Open Access Article
Open Access ArticleSynthesis, reactivity, structures, and dynamic properties of gyroscope like iron complexes with dibridgehead diphosphine cages: pre- vs. post-metathesis substitutions as routes to adducts with neutral dipolar Fe(CO)(NO)(X) rotors†
Georgette M.
Lang
a,
Dirk
Skaper
b,
Frank
Hampel
b and
John A.
Gladysz
 *a
*a
aDepartment of Chemistry, Texas A&M University, PO Box 30012, College Station, Texas 77842-3012, USA. E-mail: gladysz@mail.chem.tamu.edu
bInstitut für Organische Chemie and Interdisciplinary Center for Molecular Materials, Friedrich-Alexander-Universität Erlangen-Nürnberg, Henkestraße 42, 91054 Erlangen, Germany
First published on 10th October 2016
Abstract
Three routes are explored to the title halide/cyanide complexes trans-Fe(CO)(NO)(X)(P((CH2)14)3P) (9c-X; X = Cl/Br/I/CN), the Fe(CO)(NO)(X) moieties of which can rotate within the diphosphine cages (ΔH‡/ΔS‡ (kcal mol−1/eu−1) 5.9/−20.4 and 7.4/−23.9 for 9c-Cl and 9c-I from variable temperature 13C NMR spectra). First, reactions of the known cationic complex trans-[Fe(CO)2(NO)(P((CH2)14)3P)]+ BF4− and Bu4N+ X− give 9c-Cl/-Br/-I/-CN (75–83%). Second, reactions of the acyclic complexes trans-Fe(CO)(NO)(X)(P((CH2)mCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)3)2 and Grubbs’ catalyst afford the tris(cycloalkenes) trans-Fe(CO)(NO)(X)(P((CH2)mCH
CH2)3)2 and Grubbs’ catalyst afford the tris(cycloalkenes) trans-Fe(CO)(NO)(X)(P((CH2)mCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH2)m)3P) (m/X = 6/Cl,Br,I,CN, 7/Cl,Br, 8/Cl,Br) as mixtures of Z/E isomers (24–41%). Third, similar reactions of trans-[Fe(CO)2(NO)(P((CH2)mCH
CH(CH2)m)3P) (m/X = 6/Cl,Br,I,CN, 7/Cl,Br, 8/Cl,Br) as mixtures of Z/E isomers (24–41%). Third, similar reactions of trans-[Fe(CO)2(NO)(P((CH2)mCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)3)2]+ BF4− and Grubbs’ catalyst afford crude trans-[Fe(CO)2(NO)P((CH2)mCH
CH2)3)2]+ BF4− and Grubbs’ catalyst afford crude trans-[Fe(CO)2(NO)P((CH2)mCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH2)m)3P)]+ BF4− (m = 6, 8). However, the C
CH(CH2)m)3P)]+ BF4− (m = 6, 8). However, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogenations required to consummate routes 2 and 3 are problematic. Crystal structures of 9c-Cl/-Br/-CN are determined. Although the CO/NO/X ligands are disordered, the void space within the diphosphine cages is analyzed in terms of horizontal and vertical constraints upon Fe(CO)(NO)(X) rotation and the NMR data. The molecules pack in identical motifs with parallel P–Fe–P axes, and without intermolecular impediments to rotation in the solid state.
C hydrogenations required to consummate routes 2 and 3 are problematic. Crystal structures of 9c-Cl/-Br/-CN are determined. Although the CO/NO/X ligands are disordered, the void space within the diphosphine cages is analyzed in terms of horizontal and vertical constraints upon Fe(CO)(NO)(X) rotation and the NMR data. The molecules pack in identical motifs with parallel P–Fe–P axes, and without intermolecular impediments to rotation in the solid state.
Introduction
As has been extensively documented1 and surveyed in previous papers in this series,2–9 gyroscopes are utilized in a multitude of technologies. Older readers may recall the Sperry Rand Corporation, which ceased to exist following a hostile merger that resulted in Unisys (1986),10 had an earlier incarnation as the Sperry Gyroscope Company.11 The latter was founded in 1910 and for some time supplied most of the world's torpedo warhead and gyrocompass guidance systems. These days, dedicated “gyroscope companies” appear to be extinct, but their spirit lives on in a small cadre of research groups that seek to realize such devices at a molecular level.12–15 Representative applications for which miniaturization is especially important include drones, virtual reality headsets, mobile phone displays, and flying suits as popularized by Iron Man.16Some candidates for molecular gyroscopes that have been under study in the authors’ laboratory are represented by II and IV in Scheme 1.2–8a These consist of a P–M–P axis about which the metal based ancillary ligands (Ly) can rotate; the MLy moiety is termed the “rotator”. Steric shielding is provided by a cage like trans spanning dibridgehead diphosphine or “stator”. This is assembled via three fold intramolecular ring closing alkene metathesis. Interestingly, a variety of substitution reactions can subsequently be effected (II → IV), despite the steric shielding of the metal core.
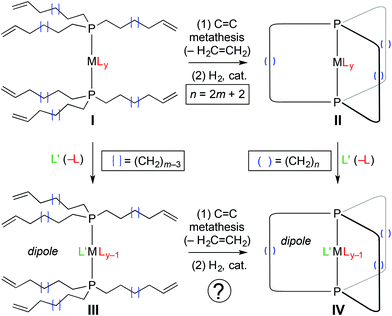 | ||
| Scheme 1 General synthetic approaches to gyroscope like complexes with different ligands on the rotator. | ||
Importantly, all of the physics that underlies the classical mechanical gyroscope17 – most fundamentally, the conservation of angular momentum – holds at the molecular level.18 One essential requirement is unidirectional rotation. In contrast, rotations about bonds in molecules are, in accord with microscopic reversibility, bidirectional or Brownian. Hence, this problem must be solved in order to attain molecular gyroscopes. Of several approaches that have been considered,18 the most easily conceptualized utilizes electric fields.19 Electric fields interact with dipoles, and it is a simple matter to prepare gyroscope like complexes in which the rotator possesses a dipole moment (e.g., IV). Application of a static electric field will orient the dipoles in the sample. The net result is, as termed by Garcia-Garibay, a “molecular compass”.20
The next level of function would be achieved with a rotating electric field, which could drive the dipole unidirectionally. Indeed, the optimal rotational frequency has already been computed for various rotational barriers.18 Importantly, if the field rotates too fast, the dipole of the rotator cannot “keep up”. For some readers, it may be easier to envision this experiment with some type of “ordered array”, such as an ensemble of surface mounted species, or a suitable crystal. Regardless, neutral compounds are preferable to ionic compounds, as any counter ions will interact with the dipoles, and invariably increase the rotational barrier. In fluid environments, counter ions would seek to “follow” the dipole, and in the solid state, the electrostatic potential would increase the energies of selected maxima and/or decrease the energies of selected minima.
We have reported iron complexes of the types II or IV with Fe(CO)3, Fe(CO)2(NO)+ and Fe(CO)3(H)+ rotators and chains of ten to eighteen methylene carbon atoms.2 The Fe(CO)3 rotators of course lack dipole moments. The others feature dipoles, but are accompanied by counter anions. Accordingly, we sought to address two major objectives in this study. First, could the Fe(CO)2(NO)+ systems be elaborated with anionic (pseudo)halide nucleophiles to neutral Fe(CO)(NO)(X) species? As reported earlier, the acyclic (pseudo)halide complexes trans-Fe(CO)(NO)(X)(P((CH2)mCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)3)2
CH2)3)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 and related bis(phosphine) adducts22–25 are easily accessed from Fe(CO)2(NO)+ precursors. Second, would the ring closing metatheses to give dibridgehead diphosphine ligands best be carried out before, during, or after the substitution sequences?
