prices. Once the industry expands, competition will force prices to adjust to markets (Table S11, ESI†) and strategies to adapt accordingly.Limited knowledge about costs on microalgal cultivation and processing at commercial scale is available, particularly concerning closed photobioreactors. Model-based simulations, combined with pilot-plant production data, can fill this gap. This study revisits economics, and thereby feasibility, by combining techno-economic models for microalgae production and biorefinery with a market analysis; a pioneering approach. The projections include six locations (The Netherlands, Canary Islands, Turkey, Curaçao, Saudi Arabia and south of Spain), but discussion is focused on south of Spain, an attractive location within Europe (Fig. 1).
Methods
See ESI.†Current status of autotrophic microalgae production: culture systems
Biomass production is the starting point for commercialization of industrial products from algae; we have estimated costs for obtaining harvested biomass in an industrial cultivation facility of 100 hectares for different culture systems. The study includes four state-of-the-art production systems: horizontal tubular photobioreactor, vertically stacked tubular photobioreactor, flat panels photobioreactor and open raceway pond. The cost projections (Fig. 1) are supported by experimental results obtained at AlgaePARC pilot facility,6,7 where non-GMO microalgae are used. AlgaePARC was designed to bridge the gap between fundamental research on algae and industrial production facilities, allowing us to extrapolate the empirical data from its operation to commercial-scale.Discussion on the best production system is an ongoing debate, due to the fact that none of the systems seems to completely surpass others. Raceway ponds are simple and imply about half of the initial investment than closed systems at the expense of also lower productivities (27 ton ha−1 year−1 for south of Spain, Fig. 1), about one order of magnitude more diluted cultures and consequently greater volumes to process. Besides, the culture is more prone to contamination and heavy rain can interfere with proper operation. Although closed systems need a greater investment, they offer higher productivities (between 34 and 61 ton ha−1 year−1 for current projections in south of Spain; Fig. 1) and more degrees of freedom in design, construction and operation (implying also more complexity); definitely closed systems have more potential for improvement. Our study shows that the flat panels reactor is the most convenient system in terms of costs (Fig. 1), as also shown recently.8 It is a finding regardless of location, with current biomass production costs that would reach 3.4 € kg−1 in Spain at 100 ha scale.
Large-scale cultivation in closed systems involves some constraints to consider. Overheating can be lethal to microalgae9,10 and temperature control in closed reactors is mandatory. This is solved in different ways; either immersing the systems in a body of water, spraying water on the surface of reactors or using heat exchangers. Build-up of oxygen must be controlled, since it could inhibit growth and cause the collapse of cultures. Oxygen control is a basic premise in the design of closed systems. This can be done by limiting the tube length in tubular photobioreactors and by designing efficient degassers, increasing therefore complexity. Cleaning is required in both open and closed systems, but closed photobioreactors require chemicals to remove the biofouling from the inner surface, since it can restrain growth.11
In open systems, water evaporation restrains excessive temperature raise. Our energy balances show that temperatures in the open ponds would not exceed 32 °C for any of the studied locations. Therefore, forced cooling is absent in raceway ponds in this study. On the contrary, a non-cooled closed system can imply culture temperatures above 60 °C, making temperature control indispensable to cultivate microalgae. This work is based on cooling using an external source of water, which implies a cost of 0.4, 0.5 and 0.8 € kg−1 for flat panels, vertical and horizontal tubular reactors respectively. Cooling towers could be used instead as source of cooling water if an external source, such as the sea, is not available. This option would result more expensive, increasing cooling cost to 2.0, 2.2 and 3.6 € kg−1 for the above-mentioned systems. Separation of the biomass from the culture medium is usually identified as one of the major bottlenecks in the process.12 This is valid for raceways, where our projections show that harvesting contributes to about 23% of cultivation cost (1.2 € kg−1), whereas it is only between 5 and 7% of total costs in closed systems (0.2 to 0.3 € kg−1) due to higher biomass concentrations.
