X20CoCrWMo10-9//Co3O4: a metal–ceramic composite with unique efficiency values for water-splitting in the neutral regime†
Abstract
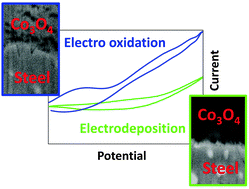
Water splitting allows the storage of solar energy into chemical bonds (H2 + O2) and will help to implement the urgently needed replacement of limited available fossil fuels. In particular, in a neutral environment electrochemically initiated water splitting suffers from low efficiency due to high overpotentials (η) caused by the anode. Electro-activation of X20CoCrWMo10-9, a Co-based tool steel resulted in a new composite material (X20CoCrWMo10-9//Co3O4) that catalyzes the anode half-cell reaction of water electrolysis with a so far, unequalled effectiveness. The current density achieved with this new anode in pH 7 corrected 0.1 M phosphate buffer is over a wide range of η around 10 times higher compared to recently developed, up-to-date electrocatalysts and represents the benchmark performance which advanced catalysts show in regimes that support water splitting significantly better than pH 7 medium. X20CoCrWMo10-9//Co3O4 exhibited electrocatalytic properties not only at pH 7, but also at pH 13, which are much superior to the ones of IrO2–RuO2, single-phase Co3O4- or Fe/Ni-based catalysts. Both XPS and FT-IR experiments unmasked Co3O4 as the dominating compound on the surface of the X20CoCrWMo10-9//Co3O4 composite. By performing a comprehensive dual beam FIB-SEM (focused ion beam-scanning electron microscopy) study, we could show that the new composite does not exhibit a classical substrate-layer structure due to the intrinsic formation of the Co-enriched outer zone. This structural particularity is basically responsible for the outstanding electrocatalytic OER performance.

 Please wait while we load your content...
Please wait while we load your content...