21 and related bis(phosphine) adducts22–25 are easily accessed from Fe(CO)2(NO)+ precursors. Second, would the ring closing metatheses to give dibridgehead diphosphine ligands best be carried out before, during, or after the substitution sequences?
In this paper, we report a detailed investigation of the aforementioned possibilities, which are embodied in Scheme 2 by routes I (1 → 2 → 3 → 4+ BF4− → 9-X), II (1 → 5+ BF4− → 6-X → 7-X → 9-X), and III (1 → 5+ BF4− → 8+ BF4− → 4+ BF4− → 9-X). In the course of these efforts, barriers to Fe(CO)(NO)(X) rotation for some of the new gyroscope like complexes could be determined, as well as three crystal structures. These reveal a number of auspicious attributes, which suggest that with continued optimization, it may be possible to realize functional molecular gyroscopes with related iron complexes.
 | ||
| Scheme 2 Three possible synthetic routes to the title complexes 9-X: a, alkene metathesis; b, hydrogenation. | ||
Results
Syntheses of title complexes
The first route to the title complexes trans-Fe(CO)(NO)(X)(P((CH2)n)3P) (9-X) in Scheme 2 begins with three efficient steps described in previous papers (a, b, c).2 Then a carbonyl ligand of the cationic dicarbonyl nitrosyl complex trans-[Fe(CO)2(NO)(P((CH2)n)3P)]+ BF4− (4+ BF4−) must be displaced by a halide or pseudohalide ion of a suitable salt.As shown in Scheme 3, solutions of trans-[Fe(CO)2(NO)(P((CH2)14)3P)]+ BF4− (4c+ BF4−),2 which features three seventeen membered macrocycles, and the ammonium salts Bu4N+ X− (X = Cl, Br, I, or CN; 1–2 equiv.) were combined. Workups gave the target halide or cyanide complexes trans-Fe(CO)(NO)(X)(P((CH2)14)3P) (9c-Cl, -Br, -I, -CN) as pale orange solids in 75–83% yields. These were always handled under inert atmospheres, but their high thermal stabilities were reflected by melting points (no decomposition) of at least 140 °C.
 | ||
| Scheme 3 Route I to the title complexes; substitution of the carbonyl ligand by (pseudo)halide nucleophiles following the alkene metathesis/hydrogenation/nitrosylation sequence. | ||
As summarized in Table 1 and the Experimental section, the new complexes were characterized by IR and NMR (1H, 13C{1H}, 31P{1H}) spectroscopy, microanalyses, and mass spectrometry. The IR spectra of 9c-Cl, -Br, -I, -CN (Table 1) exhibited strong νCO (1905–1917 cm−1) and νNO (1678–1703 cm−1) bands; that of 9c-CN furthermore showed a weak νCN band (2098 cm−1). Consistent with related compounds,22–25 the frequencies were lower than those of the cationic precursor 4c+ BF4−, and increased in the order X = Cl/Br/I/CN.
| Complex | IRa [cm−1] | 31P{1H} (δ/ppm)b | 13C{1H} (δ/ppm)b,c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ν CO (s) | ν NO (s) | ν CN (w) | C O | C N | PCH2 | PCH2CH2 | PCH2CH2CH2 | ||
| [2JCP Hz] | [2JCP Hz] | [1JCP Hz] | [3JCP Hz] | ||||||
| a Powder film (s = strong, w = weak). b Spectra were recorded in CDCl3 at ambient probe temperature. c All signals for which J values are given correspond to a triplet or a virtual triplet. d The spectrum of 9c-I exhibits three sets of P(CH2)n/2 signals at ambient probe temperatures. These coalesce at higher temperatures as exemplified in Fig. 5. The PCH2 signals overlap with other CH2 signals and could not be assigned. | |||||||||
| 3c | 1861 | — | — | 66.9 | 215.6 [29.0] | — | 31.6 [15.1] | 24.0 | 31.0 [6.6] |
| 4c + BF4– | 1965 | 1764 | — | 56.8 | 208.2 [25.3] | — | 28.7 [15.4] | 24.6 | 30.7 [7.6] |
| 9c-Cl | 1905 | 1678 | — | 48.7 | 218.9 [37.4] | — | 26.8 [12.9] | 23.5 | 30.6 [6.5] |
| 9c-Br | 1907 | 1683 | — | 45.2 | 218.7 [36.7] | — | 26.5 [12.9] | 23.2 | 30.6 [6.1] |
| 9c-I | 1911 | 1691 | — | 41.7 | 218.7 [35.5] | — | 24.1/23.8/23.5d | 30.5/30.2/29.9d | |
| 9c-CN | 1917 | 1703 | 2098 | 58.2 | 218.3 [30.1] | 140.7 [40.6] | 29.3 [13.5] | 23.8 | 30.7 [6.9] |
In each 13C{1H} NMR spectrum, the CO signal was coupled to both phosphorus atoms (t, 218.9–218.3 ppm), with the 2JCP values (37.4–30.1 Hz) decreasing in the series X = Cl/Br/I/CN. These were downfield from that of 4c+ BF4− (208.2 ppm), which showed a lower 2JCP value (25.3 Hz). The CN signal of 9c-CN (140.7 ppm) was similarly coupled to phosphorus (t, 2JCP = 40.6 Hz). The 13C{1H} NMR spectra of 9c-Cl, -Br, -CN gave one set of P(CH2)n/2 signals. The PCH2CH2CH2 resonances were assigned according to chemical shift and coupling trends established earlier for related complexes using 2D NMR experiments.2 The PCH2 and PCH2CH2CH2 peaks were apparent triplets due to virtual coupling (JCP 12.9–13.5 and 6.1–6.9 Hz).26 However, the spectrum of 9c-I was more complicated, showing three sets of P(CH2)n/2 signals. This difference is further analyzed in connection with variable temperature NMR data below.
As sketched in Scheme 2, route II to 9-X involves ring closing metatheses about the dipolar rotators of the previously reported adducts trans-Fe(CO)(NO)(X)(P((CH2)6CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)3)2 (6c-X),21 followed by hydrogenations of the C
CH2)3)2 (6c-X),21 followed by hydrogenations of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C linkages (steps a′, b′). Many gyroscope like complexes with halide ligands have been similarly accessed.3,5,6,8a Accordingly, as shown in Scheme 4, dilute CH2Cl2 solutions of 6c-Cl, -Br, -I, -CN (0.0020 M) and Grubbs’ catalyst (first generation; 18 mol% or 6 mol%/new C
C linkages (steps a′, b′). Many gyroscope like complexes with halide ligands have been similarly accessed.3,5,6,8a Accordingly, as shown in Scheme 4, dilute CH2Cl2 solutions of 6c-Cl, -Br, -I, -CN (0.0020 M) and Grubbs’ catalyst (first generation; 18 mol% or 6 mol%/new C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C linkage) were refluxed. After 24 h, aliquots were analyzed by 1H NMR (CDCl3). Except in the case of 6c-I, the terminal alkene signals (5.87–5.73/5.09–4.96 ppm,
C linkage) were refluxed. After 24 h, aliquots were analyzed by 1H NMR (CDCl3). Except in the case of 6c-I, the terminal alkene signals (5.87–5.73/5.09–4.96 ppm, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH/
CH/![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) were no longer detected. For 6c-I, additional catalyst was required for complete conversion as described in the Experimental section.