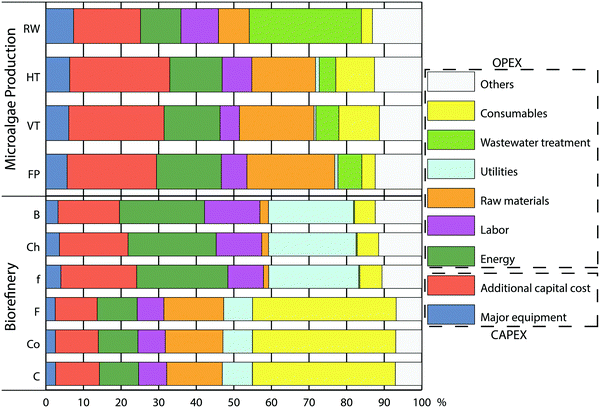
Fig. 2 shows a detailed breakdown of costs, where major equipment and additional capital cost define capital costs in Fig. 1 (CAPEX), while the rest of contributors account for operational costs (OPEX). The most influential factors on the cost of microalgae production vary with production system, but our estimations identify different trends in open and closed systems (Fig. 2). Cost of wastewater treatment plays an important role in the raceway pond, but has only limited influence in closed systems. It is a consequence of greater volumes processed in raceways due to a more diluted culture. On the other hand, costs of raw materials (17–23%; mainly due to cleaning) and energy consumption (14–17%) become relevant items in closed systems. A closer look to these systems would show that energy for mixing represents more than 80% of total energy consumption. In particular, tubes in tubular systems are replaceable and therefore considered OPEX, as described in ESI† accounting for more than 10% of total biomass production cost (consumables in Fig. 2).
Photosynthetic efficiency (PE; percentage of the solar irradiation converted to biomass) is greater in vertical systems, with values of 2.7% and 2.4% for flat panels and vertical tubular photobioreactors respectively (Table S5, ESI†). Due to the principle of light dilution, placing the reactors vertically increases the volume per ground area, thereby average light intensity impinging on reactor surface is decreased, leading to these enhanced productivities.5 Thus building upright is a convenient strategy that cost-wise seems to compensate the extra costs involved in frames, materials and energy, by increased productivity. Consequently, there is a reduction in the final cost of produced biomass (Fig. 1).
Selective separation of products: biorefinery
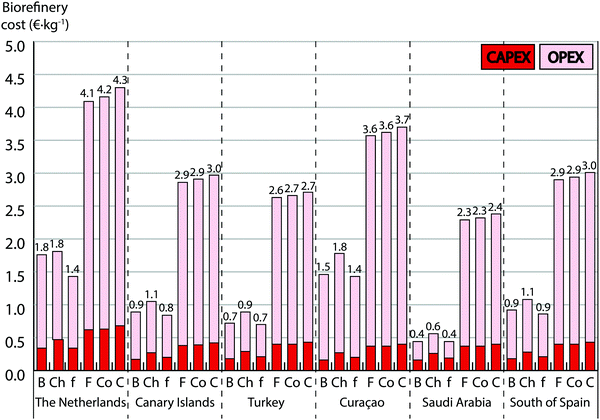
Biorefinery of microalgal biomass is the next step for the commercialization of products from algae. Nowadays the process is at an early stage, and most commercial facilities focus on one single product. Commercial products are currently simply based on harvesting and drying the biomass or on extracting and purifying special lipids as omega-3 fatty acids or pigments such as astaxanthin.13 The biorefinery process strategy depends on the product portfolio, and may result in different market scenarios for biofuels, chemicals, food/feed, food additives and cosmetics/healthcare. In addition, a complete biorefinery aiming at the optimum exploitation of the biomass components allocating them in different markets may lead to several market combinations (see ESI†). We have designed specific biorefinery processes for the different market scenarios (Fig. S2–S3-E, ESI†) and identified expected economic bottlenecks and opportunities.At 100 ha cultivation scale in south of Spain, the production of bulk commodities as fuels, chemicals or food results in a biorefinery cost of about 1 € kg−1 (Fig. 3). Two main economic bottlenecks are identified: energy involved in bead-milling for cell disruption, which is largely dissipated in the liquid as heat (∼1 kW h kg−1 corresponding to 0.15 € kg−1) as reported also in14 and the use of heat for biomass drying, lipid extraction and solvent recovery (0.18 € kg−1). Novel controlled cell disruption techniques as those patented by several companies, involving hydrodynamic cavitation (SoniqueFlo technology developed by Cellulac Inc.15), electrical fields (Pulsed Electric Field technology developed by Diversified Technologies Inc.16), electromagnetic fields (technology developed by Origin Oil Inc.17) and acoustic cavitation (technology developed by Los Alamos Laboratories, Solix Biofuels and Cavitation Technologies Inc.18) could offer an alternative. Up to 90% of reduction of energy involved in cell disruption can be theoretically achieved with these technologies.19 Another challenge is achieving a good yield for wet extraction of lipids. This could be achieved using solvents which require less energy when recovered as supercritical fluids and switchable solvents.
