Lithium-excess olivine electrode for lithium rechargeable batteries†
Abstract
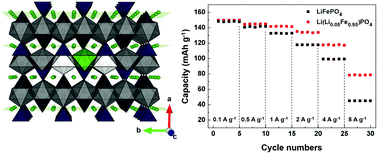
Lithium iron phosphate (LFP) has attracted tremendous attention as an electrode material for next-generation lithium-rechargeable battery systems due to the use of low-cost iron and its electrochemical stability. While the lithium diffusion in LFP, the essential property in battery operation, is relatively fast due to the one-dimensional tunnel present in the olivine crystal, the tunnel is inherently vulnerable to the presence of FeLi anti-site defects (Fe ions in Li ion sites), if any, that block the lithium diffusion and lead to inferior performance. Herein, we demonstrate that the kinetic issue arising from the FeLi defects in LFP can be completely eliminated in lithium-excess olivine LFP. The presence of an excess amount of lithium in the Fe ion sites (LiFe) energetically destabilizes the FeLi-related defects, resulting in reducing the amount of Fe defects in the tunnel. Moreover, we observe that the spinodal decomposition barrier is notably reduced in lithium-excess olivine LFP. The presence of LiFe and the absence of FeLi in lithium-excess olivine LFP additionally induce faster kinetics, resulting in an enhanced rate capability and a significantly reduced memory effect. The lithium-excess concept in the electrode crystal brings up unexpected properties for the pristine crystal and offers a novel and interesting approach to enhance the diffusivity and open up additional diffusion paths in solid-state ionic conductors.

 Please wait while we load your content...
Please wait while we load your content...