CH2) were no longer detected. For 6c-I, additional catalyst was required for complete conversion as described in the Experimental section.
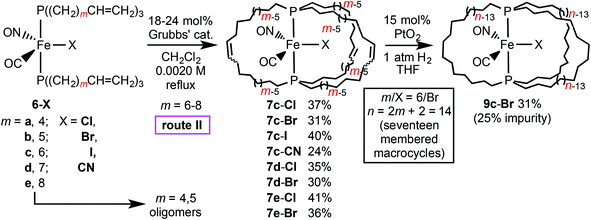 | ||
| Scheme 4 Route II to the title complexes; alkene metathesis after completion of the substitution sequence. | ||
Chromatographic workups afforded trans-Fe(CO)(NO)(X)(P((CH2)6CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH2)6)3P) (7c-Cl, Br, I, CN), which feature three unsaturated seventeen membered macrocycles, as air sensitive red-brown waxy solids in 24–40% yields. Both 1H and 31P{1H} NMR spectra indicated mixtures of E/Z C
CH(CH2)6)3P) (7c-Cl, Br, I, CN), which feature three unsaturated seventeen membered macrocycles, as air sensitive red-brown waxy solids in 24–40% yields. Both 1H and 31P{1H} NMR spectra indicated mixtures of E/Z C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C isomers. The synthesis of 6c-I was found to be poorly reproducible and the yield of 7c-CN was consistently low. Hence, studies of substrates with shorter (a,b) or longer (d,e) methylene segments were limited to bromide and chloride complexes.
C isomers. The synthesis of 6c-I was found to be poorly reproducible and the yield of 7c-CN was consistently low. Hence, studies of substrates with shorter (a,b) or longer (d,e) methylene segments were limited to bromide and chloride complexes.
In the cases of 6d,e-Cl and 6d,e-Br, workups gave the larger nineteen and twenty-one membered macrocycles trans-Fe(CO)(NO)(X)(P((CH2)mCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH2)m)3P) (7d,e-Cl or 7d,e-Br) in 30–41% yields. However, conversions of 6a,b-Cl and 6a,b-Br to the smaller macrocycles 7a,b-Cl and 7a,b-Br could not be verified, even though yields were high for the Fe(CO)3 analogs.2 The 1H NMR spectra always showed residual CH
CH(CH2)m)3P) (7d,e-Cl or 7d,e-Br) in 30–41% yields. However, conversions of 6a,b-Cl and 6a,b-Br to the smaller macrocycles 7a,b-Cl and 7a,b-Br could not be verified, even though yields were high for the Fe(CO)3 analogs.2 The 1H NMR spectra always showed residual CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 signals, and although CH
CH2 signals, and although CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH signals were apparent these could equally well arise from oligomers.27
CH signals were apparent these could equally well arise from oligomers.27
As illustrated in Schemes 2 and 4, route II would conclude with a three fold C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogenation to give 9-X. This proved challenging. The hydrogenation catalyst used in route 1, ClRh(PPh3)3, gave only partial conversions, despite extensive optimization efforts. Crabtree's catalyst and Pd/C also proved problematic. Adams’ catalyst, PtO2, has been successfully employed with other families of gyroscope like complexes.3–6,8 Indeed, complete conversion of 7c-Br could be effected with 15 mol% under 1 atm of H2 at room temperature. A chromatographic workup gave trans-Fe(CO)(NO)(Br)(P((CH2)14)3P) (9c-Br) in 31% yield. However, the 31P{1H} NMR spectrum exhibited two signals (45.1, 48.2 ppm; 75
C hydrogenation to give 9-X. This proved challenging. The hydrogenation catalyst used in route 1, ClRh(PPh3)3, gave only partial conversions, despite extensive optimization efforts. Crabtree's catalyst and Pd/C also proved problematic. Adams’ catalyst, PtO2, has been successfully employed with other families of gyroscope like complexes.3–6,8 Indeed, complete conversion of 7c-Br could be effected with 15 mol% under 1 atm of H2 at room temperature. A chromatographic workup gave trans-Fe(CO)(NO)(Br)(P((CH2)14)3P) (9c-Br) in 31% yield. However, the 31P{1H} NMR spectrum exhibited two signals (45.1, 48.2 ppm; 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 area ratio). The chemical shift of the major peak agreed with that of 9c-Br from route I, and a mass spectrum showed an abundant ion of the correct mass (and no ions of higher masses). However, efforts to separate the byproduct were unsuccessful. Considering the moderate yields of the ring closing metatheses in Scheme 4, and the 43–64% yield range for the conversion of 1 to 6-X (Scheme 2) as described earlier,21 route II falls far short of route I in terms of overall yields.
25 area ratio). The chemical shift of the major peak agreed with that of 9c-Br from route I, and a mass spectrum showed an abundant ion of the correct mass (and no ions of higher masses). However, efforts to separate the byproduct were unsuccessful. Considering the moderate yields of the ring closing metatheses in Scheme 4, and the 43–64% yield range for the conversion of 1 to 6-X (Scheme 2) as described earlier,21 route II falls far short of route I in terms of overall yields.
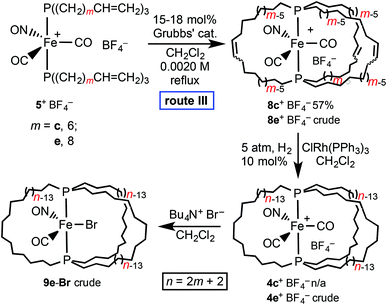
As depicted in Schemes 2 and 5, route III to 9-X involves ring closing metathesis after the first but before the second ligand substitution at iron – i.e., at the stage of the cationic dicarbonyl nitrosyl complex 5+ BF4−. As shown in Scheme 5, a 0.0020 M CH2Cl2 solution of trans-[Fe(CO)2(NO)(P((CH2)6CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)3)2]+ BF4− (5c+ BF4−) was refluxed with Grubbs’ catalyst. Workup afforded trans-[Fe(CO)2(NO)(P((CH2)6CH
CH2)3)2]+ BF4− (5c+ BF4−) was refluxed with Grubbs’ catalyst. Workup afforded trans-[Fe(CO)2(NO)(P((CH2)6CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH2)6)3P)]+ BF4− (8c+ BF4−) in 57% yield. The 31P{1H} spectrum exhibited two signals (60.9, 60.1 ppm; 82
CH(CH2)6)3P)]+ BF4− (8c+ BF4−) in 57% yield. The 31P{1H} spectrum exhibited two signals (60.9, 60.1 ppm; 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 area ratio), consistent with Z/E C
18 area ratio), consistent with Z/E C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C isomers. A similar reaction of 5e+ BF4− afforded the crude higher homolog 8e+ BF4−, which was not further analyzed.