The purification of high-value products as omega-3 fatty acids, pigments and water-soluble proteins results in a cost close to 3 € kg−1 in Spain (Fig. 3). Food additives and cosmetics markets require complete techno-functionality of soluble proteins and to achieve this, the biorefinery must follow a cascade approach. This cascade consists of three sections: first extraction of hydrophilic components (proteins and carbohydrates), followed by extraction and fractionation of lipids and final recovery of insoluble components.4 We propose, after cell disruption, to use aqueous two phase extraction (ATPE) with polyethylene glycol and potassium phosphate20 followed by a cascade of ultrafiltration/diafiltration membrane purification. These two steps are commonly used in mild extraction and purification of proteins from other feedstocks in order to preserve their techno-functionality and have also recently been proposed for a complete biorefinery of microalgae.20,21 However, our cost analysis indicates that for the complete exploitation of the biomass a large part of the biorefinery cost (70–80%) is due to the first step of the cascade: the extraction and purification of soluble proteins. Concretely, consumables (membranes) and chemicals, both involved in protein biorefinery, represent a large part of the OPEX: 1.14 € kg−1 and 0.44 € kg−1, respectively (Fig. 2).
On the one hand the biorefinery of functional proteins, requiring a process characterized by more mild conditions, is in practice still in the R&D stage for microalgae; hence the high cost projected. However, a biorefinery process addressing bulk commodities markets (biofuel, chemical and food/feed) does not require the use of ATPE, dropping the biorefinery cost to 0.9–1.1 € kg−1 (Fig. 3).
The biorefinery of lipids is a well-established technology adopted at industrial scale for vegetal oil and oilseeds (extraction, degumming, winterization and bleaching).
To reduce the cost and establish a feasible technology, we address as key challenges the use of better extractants in ATPE. The extractants should be characterized by a higher protein partition coefficient, such as ionic liquids and surfactants.22 However, some issues about the toxicity and food-grade quality of these solvents still have to be solved.
When considering the evolution in membrane technology, important reductions in membrane costs seem feasible. In the last 20 years new technologies as low-fouling, reversible spiral and capillary ultrafiltration have been set up and developed, achieving an improvement in the life-time of membranes and cleaning efficiency.23
Valuing the product: market
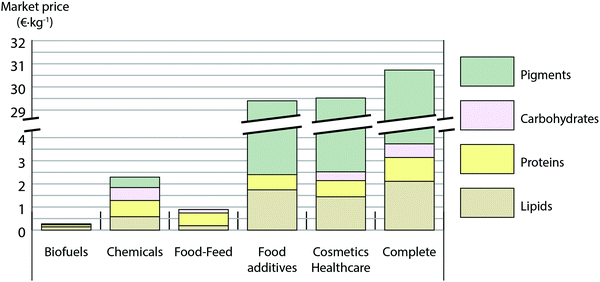
To investigate potential commercialization of microalgae we identify two main business cases for the exploitation of the microalgal biomass: (1) bulk commodities as biofuels, chemicals or food/feed and (2) special markets for food additives and cosmetics/health care (high-value products).The EU is in need of renewable sources for commodities. In terms of potential market demand in Europe, the food and fuel demand is mainly covered by foreign import: the EU imports each year 12 × 106 tons (44% of total supply) of lipids and 37 × 106 tons (68% of total supply) of proteins (source FAOSTAT, 2013). The market of bulk chemicals has a substantial demand in EU (2.4 × 106 tons year−1) including biopolymers, biolubricants, biosolvents and surfactants (source Eurostat, 2010). Regarding the value of biomass in the different markets, the message is clear (Fig. 4): the lowest revenue per unit of biomass comes from biofuel (0.3 € kg−1) and the application of microalgae for food is more attractive with a three times higher potential value. The market scenario aiming to bulk chemicals is more interesting, with a revenue per unit of biomass greater than 2 € kg−1.
