A simultaneous disulfide bond cleavage, N,S-bialkylation/N-protonation and self-assembly reaction: syntheses, structures and properties of two hybrid iodoargentates with thiazolyl-based heterocycles†
Abstract
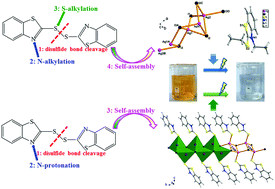
Solvothermal reactions of AgI with the vulcanization accelerator 2,2′-dibenzothiazolyl disulfide and hydroiodic acid in alcohols afford two hybrid iodoargentates with one-dimensional structures, namely (Et2mbt)[Ag2I3] (1, Hmbt = 2-mercaptobenzothiazole) and {(Hmbt)[AgI]}n (2). The syntheses of both 1 and 2 involve unprecedented multiple in situ reactions. Specifically, a simultaneous disulfide bond cleavage, N and S donor atoms bialkylation, and self-assembly reaction lead to 1 that contains a discrete N,S-biethylated cation (Et2mbt)+ and a rare inorganic (Ag2I3)− anionic chain, while a simultaneous disulfide bond cleavage, N-protonation and self-assembly reaction affords 2 which features zwitterionic Hmbt molecules coordinating with the opposite side Ag atoms of a neutral inorganic (AgI)n chain via forming Ag–S coordination bonds. The simultaneous alkylation on a thiazolyl-N donor and a thiol-S donor atom for a thiazolyl-based heterocycle using inexpensive alcohols and haloids under solvothermal conditions instead of traditional two-step organic synthesis was found for the first time. The two compounds have band gaps of 2.95 eV for 1, and 2.78 eV for 2 exhibiting an observed blue shift compared with bulk AgI. Also, 1 and 2 can be used as effective heterogeneous photocatalysts for methyl orange dye treatment under UV light irradiation.


 Please wait while we load your content...
Please wait while we load your content...