C isomers. A similar reaction of 5e+ BF4− afforded the crude higher homolog 8e+ BF4−, which was not further analyzed.
 | ||
| Scheme 5 Route III to the title complexes; alkene metathesis during the course of the substitution sequence. | ||
Hydrogenations of 8c,e+ BF4− under the conditions employed for other metathesis products above gave variable results. With 8c+ BF4−, substantial amounts of starting material were always recovered under a variety of conditions. In contrast, when 8e+ BF4− and H2 (5 atm) were combined in the presence of ClRh(PPh3)3, a 1H spectrum of an aliquot showed no residual CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH signals. However, a 31P{1H} NMR spectrum of the crude trans-[Fe(CO)2(NO)(P((CH2)18)3P)]+ BF4− (4e+ BF4−) indicated a purity of only 80%. Nevertheless, it was further reacted with Bu4N+ Br−. A chromatographic workup gave crude trans-Fe(CO)(NO)(Br)(P((CH2)18)3P) (9e-Br), which exhibited 31P{1H} NMR signals at 45.4, 45.0, and 44.8 ppm (5
CH signals. However, a 31P{1H} NMR spectrum of the crude trans-[Fe(CO)2(NO)(P((CH2)18)3P)]+ BF4− (4e+ BF4−) indicated a purity of only 80%. Nevertheless, it was further reacted with Bu4N+ Br−. A chromatographic workup gave crude trans-Fe(CO)(NO)(Br)(P((CH2)18)3P) (9e-Br), which exhibited 31P{1H} NMR signals at 45.4, 45.0, and 44.8 ppm (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 area ratio). The major peak agrees well with what would be expected based upon the chemical shift of the lower homolog 9c-Br (45.2 ppm). The mass spectrum showed a molecular ion and fragmentation pattern consistent with the presence of 9e-Br. Nonetheless, although the starting materials in Scheme 5 (5c,e+ BF4−) are available in 96% yields from 1 (Scheme 2), it is clear that route III to the title complexes is not competitive with route I.
15 area ratio). The major peak agrees well with what would be expected based upon the chemical shift of the lower homolog 9c-Br (45.2 ppm). The mass spectrum showed a molecular ion and fragmentation pattern consistent with the presence of 9e-Br. Nonetheless, although the starting materials in Scheme 5 (5c,e+ BF4−) are available in 96% yields from 1 (Scheme 2), it is clear that route III to the title complexes is not competitive with route I.
Molecular and lattice structures
Structural data were sought for as many gyroscope like complexes as possible. Single crystals of 9c-Cl, -Br, -CN could be grown. X-ray data were collected, and the structures were solved as summarized in the Experimental section and Table 2. Key metrical parameters are given in Table 3.| Complex | 9c-Cl | 9c-Br | 9c-CN |
|---|---|---|---|
| a Data common to all structures: T = 173(2) K; λ = 0.71073 Å. | |||
| Empirical formula | C43H84ClFeNO2P2 | C43H84BrFeNO2P2 | C44H84FeN2O2P2 |
| Formula weight | 800.35 | 844.81 | 790.92 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | C2/c | C2/c | C2/c |
| Unit cell dimensions | |||
| a [Å] | 21.4367(4) | 21.2190(8) | 21.4114(6) |
| b [Å] | 13.8586(4) | 13.9660(8) | 13.9998(2) |
| c [Å] | 18.3518(5) | 18.1727(2) | 18.2960(5) |
| α [°] | 90 | 90 | 90 |
| β [°] | 122.459(1) | 121.002(4) | 122.681(9) |
| γ [°] | 90 | 90 | 90 |
| Volume [Å3] | 4600.3(2) | 4616.1(3) | 4616.1(3) |
| Z | 4 | 4 | 4 |
| ρ calcd [Mg m−3] | 1.1560.489 | 1.2160.489 | 1.1380.489 |
| μ [mm−1] | 0.489 | 1.295 | 0.431 |
| F(000) | 1752 | 1824 | 1736 |
| Crystal size [mm3] | 0.35 × 0.30 × 0.20 | 0.30 × 0.30 × 0.30 | 0.35 × 0.20 × 0.20 |
| Range for data collection | 2.37 to 27.44 | 2.92 to 27.57 | 2.26 to 27.50 |
| Index ranges | –27 ≤ h ≤ 27 | –27 ≤ h ≤ 27 | –27 ≤ h ≤ 27 |
| –17 ≤ k ≤ 17 | –18 ≤ k ≤ 18 | –18 ≤ k ≤ 17 | |
| –23 ≤ l ≤ 23 | –23 ≤ l ≤ 23 | –23 ≤ l ≤ 23 | |
| Reflections collected | 9763 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 777 777 |
9935 |
| Independent reflections | 5244 | 5278 | 5286 |
| Data/restraints/parameters | 5244/3/234 | 5278/0/230 | 5286/0/232 |
| Goodness-of-fit on F2 | 1.062 | 1.026 | 0.984 |
| Final R indices [I > 2σ(I)] | R 1 = 0.0503, wR2 = 0.1496 | R 1 = 0.0613, wR2 = 0.1595 | R 1 = 0.0346, wR2 = 0.0900 |
| R indices (all data) | R 1 = 0.0648, wR2 = 0.1612 | R 1 = 0.0857, wR2 = 0.1774 | R 1 = 0.0464, wR2 = 0.0960 |
| Largest diff. peak & hole [e Å−3] | 0.975/−1.014 | 2.200/−2.197 | 0.219/−0.772 |
| Complex | 9c-Cl | 9c-Br | 9c-CN |
|---|---|---|---|
a These values have considerable uncertainty given the disorder and refinement constraints described in the Experimental section; at the same time, it is unlikely that they lead to an overestimation of the rotator radius.
b The longest of the ![[F with combining low line]](https://www.rsc.org/images/entities/char_0046_0332.gif) ![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif) C C![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif) , , ![[F with combining low line]](https://www.rsc.org/images/entities/char_0046_0332.gif) ![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif) N N![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif) , or , or ![[F with combining low line]](https://www.rsc.org/images/entities/char_0046_0332.gif) ![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif) C C![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif) distances, plus the van der Waals radius of the terminal atom (oxygen, 1.52 Å).
c The distance from iron to the two remote carbon atoms of the three macrocycles (a, b, c; the first contains the idealized C2 axis) that are closest to the plane of the rotator (Fe–Cdistal).
d The shortest of the previous six distances, minus the van der Waals radius of the carbon atom (1.70 Å).
e The longest of the previous six distances, minus the van der Waals radius of the carbon atom.
f The distance from iron to the nearest carbon atom of a neighboring gyroscope like molecule.
g The previous entry minus the van der Waals radius of the carbon atom.
h The distance between planes defined by the three carbon atoms attached to each phosphorus atom, taken at the centroid of the three carbon atoms.
i The previous value minus the van der Waals radii of two carbon atoms.
j The distance between the P distances, plus the van der Waals radius of the terminal atom (oxygen, 1.52 Å).
c The distance from iron to the two remote carbon atoms of the three macrocycles (a, b, c; the first contains the idealized C2 axis) that are closest to the plane of the rotator (Fe–Cdistal).
d The shortest of the previous six distances, minus the van der Waals radius of the carbon atom (1.70 Å).
e The longest of the previous six distances, minus the van der Waals radius of the carbon atom.
f The distance from iron to the nearest carbon atom of a neighboring gyroscope like molecule.
g The previous entry minus the van der Waals radius of the carbon atom.
h The distance between planes defined by the three carbon atoms attached to each phosphorus atom, taken at the centroid of the three carbon atoms.
i The previous value minus the van der Waals radii of two carbon atoms.
j The distance between the P![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2 carbon atoms of each macrocycle.
k The C–P–P–C torsion angle within the three macrocycles (a, b, c). H2 carbon atoms of each macrocycle.
k The C–P–P–C torsion angle within the three macrocycles (a, b, c).