Whereas there is a vast demand for commodities, markets selling high-value products are more restrained. In case of natural pigments, which are sold as strong antioxidants (beta-carotene, lutein and astaxanthin), the market volume worldwide is estimated at almost 1 bn $,24 corresponding to some 1000 ton year−1, still far from the 183 ton year−1 of pigments produced at 100 hectares scale. It is expected that at larger production facilities, these pigments should be sold in the synthetic pigment market, which is 2 orders of magnitude bigger in volume.
Results from Fig. 4 indicate why algae are now exclusively commercialized in special markets. Actually natural pigments are the most influential compounds in the overall revenue from the microalgal biomass (Fig. 4). This is very attractive since consumers are trending towards natural products. Indeed the complete scenario, where each product would be allocated in the market that optimizes its revenue, yields the highest achievable revenue per unit of biomass (30.4 € kg−1). It is remarkable, that functional proteins do not play a key role in biomass value (Fig. 4), while they are the most challenging and costly product to extract as addressed in the previous section.
Industrial microalgae chains: current status, future potential and roadmap
The commercial production costs of microalgal products can be estimated by combining both models: cultivation and biorefinery. Projected costs vary greatly depending on scale; cost of cultivation, harvesting and subsequent complete biorefinery would be 6.4 € kg of biomass−1 for a facility of 100 hectares in south of Spain (Fig. 1 and 3). Whereas, according to our projections, a facility of 1 hectare would imply costs of 64.2 € kg−1 for the same scenario (28.4 € kg−1 for cultivation and harvesting and 35.8 € kg−1 for biorefining). Parameters associated to scale effects such as labor demand, price and efficiency of equipment are responsible for this higher cost. Increasing scale even further (>100 hectares) should not result in a relevant reduction in costs; equipment and production systems in the cultivation are modular and more units with identical design would be used, while a facility of 1000 hectares would only reduce biorefinery cost by 14%. Location is not trivial either, factors as productivity, temperature or cost of labor and energy makes choosing a suitable location essential (Fig. 1 and 3).The integration of cultivation and biorefinery costs with market analysis allows pinpointing industrially profitable scenarios, those showing a positive net present value (NPV) in 15 years lifetime. Scenarios are built on cultivation performed in south of Spain using flat panels. Our projections confirm that business cases aimed at high-value products would currently be a profitable activity. Examples are food additives, cosmetics or the complete biorefinery (strategy that maximizes revenues allocating the products in different markets). Estimated total investment, NPV, payback period and internal rate of return are 113 M€, 631 M€, 2 years, 61% for food additives, 113 M€, 633 M€, 2 years, 61% for cosmetics and 115 M€, 657 M€, 2 years, 62% for complete biorefinery. The complete biorefinery case results in the most promising strategy, with a net profit of 7.6 € kg−1. In addition, the production system can be placed in land unsuitable for agriculture, being otherwise unproductive, making investments even more appealing.
In these cases, pigments are the main source of revenues (Fig. 4), but as noted earlier, the market volume for natural pigments is limited. In case of market saturation the surplus of pigments could be sold as synthetic instead of as natural pigments at the expense of a lower market price. These scenarios would maintain economic feasibility as long as at least 21% of pigments were sold as natural pigments.