|
|||
| Fe–P | 2.2527(6) | 2.2705(8) | 2.2310(3) |
![[F with combining low line]](https://www.rsc.org/images/entities/char_0046_0332.gif) ![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif) C C![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif) |
2.90a | 2.70a | 2.99a |
![[F with combining low line]](https://www.rsc.org/images/entities/char_0046_0332.gif) ![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif) N N![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif) |
2.75a | 3.00a | 2.99a |
![[F with combining low line]](https://www.rsc.org/images/entities/char_0046_0332.gif) ![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif) ![[X with combining low line]](https://www.rsc.org/images/entities/char_0058_0332.gif) |
2.363(4) | 2.5280(19) | 2.88a |
| Radius of rotatorb | 4.42 | 4.52 | 4.51 |
Fe–Ca![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
7.89 | 7.91 | 7.93 |
Fe–C′a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
7.89 | 7.91 | 7.93 |
Fe–Cb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
6.65 | 6.73 | 6.62 |
Fe–C′b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
7.46 | 7.37 | 7.43 |
Fe–Cc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
7.46 | 7.37 | 7.43 |
Fe–C′c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
6.65 | 6.73 | 6.62 |
| Fe–Cdistal–vdWd | 4.95 | 5.03 | 4.92 |
| Fe–Cdistal′–vdWe | 6.19 | 6.21 | 6.23 |
Fe–Cneighbor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f f |
5.99 | 6.06 | 6.04 |
| Fe–Cneighbor–vdWg | 4.29 | 4.36 | 4.34 |
| ∠P–Fe–P | 177.9 | 178.2 | 176.1 |
P(![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2)3/P( H2)3/P(![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2)3 H2)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h h |
6.02 | 6.08 | 5.98 |
P(![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2)3/P( H2)3/P(![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2)3–2vdWi H2)3–2vdWi |
2.62 | 2.68 | 2.58 |
![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) aP–Fe–P
aP–Fe–P![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) a a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j j |
6.04 | 6.12 | 5.98 |
![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) bP–Fe–P
bP–Fe–P![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) b b |
6.16 | 6.20 | 6.12 |
![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) cP–Fe–P
cP–Fe–P![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) c c |
6.16 | 6.20 | 6.12 |
Ca–P–P–Ca![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k k |
38.4 | 38.2 | 38.5 |
| Cb–P–P–Cb | 37.9 | 37.5 | 38.2 |
| Cc–P–P–Cc | 37.9 | 37.5 | 38.2 |
Thermal ellipsoid representations of 9c-Cl, -Br, -CN are provided in Fig. 1. In all three structures, the CO/NO/X ligands were disordered over three positions. In the case of 9c-Cl, the ligands could nonetheless be distinguished (33% occupancy, each position); for refinement, the CO and NO positions were arbitrarily assigned in accord with the overall symmetry. With 9c-Br, the bromide ligand exhibited 33% occupancy at each position, but the CO and NO ligands could not be distinguished. For refinement, they were assigned equal occupancies in each position. In the case of 9c-CN, none of the ligands could be distinguished and were presumed to be equally disordered over three positions. Refinement was carried out with fixed occupancy factors as described in the Experimental section.
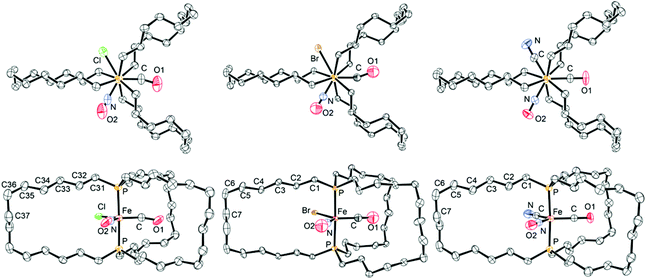 | ||
| Fig. 1 Thermal ellipsoid plots (50% probability) of the molecular structures of 9c-Cl, 9c-Br, and 9c-CN (left to right; all Fe(CO)(NO)(X) moieties are disordered). | ||
As a result, the positions of the Fe(CO)(NO)(X) ligands in Fig. 1 are arbitrary, and their bond lengths must be cautiously interpreted. In each structure, the dibridgehead diphosphine ligand exhibits a crystallographic C2 axis coincident with the ![[F with combining low line]](https://www.rsc.org/images/entities/char_0046_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif) –
–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) O linkage (the numbered carbon atoms in Fig. 1 thereby exchange between the upper and lower hemispheres). In all complexes, the CH2/(CO)(NO)(X)/CH2 substituents along the P–Fe–P axes adopt staggered conformations (Fig. 1, top). The crystal structure of the tricarbonyl complex 3c (Scheme 2), which has been analyzed in previous papers,2 exhibits an analogous symmetry axis and diphosphine and P–Fe–P conformations.
O linkage (the numbered carbon atoms in Fig. 1 thereby exchange between the upper and lower hemispheres). In all complexes, the CH2/(CO)(NO)(X)/CH2 substituents along the P–Fe–P axes adopt staggered conformations (Fig. 1, top). The crystal structure of the tricarbonyl complex 3c (Scheme 2), which has been analyzed in previous papers,2 exhibits an analogous symmetry axis and diphosphine and P–Fe–P conformations.
In all three structures, the lattice packing is analogous to that in 3c (monoclinic, C2/c, Z = 4).2 A representative example (9c-Br) is shown in Fig. 2. The molecules align in well defined layers in which all P–Fe–P axes are parallel (Fig. 2, left). Within each layer the individual molecules alternate between two different orientations (Fig. 2, right). As would be expected, the unit cell dimensions are virtually identical (Table 2), with the volumes spanning the narrow range of 4600.3 to 4616.1 Å3 (3c: 4633.5 Å3).2
 | ||
| Fig. 2 The crystal lattice of 9c-Br as viewed along (left) and perpendicular (right) to the b axis (the Fe(CO)(NO)(Br) moieties are disordered). | ||
A model for approximating the void space available to the rotator within the dibridgehead diphosphine cages has been developed in several papers.2b,3b,8a,9Fig. 3 applies this paradigm to 9c-X. The first step is to determine the rotator radius. Towards this end, one takes the distance from the metal to the most remote atom of each ligand on the rotator (e.g., ![[F with combining low line]](https://www.rsc.org/images/entities/char_0046_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif) C
C![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif) ,
, ![[F with combining low line]](https://www.rsc.org/images/entities/char_0046_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif) N
N![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif) ,
, ![[F with combining low line]](https://www.rsc.org/images/entities/char_0046_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif) C
C![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif) for the diatomic ligands). Then the van der Waals radius of the remote atom is added (oxygen, 1.52 Å; nitrogen, 1.55 Å; chloride, 1.75 Å; bromide, 1.85 Å; iodide, 1.98 Å).28 The ligand that yields the greatest value can be termed “radius determining”.
for the diatomic ligands). Then the van der Waals radius of the remote atom is added (oxygen, 1.52 Å; nitrogen, 1.55 Å; chloride, 1.75 Å; bromide, 1.85 Å; iodide, 1.98 Å).28 The ligand that yields the greatest value can be termed “radius determining”.
 | ||
| Fig. 3 Spatial relationships involving the iron atom, rotator, and (CH2)14 bridges in 9c-X; see text and Table 3 (vdW = van der Waals; A/B/C refer to the macrocycle labels in Table 3). | ||
Given the complexities introduced by the crystallographic disorder, boxes are presented for each complex of interest in Fig. 3. These summarize the radii associated with each ligand based upon either the data in Table 3 or model complexes in the literature.29 The radius determining values are denoted in red, and in three cases correspond to FeCO or FeNO ligands (4.42–4.52 Å). For 9c-I, which could not be structurally characterized, the iodide ligand should be radius determining (4.68 Å).29 Regardless, it can be confidently concluded that all rotator radii fall into the relatively narrow range of 4.42–4.68 Å.