On the other hand, our projections show that commercialization of bulk commodities from microalgae is not profitable yet. Market price of commodities is low (Fig. 4) and revenues do not counterweight current production costs (Fig. 1 and 3). However, during the next decade, R&D will bring improvements in the process that could change this trend. Future projections, where the expected improvements in cultivation are implemented (see ESI†), show that cultivation costs could drop to 0.5 € kg−1 (Fig. 1). This achievement would make the production of bulk chemicals feasible, even at current biorefinery costs. A break-even analysis (point at which revenues equal the costs) shows that production of commodities for the food and feed sector requires a combined cultivation and biorefinery cost below 0.6 € kg−1. This could be reached by major advances like better process strategies, improved versions of the studied production systems or even completely new reactor concepts combined with biorefinery chains that are still under development in several EU projects (FP7-EU-MIRACLES, FP7-EU-D-Factory, FP7-EU-PUFA-Chain, FP7-EU-SPLASH).
Lately, biofuels have probably been the most discussed topic on algae; algae based fuels could overcome some of the negative impacts of the oil palm industry like competition with food production, removing at the same time pollutants from wastewater. However, at current fuel prices, our projections of microalgae production for energetic purposes show economic losses. A trend that, according to our models, would not change in the short term. On top of that, the energetic analysis shows that operational energy (direct energy) in the process exceeds energy yielded as biofuels (ratio energy produced as biofuels/electricity needed is 0.8 for our best future projection), despite excluding the energy embedded in the process. It results in an energy production cost of 541 € GJ−1 as biofuels, in contrast to 11–18 € GJ−1 of typical biofuels and 4–11 € GJ−1 of fossil fuels.25 Nevertheless, reduced biomass production cost from a different approach like wastewater treatment combined with environmental pressure and an increasing trend of fossil oil prices26 could make it possible. Also, combination with other commodities could be an alternative, using for instance the lipids for biofuels and the remaining components for chemicals; this would increase the biomass market price to 1.85 € kg−1 (Table S11, ESI†).
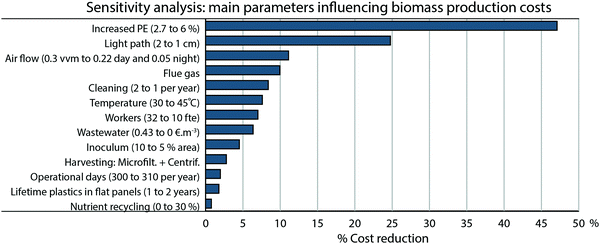
In 2011, the costs of algal cultivation and harvesting in flat panels were estimated on 5.96 € kg−1.27 There has been an important improvement in biomass production costs (Fig. 1), due to better insight in the process and operation strategies. Nevertheless, we are still facing immature technologies for production and technologies not specifically designed for algae biorefinery.28 Accordingly, this field is continuously evolving and further reductions in costs can be expected in the coming years. The progress in cultivation is particularly important since improvements in productivity, quality and composition of the biomass are not only relevant to the upstream processing, but have pronounced effects on the downstream and market price as well. A sensitivity analysis (Fig. 5) aids us to draw an interdisciplinary roadmap for long-term research on microalgae production, pinpointing the major obstacles towards market penetration. Establishing flat panels systems in south of Spain as base case (3.4 € kg−1 as aforementioned), individual parameters are changed to the value expected for the future, which in combination lead to the mentioned 0.5 € kg−1. It pinpoints PE as the most influential parameter on production cost, with a potential reduction of 1.6 € kg−1 (47% reduction in cost from base case) (Fig. 5). Greater industrial PE in outdoors conditions than our future projection have been achieved using engineered cyanobacteria and improved systems.29 Both cases are still below the theoretical maximum PE of 8 to 10%.30 It leads us to think that projected enhancements in PE are foreseeable. In this regard, strain improvement on performance (robustness, increased tolerance to photoinhibition, photosaturation, oxygen saturation and capture and conversion of CO2) is essential for the achievement of higher year-round PE.
The base for design of current available commercial photobioreactors is primarily empirical; nonetheless recent engineering tools, arisen from a deeper knowledge on microalgal biotechnology, enable step-change designs that will foster more effective technologies.31 Efforts should not be spared on improvements in reactors; in fact there is an array of new patents, reactor designs and materials on photobioreactors.31–33 Efficient thinner systems that could be operated at higher biomass concentration, less prone to fouling (cleaning) and more automated (labor) could drop costs by 1.3 € kg−1 (Fig. 5). In general, high purity CO2 gas is not essential; microalgae have demonstrated the ability to directly use flue gas to grow, using some combustion gasses as source of nutrients such as NOx.34 Use of flue gas should not be an issue even in those specific cultures requiring a purified gas, since the capture of CO2 from these currents is a mature technology commercially available.35 Therefore, a strategy directed to combining microalgae growth and carbon fixation from flue gas can be realistic. Nutrient recycling from the downstream is required for standalone operation of algae biorefineries and is likely a key factor in sustainability.36 However, it still remains a relatively unknown topic, as a consequence of the limited experience on downstream processes and a wide range of options. Feasibility of nutrient recycling largely depends on the selected catalysts and operating conditions, being the accumulation of growth inhibitors the main barrier.37,38 Nevertheless, reuse of nutrient shows high potential.36–38 The use of carbon dioxide from flue gas and recycling part of the nutrients from the downstream would make a hefty contribution, saving 0.4 € kg−1. There is not a consensus regarding standard operating procedures in microalgae production. On the contrary, process operation shows considerable divergences among authors and sources. Relevant parameters in operation are numerous; aeration is one of them and can illustrate the diversity in practices. The range of aeration per unit of volume in flat panels is very wide, being the difference as large as 1 order of magnitude for outdoors systems.39,40 Therefore, a “best practice” to operate microalgal facilities, used as a benchmark, could be a valuable tool to increase productivity, drop costs and energy consumption. Proper operation strategies are essential; reduced aeration and night “cutbacks” result in savings of 0.4 € kg−1 and up to 51% reduction of energy use. Wastewater treatment is a burden, particularly in raceway ponds (Fig. 2); this could be reduced or avoided with a complete recycling of the spent culture medium or keeping pollutants below discharge limits. Indeed researchers are studying the subject in considerable depth. While further studies are still essential to elucidate the challenges of water recycling on large scale, several authors point to its benefits, even enhancing the growth in some cases.41–43
Temperature control deserves a special focus, since it has a substantial effect on cost, adds complexity to the process and needs a source of cooling water. Reactor design, floating cultivation systems, and strain improvement are the main approaches to reduce the costs involved with temperature control. Regarding reactor design, materials reflecting near-infrared light, known as “heat radiation”, will avoid part of the heat inflow to the culture without effects on productivity.5 Offshore cultivation, like the OMEGA system from NASA,44 where surrounding water acts as temperature buffer, controlling reactor temperature could be a future alternative. From a biological perspective, temperature acclimation by a microalgal strain is complex and specific strategies are involved.45 The biotechnology of microalgae has entered into a rapid developing phase,46 and although genetic engineering remains a challenge, an increase of optimal temperature has already been proved with long term adaptation strategies.45 Culture of strains adapted to temperatures of 45 °C could reduce costs by 0.3 € kg−1 (8%) (Fig. 5).
Regarding specific biorefinery processes, the existing technology for lipids is well-established and robust. On the other hand, efficient, mild extraction and separation of proteins and carbohydrates from lipids requires a breakthrough. Research must pursue two major points: to increase the efficiency of aqueous extraction using novel solvents as ionic liquids or surfactants; and to develop novel technologies or cheaper filtration systems (membranes) for proteins-carbohydrates fractionation. This last issue should result from increasing lifetimes of membranes with low-fouling systems, decreasing the transmembrane pressure and improving the selectivity of permeation. Last but not least, there is need for more sustainable and easy-to-be recovered solvents for protein and lipid extraction. Indeed some companies as Algatechnologies Ltd and Cyanotech Corporation are developing the use of supercritical CO2 as solvent for extracting pigments from algae. In that respect, the use of ionic liquid and surfactants has to be validated checking their toxicity and food-grade quality.
In sum, we face a process that despite the long road ahead to reach maturity appears already to be lucrative. We know the existing shortcomings and how to approach them, let's keep on the right path; the benefits will justify the effort.
References
- R. H. Wijffels, O. Kruse and K. J. Hellingwerf, Curr. Opin. Biotechnol., 2013, 24, 405–413 CrossRef CAS PubMed.
- L. Zhu, Renewable Sustainable Energy Rev., 2015, 41, 1376–1384 CrossRef.