The “horizontal clearance” within the diphosphine cage is then estimated. First, the distances from iron to the distal carbon atoms of all three methylene chains are determined (per Table 3 and Fig. 3, the chains are labeled a/b/c). Since the P(CH2)14P linkages are roughly symmetric with respect to the rotator plane, these should normally be C7 and C8. The van der Waals radius of a carbon atom (1.70 Å)28 is then subtracted, giving six values as summarized in Table 3.30 In order to reflect the minimum clearance available, the shortest is normally used for analysis (Fe–Cdistal–vdW, Table 3), although other approaches have been considered.2,9,31 In any case, the horizontal clearances for 9c-Cl, -Br, -CN fall in the range of 4.92–5.03 Å, as highlighted in Fig. 3 (red), and a similar value would be expected for crystalline 9c-I. Hence, the clearances are significantly greater than the radii of the four rotators. Implications regarding the NMR data presented above or below are analyzed in the Discussion section.
Another issue concerns the feasibility of rotator rotation in the crystal lattice. Here, there is the additional consideration of interference from neighboring molecules. Of special interest are cases where the distances from the metal atom of one complex to the nearest non-hydrogen atoms of a neighboring complex are greater than the radius of the rotator, after adjusting for the van der Waals radius of the non-hydrogen atom.30 In this limit, sufficient steric spacing is assured.
Data are obtained from the crystal structures as exemplified for 9c-Cl in Fig. 4 and summarized in Table 3. The intermolecular distances are quite similar for all three complexes (5.99–6.06 Å; “Fe–Cneighbor”), giving 4.29–4.36 Å (“Fe–Cneighbor–vdW”) after the van der Waals correction.30 These values are comparable to the rotator radii (4.42–4.52 Å). Furthermore, the atom of the neighboring molecule that exhibits the shortest distance (C23 in Fig. 4) is markedly removed from the plane of the rotator, attenuating the degree of interaction. The next nearest atom, which is barely removed from the rotator plane (C18 in Fig. 4), allows for more clearance (4.44 Å, 4.48 Å, and 4.55 Å in 9c-Cl, -Br, -CN after van der Waals corrections). In any case, any impediment to rotator rotation imposed by the crystal lattice is not likely to be significantly greater than that imposed by the horizontal dimension of the diphosphine cage.
 | ||
| Fig. 4 Distances from the iron atom of crystalline 9c-Cl to the six nearest non-hydrogen atoms of a neighboring molecule. | ||
Variable temperature NMR data
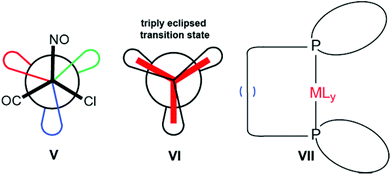
13C{1H} NMR spectra of 9c-Cl were recorded in CD2Cl2 at temperatures ranging from 25 to −85 °C. The chemical shifts of the PCH2CH2CH2 carbon atoms varied moderately (Δδ 0.3–0.6 ppm) as they broadened and ultimately decoalesced into three peaks, as exemplified for the PCH2CH2 signal in Fig. 5 (left). This corresponds to Fe(CO)(NO)(Cl) rotation becoming slow on the NMR time scale, leading to three symmetry inequivalent macrocycles with different sets of NMR chemical shifts, as represented in V (Fig. 6). In order for a methylene group to sample all environments in V, the Fe(CO)(NO)(Cl) moiety must rotate by 240° through transition states of the type VI. Alternative processes that might render the methylene signals equivalent, such as mechanisms involving CO or phosphine dissociation (or with 9c-CN, cyanide dissociation) can be excluded due to coupling constants that are maintained at the high temperature limit (JCP, Table 1). | ||
| Fig. 5 Partial 13C NMR spectra (PCH2CH2 signals) of 9c-Cl (CD2Cl2, left), and 9c-I (C6D5Cl, right) as a function of temperature. Each spectrum is paired with simulated line shapes (red). | ||
The spectra of 9c-Cl were simulated using gNMR (Fig. 5, red traces),32 which afforded the rate constant at each temperature. An Eyring plot (Fig. s1, ESI†) gave ΔH‡ and ΔS‡ values of 5.9 kcal mol−1 and −20.1 eu for the 240° rotation of the Fe(CO)(NO)(Cl) moiety. Other examples have been reported where three identical groups similarly exchange between three distinct environments and the rate constants determined by line shape analysis.33
Next, 13C NMR spectra of 9c-I were recorded in C6D5Cl between −20 and 100 °C (Fig. 5, right). As noted in connection with Table 1, Fe(CO)(NO)(I) rotation was slow on the NMR time scale at room temperature, as evidenced by three peaks of equal area for the PCH2CH2 and other methylene signals. However, a well defined three fold coalescence was observed near 55 °C. The data were treated as for 9c-Cl, giving ΔH‡ and ΔS‡ values of 7.6 kcal mol−1 and −23.9 eu. This yields a ΔG‡298 K value (14.7 kcal mol−1) that is as expected higher than that of 9c-Cl (11.9 kcal mol−1). The activation parameters are summarized in Table 4, together with those of other trigonal bipyramidal iron gyroscope like complexes.2,9 Additional aspects of these data are analyzed in the Discussion section.
| Compound | X/E/n/z | ΔH‡ (kcal mol−1) | ΔS‡ (eu)a | ΔG‡298 K (kcal mol−1) | ΔG‡383 K (kcal mol−1) |
|---|---|---|---|---|---|
| a cal mol−1 K−1. b Spectra were recorded in CD2Cl2. c Spectra were recorded in C6D5Cl. d Data are from ref. 2. e Spectra were recorded in CDFCl2. f Diarsenic analog of 4a+ BF4− or 4b+ BF4−; data are from ref. 9. | |||||
| 9c-Cl | Cl/P/14/0 | 5.9 | −20.4 | 11.9 | 13.6 |
| 9c-I | I/P/14/0 | 7.6 | −23.9 | 14.7 | 16.8 |
| 4b+ BF4–c,d | CO/P/12/1 | 8.3 | −28.4 | 16.7 | 19.2 |
| 4c+ BF4–d,e | CO/P/14/1 | 9.5 | −6.5 | 11.4 | 11.9 |
| 4a-As2+ BF4−c,f | CO/As/10/1 | — | — | — | >19.4 |
| 4b-As2+ BF4−b,f | CO/As/12/1 | 7.7 | −22.1 | 14.2 | 16.2 |
Discussion
Synthetic strategies and cage effects
The major objective of this study was to efficiently access gyroscope like complexes based upon cage like dibridgehead diphosphines that contain neutral dipolar Fe(CO)(NO)(X) rotators (9-X).34 A companion objective was to determine whether the ring closing metatheses that afford the diphosphine cages are best carried out before (route I), after (route II), or during (route III) substitution sequences involving various Fe(CO)3 precursors. As shown in Schemes 3–5, route I proved to be unequivocally superior. Routes II and III suffered from lower yields in both the metathesis and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogenation steps. Thus, perhaps counterintuitively, it proves best to effect substitution of the rotator in the most sterically shielded adduct.
C hydrogenation steps. Thus, perhaps counterintuitively, it proves best to effect substitution of the rotator in the most sterically shielded adduct.