- P. M. Schenk, S. R. Thomas-Hall, E. Stephens, U. C. Marx, J. H. Mussgnug, C. Posten, O. Kruse and B. Hankamer, BioEnergy Res., 2008, 1, 20–43 CrossRef.
- M. Vanthoor-Koopmans, R. H. Wijffels, M. J. Barbosa and M. H. M. Eppink, Bioresour. Technol., 2013, 135, 142–149 CrossRef CAS PubMed.
- R. H. Wijffels and M. J. Barbosa, Science, 2010, 329, 796–799 CrossRef CAS PubMed.
- R. Bosma, J. H. de Vree, P. M. Slegers, M. Janssen, R. H. Wijffels and M. J. Barbosa, Algal Res., 2014, 6, 160–169 CrossRef.
- J. H. de Vree, R. Bosma, M. Janssen, M. J. Barbosa and R. H. Wijffels, Biotechnol. Biofuels, 2015, 8, 215 CrossRef PubMed.
- M. S. Chauton, K. I. Reitan, N. H. Norsker, R. Tveterås and H. T. Kleivdal, Aquaculture, 2014, 436, 95–103 CrossRef.
- A. Richmond and Q. Hu, Handbook of Microalgal Culture: Applied Phycology and Biotechnology, Wiley, Oxford, UK, 2013 Search PubMed.
- Y. C. Sharma, B. Singh and J. Korstad, Green Chem., 2011, 13, 2993 RSC.
- Z. Arbib, J. Ruiz, P. Álvarez-Díaz, C. Garrido-Pérez, J. Barragan and J. A. Perales, Ecol. Eng., 2013, 52, 143–153 CrossRef.
- N. Uduman, Y. Qi, M. K. Danquah, G. M. Forde and A. Hoadley, J. Renewable Sustainable Energy, 2010, 2, 012701 CrossRef.
- S. P. Cuellar-Bermudez, I. Aguilar-Hernandez, D. L. Cardenas-Chavez, N. Ornelas-Soto, M. A. Romero-Ogawa and R. Parra-Saldivar, J. Microbiol. Biotechnol., 2015, 8, 190–209 CrossRef CAS PubMed.
- E. Günerken, E. D'Hondt, M. H. M. Eppink, L. Garcia-Gonzalez, K. Elst and R. H. Wijffels, Biotechnol. Adv., 2015, 33, 243–260 CrossRef PubMed.
- M. B. M. Fenton, O. Koroleva, M. G. E. Gothard and C. Drake, An apparatus and method of processing microorganisms, EU Pat., 2723849A1, US Pat., 20130337523A1, Marcus Brian Mayhall Fenton, 2014 Search PubMed.
- M. A. Kempkes, I. Roth and M. P. J. Gaudreau, A pulsed electric field (pef) method for continuous enhanced extraction of oil and lipids from small aquatic plants, WO Pat. 2011056223A1, EU Pat. 2496703A4, US Pat. 20110107655A1, Diversified Technologies, Inc., 2011.
- P. Reep and M. P. Green, Procedure for extracting of lipids from algae without cell sacrifice, US Pat., 20120040428A1, WO Pat., 2012021831A2, Paul Reep, Michael Phillip Green, 2012 Search PubMed.
- R. Gordon, I. Gorodnitsky and V. Grichko, Method for processing an algae medium containing algae microorganisms to produce algal oil and by-products, US Pat., 8709750B2, Roman Gordon, Igor Gorodnitsky, Varvara Grichko, 2014 Search PubMed.
- A. K. Lee, D. M. Lewis and P. J. Ashman, Bioresour. Technol., 2013, 128, 199–206 CrossRef CAS PubMed.
- T. Cunha and R. Aires-barros, Mol. Biotechnol., 2002, 20, 29–40 CrossRef CAS PubMed.
- M. L. Gerardo, D. L. Oatley-Radcliffe and R. W. Lovitt, J. Membr. Sci., 2014, 464, 86–99 CrossRef CAS.
- R. K. Desai, M. Streefland, R. H. Wijffels and M. H. M. Eppink, Green Chem., 2014, 16, 2670 RSC.
- I. Wilf, Hydranautics Tech. Pap. http://http//hydracap.net/index.php?pagename = tech_papers.
- Marketsandmarkets, Natural Colors & Flavors Market by Types, Applications and Geography: Global Trends & Forecasts up to 2017. Report code: FB 1141, 2012.
- J.-P. Lange, Biofuels, Bioprod. Biorefin., 2007, 1, 39–48 CrossRef CAS.
- S. Shafiee and E. Topal, Appl. Energy, 2010, 87, 988–1000 CrossRef.
- N.-H. Norsker, M. J. Barbosa, M. H. Vermuë and R. H. Wijffels, Biotechnol. Adv., 2011, 29, 24–27 CrossRef CAS PubMed.
- R. B. Draaisma, R. H. Wijffels, P. M. E. Slegers, L. B. Brentner, A. Roy and M. J. Barbosa, Curr. Opin. Biotechnol., 2013, 24, 169–177 CrossRef CAS PubMed.
- C. E. de Farias Silva and A. Bertucco, Process Biochem., 2016 DOI:10.1016/j.procbio.2016.02.016.
- A. Melis, Plant Sci., 2009, 177, 272–280 CrossRef CAS.
- J. Pruvost, F. Le Borgne, A. Artu and J.-F. Cornet, Photobioreaction Engineering, Elsevier, 2016, vol. 48 Search PubMed.
- A. Léonard, J. C. Rooke, C. F. Meunier, H. Sarmento, J.-P. Descy and B.-L. Su, Energy Environ. Sci., 2010, 3, 370 Search PubMed.
- A. Melis, Energy Environ. Sci., 2012, 5, 5531–5539 CAS.
- H.-W. Yen, S.-H. Ho, C.-Y. Chen and J.-S. Chang, Biotechnol. J., 2015, 10, 829–839 CrossRef CAS PubMed.
- M. Mikkelsen, M. Jørgensen and F. C. Krebs, Energy Environ. Sci., 2010, 3, 43–81 CAS.
- Y. Zhang, A. Kendall and J. Yuan, Resour., Conserv. Recycl., 2014, 88, 13–20 CrossRef.
- L. Garcia Alba, PhD thesis, Universiteit Twente, 2013.
- P. Biller, A. B. Ross, S. C. Skill, A. Lea-Langton, B. Balasundaram, C. Hall, R. Riley and C. A. Llewellyn, Algal Res., 2012, 1, 70–76 CrossRef CAS.
- J. Wolf, E. Stephens, S. Steinbusch, J. Yarnold, I. L. Ross, C. Steinweg, A. Doebbe, C. Krolovitsch, S. Müller, G. Jakob, O. Kruse, C. Posten and B. Hankamer, Algal Res., 2016, 15, 187–201 CrossRef.
- L. Rodolfi, G. Chini Zittelli, N. Bassi, G. Padovani, N. Biondi, G. Bonini and M. R. Tredici, Biotechnol. Bioeng., 2009, 102, 100–112 CrossRef CAS PubMed.
- S. Fon Sing, A. Isdepsky, M. A. Borowitzka and D. M. Lewis, Bioresour. Technol., 2014, 161, 47–54 CrossRef CAS PubMed.
- J. Kim, B.-G. Ryu, Y.-J. Lee, J.-I. Han, W. Kim and J.-W. Yang, Bioprocess Biosyst. Eng., 2014, 37, 1249–1259 CrossRef CAS PubMed.
- M. S. Farid, A. Shariati, A. Badakhshan and B. Anvaripour, Bioresour. Technol., 2013, 131, 555–559 CrossRef CAS PubMed.
- J. Trent, T. Embaye, P. Buckwalter, T.-M. Richardson, H. Kagawa, S. Reinsch and M. Martis, Renewable Energy, Yokohama, 2010, p. 4 Search PubMed.
- M. Ras, J.-P. Steyer and O. Bernard, Rev. Environ. Sci. Bio/Technol., 2013, 12, 153–164 CrossRef CAS.
- J. Lü, C. Sheahan and P. Fu, Energy Environ. Sci., 2011, 4, 2451 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ee01493c |
| This journal is © The Royal Society of Chemistry 2016 |