With additional experimentation, an effective hydrogenation catalyst might have been found for routes II and III. However, conditions successfully employed with other types of gyroscope like complexes gave mediocre results. In the metathesis step, an alternative cyclization mode is possible involving a combination of interligand and intraligand coupling. After hydrogenation, species of the type VII would result (Fig. 6). These are routinely encountered as byproducts in metatheses of related octahedral complexes (as well as a few square planar complexes),2b,8a and exhibit distinctive NMR properties. However, they have not yet been detected in any reactions of trigonal bipyramidal precursors, which as analyzed in previous papers can be viewed as conformationally pre-organized for three fold interligand metatheses.2,9
With the ready availability of 9c-X (Scheme 3), there is an opportunity to probe for “cage effects” relative to the acyclic analogs 6c-X (Scheme 2).21 However, any perturbations on spectroscopic properties appear to be modest. The 31P{1H} NMR signals of 9c-Cl, -Br, -I, -CN (Table 1) are generally 2–3 ppm downfield of those of 6c-Cl, -Br, -I, -CN. In contrast, the CO 13C{1H} signals of 9c-Cl, -Br, -I, -CN are always ∼2 ppm upfield. The IR νCO and νNO values are greater for 9c-Cl, -Br, -I, -CN; the trend is most pronounced for 9c-Ivs. 6c-I (1911 and 1691 cm−1vs. 1905 and 1681 cm−1). A more interesting type of cage effect would involve possible changes in the mechanisms of substitution of 5+ BF4−vs. 4+ BF4− (Scheme 2), and conditions for monitoring these rates are currently being optimized.
Structures and dynamic properties
Apart from the disorder of the rotators, the homology exhibited by the molecular structures and crystal lattices of the gyroscope like complexes 9c-Cl, -Br, -CN (Fig. 1 and 2) is striking. Considering that the tricarbonyl complex 3c exhibits analogous structural features,2 it seems that – at least for species with seventeen membered macrocycles – all neutral iron adducts with rotators comprised of three small ligands (e.g., H, F, CS, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH, CH3, etc.) should crystallize similarly. This is supported by the closely comparable structures of the corresponding dibridgehead diarsine9 and Os(CO)3 complexes.8a Thus, a diverse parameter space has been defined by which various properties of trigonal bipyramidal molecular gyroscopes can be optimized while maintaining homologous solid state structures.
CH, CH3, etc.) should crystallize similarly. This is supported by the closely comparable structures of the corresponding dibridgehead diarsine9 and Os(CO)3 complexes.8a Thus, a diverse parameter space has been defined by which various properties of trigonal bipyramidal molecular gyroscopes can be optimized while maintaining homologous solid state structures.
The activation parameters for Fe(CO)(NO)(X) rotation in 9c-Cl, -I can be compared to those reported earlier for Fe(CO)2(NO)+ rotation in 4c+ BF4− (Table 4).2 At first glance, the ΔG‡298 K values for 9c-Cl, -I follow the trend expected based upon the relative sizes of the chloride and iodide ligands (11.9 and 14.7 kcal mol−1). However, in some ways this is a misleading comparison, as Fig. 3 shows that the carbonyl ligand – not chloride – is radius determining in the former (4.42 vs. 4.11 Å). Importantly, the carbonyl ligand is also radius determining in 4c+ BF4−, as gauged by the crystal structure of the tricarbonyl complex 3c (4.45 Å)2 or the diarsine analog of the lower homolog 4b+ BF4− (4.42 Å; 4b-As2+ BF4−).9 Accordingly, 9c-Cl and 4c+ BF4− exhibit very similar ΔG‡298 K values (11.9 and 11.4 kcal mol−1). One also sees in Table 4 that the ΔG‡298 K value for 4c+ BF4− is much lower than that for 4b+ BF4− (11.4 vs. 16.7 kcal mol−1). The latter features smaller fifteen membered macrocycles and diminished horizontal clearance.
As illustrated by 4b+ BF4− and 4b-As2+ BF4− in Table 4, ΔG‡298 K values significantly decrease when the bridgehead phosphorus atoms are replaced by arsenic atoms (16.7 vs. 14.2 kcal mol−1). This has been interpreted in terms of increased “vertical clearance” provided by the iron arsenic bonds, which are typically 3–4% longer than iron phosphorus bonds. Since, in principle, there is sufficient horizontal clearance to accommodate the iodide ligand of 9c-I within the diphosphine cage (Fig. 3), we suggest that the increased ΔG‡298 K value vs. that of 9c-Cl is largely derived from steric interactions in the vertical dimension. This can be analyzed as exemplified in Fig. 7, which focuses on the spatial relationships between the rotator ligands and the C–P–Fe–P–C linkages of 9c-Cl.
First, all of the ligands on the rotator must “squeeze” between the upper and lower PCH2 groups as they sweep through any arc of ≥120°. In the transition states (VI, Fig. 6), these will be PCH2 groups from the same macrocycle. In the ground states sampled by the crystal structures, the PCH2 groups nearest to a given ligand will be from different macrocycles (Fig. 1, top). In any case, planes may be defined by the three upper and three lower PCH2 groups, as shown in VIII in Fig. 7. Given the crystallographic C2 axes of the diphosphine ligands, these will be parallel. As summarized in Table 3, the plane/plane distances range from 5.98 to 6.08 Å for 9-Cl, -Br, -CN. When the van der Waals radii of two PCH2 carbon atoms are subtracted (3.40 Å),28 vertical clearances of 2.58 to 2.68 Å are obtained.30
Another measure of vertical clearance is provided by the distance between the PCH2 carbon atoms on each macrocycle, as illustrated in X. For 9c-Cl these distances range from 6.04 to 6.16 Å, as summarized in Table 3. For 9c-Br, -CN, the corresponding ranges are 6.08–6.20 Å and 5.98–6.12 Å. When the van der Waals radii of the two PCH2 carbon atoms are subtracted, vertical clearances of 2.64–2.76 Å (9c-Cl), 2.68–2.80 Å (9c-Br), and 2.58–2.72 Å (9c-CN) are obtained. These distances are slightly longer than the plane/plane distances or clearances in VIII as the vector between the two PCH2 atoms is not perpendicular to the plane.
In the vertical dimension, what counts is not the radius defined by a given ligand but its “fatness”. This would be approximated by the van der Waals diameter. Every polyatomic ligand is characterized by a fatness determining atom, and every rotator by a fatness determining ligand. The diameters of the ligands in Fig. 3 increase from NO (3.10 Å), CO and CN (3.40 Å), Cl (3.50 Å), Br (3.70 Å), to I (3.96 Å).26 Hence, for the rotors of the halide complexes, the halide ligands are fatness determining, and for the cyanide complex the slightly more svelte CO and CN ligands are fatness determining. However, there is not much difference between the fatness of the Cl, CN, and CO ligands.
Regardless, all of the preceding values are somewhat greater than the vertical clearances represented in VIII, X, or the analogous structures for 9c-Br, -CN. This is particularly evident in IX, which is simply a partial view of the crystal structure with selected atoms at van der Waals radii. In solution, the tightness of the “squeeze” associated with the transition state VI (Fig. 6) can be ameliorated by simultaneous conformational changes within the macrocycles, as previously discussed.2b However, we suggest that the residual steric interactions are responsible for the major part of the activation barriers – more than any interactions connected with horizontal clearance in seventeen membered macrocycles. Thus, the introduction of the larger iodide ligand in 9c-I leads to top/bottom steric interactions that no longer allow Fe(CO)(NO)(X) rotation to be rapid on the NMR time scale at room temperature.
A final point concerns the negative ΔS‡ values in Table 4. These are consistent with the loss of entropy that would be anticipated from the three fold eclipsing interaction in transition state VI. Furthermore, this interaction would presumably be accompanied by correlated changes in the macrocycle conformations, which as noted above could attenuate van der Waals contacts originating from the tight vertical clearances.
Towards molecular gyroscopes
In the introduction, the use of external electric fields to orient dipolar rotators or compel unidirectional motion19 was discussed. This study, coupled with earlier installments,2,9 has provided a clear road map for approaching these objectives. First, facile routes to neutral gyroscope like iron complexes with dipolar rotors and cage like dibridgehead diphosphine stators have been developed. Second, these complexes can easily be expanded in the vertical dimension by switching from phosphorus to arsenic donor atoms,9 and very likely to antimony and bismuth donor atoms. The iron bismuth bond length in Fe(CO)4(Bit-Bu3) is 2.6269(9) Å,35 as compared to a range of 2.2310(3) to 2.2705(8) Å for the iron phosphorus bonds in Table 3. This 14–18% increase should greatly attenuate the types of steric interactions analyzed in Fig. 7. Metal fragments that give longer metal/donor atom bonds, such as Os(CO)3, can also be bought into play.8a Both the radius and fatness of the rotator can be decreased by introducing still smaller ligands such as hydride and fluoride. All of these strategies, and a number of others,2,9 hold great promise for achieving very low rotational barriers, analogous to the idealized frictionless limit for flywheels of classical mechanical gyroscopes.The reliably reproducible solid state properties of gyroscope like complexes based upon trigonal planar rotators and dibridgehead bis(donor atom) stators that define three seventeen membered macrocycles constitute another bonus. Since there are no substantial intermolecular impediments to M(L)(L′)(L′′) (Fe(CO)(NO)(X)) rotation in the crystal lattices, and all E–M–E (P–Fe–P, As–Fe–As, P–Os–P, etc.) axes are parallel, it is easy to envision how applying a rotating electric field to a properly oriented crystal could simultaneously transform 1017 molecules (ca. 1 μmol) into an array of functioning gyroscopes. Indeed, solid state 13C NMR studies of the tricarbonyl complex 3c have established rapid rotation between −60 and 95 °C.2b,36 Furthermore, this substrate is, in view of the insights gleamed from this and previous papers, by no means optimized for a low rotational barrier.
Promising leads for molecular gyroscopes have also been developed with other metal coordination geometries,3,5,8 other types of expanded and/or conformationally restricted stators,4 and organic or organosilicon systems under study by Garcia-Garibay12 and Setaka.13 However, variations on the trigonal bipyramidal complexes described herein are viewed as having particular potential, and further studies will be reported in due course.
Experimental section
General data
All reactions were conducted under N2 or H2 atmospheres. Chemicals were treated as follows: CH2Cl2, ether, and hexanes, dried and degassed using a Glass Contour solvent purification system; CDCl3, C6D6, (2 × Cambridge Isotope Laboratories), Bu4N+ X− (X = Cl or CN, Aldrich, 95–97%; Br, TCI, 99%; I, Alfa Aesar, 98%), Grubbs’ catalyst (Ru(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh)(PCy3)2(Cl)2; Strem), SiO2 (Silicycle, 40–63 μm, 230–400 mesh), neutral Al2O3 (Macherey-Nagel), Celite (EMD), used as received.
CHPh)(PCy3)2(Cl)2; Strem), SiO2 (Silicycle, 40–63 μm, 230–400 mesh), neutral Al2O3 (Macherey-Nagel), Celite (EMD), used as received.
NMR spectra were recorded on standard 400 or 500 MHz spectrometers at ambient probe temperatures and referenced as follows (δ, ppm): 1H, residual internal C6D5H (7.15) or CHCl3 (7.26); 13C, internal C6D6 (128.0) or CDCl3 (77.0); 31P, external H3PO4 (0.00). IR spectra were recorded using a Shimadzu IRAffinity-1 spectrometer with a Pike MIRacle ATR system (diamond/ZnSe crystal). Mass spectra and melting points were determined per earlier papers.2b,9 Microanalyses were conducted on a Carlo Erba EA1110 instrument or by Atlantic Microlab.
NMR (CDCl3, δ in ppm): 1H NMR (500 MHz): 1.98–1.86 (m, 12H, CH2), 1.66–1.48 (m, 12H, CH2), 1.44–1.37 (m, 12H, CH2), 1.36–1.27 (m, 48H, CH2); 13C{1H} NMR (126 MHz) 218.9 (t, 2JCP = 37.4 Hz, CO), 30.6 (virtual t, 3JCP, 5JCP′ = 6.5 Hz,26 PCH2CH2CH2), 27.9 (s, CH2), 27.6 (s, CH2), 26.9 (s, CH2), 26.80 (s, CH2), 26.75 (virtual t, 1JCP, 3JCP′ = 12.9 Hz,26 PCH2), 23.5 (s, CH2); 31P{1H} NMR (202 MHz): 48.7 (s).
IR (powder film, cm−1): 2926 (w), 2856 (w), 1905 (s, νCO), 1678 (s, νNO), 1458 (w), 1409 (w), 1087 (w), 803 (w), 760 (w), 719 (w). MS:37 801 (9c-Cl+, 5%), 771 ([9c-Cl − CO]+, 30%), 741 ([9c-Cl − CO − NO]+, 100%), 738 ([9c-Cl − NO − Cl]+, 65%), 684 ([9c-Cl − CO − NO − Cl − Fe + 2O]+, 65%), 668 ([9c-Cl − CO − NO − Cl − Fe + O]+, 40%), 652 ([9c-Cl − CO − NO − Cl − Fe]+, 20%).
NMR (CDCl3, δ in ppm): 1H NMR (500 MHz): 2.17–1.80 (m, 12H, CH2), 1.68–1.47 (m, 12H, CH2), 1.44–1.36 (m, 12H, CH2), 1.36–1.18 (m, 48H, CH2); 13C{1H} NMR (100 MHz): 218.6 (t, 2JCP = 36.5 Hz, CO), 30.3 (virtual t, 3JCP, 5JCP′ = 7.8 Hz,26 PCH2CH2CH2), 27.7 (s, CH2), 27.3 (s, CH2), 26.8 (s, CH2), 26.6 (s, CH2), 26.5 (virtual t, 1JCP, 3JCP′ = 12.9 Hz,26 PCH2), 23.2 (s, CH2); 31P{1H} NMR (202 MHz): 45.2 (s); for sample from route II (162 MHz) 48.2 (s, 25% impurity), 45.1 (s, 75%).
IR (powder film, cm−1): 2926 (w), 2853 (w), 1907 (s, νCO), 1683 (s, νNO), 1456 (w), 1409 (w), 1234 (w), 1087 (w), 791 (w), 758 (w), 718 (w). MS:37 815 ([9c-Br − CO]+, 20%), 785 ([9c-Br − CO − NO]+, 100%), 736 ([9c-Br − CO − Br]+, 35%), 734 ([9c-Br − NO − Br]+, 40%), 704 ([9c-Br − CO − NO − Br]+, 5%), 664 ([9c-Br − CO − NO − Br − Fe + O]+, 10%), 648 ([9c-Br − CO − NO − Br − Fe]+, 25%).
NMR (CDCl3, δ in ppm): 1H NMR (500 MHz): 2.34–1.89 (m, 24H, CH2), 1.78–1.58 (m, 24H, CH2), 1.52–1.46 (m, 12H, CH2), 1.46–1.39 (m, 12H, CH2), 1.39–1.30 (m, 12H, CH2); 13C{1H} NMR (125 MHz): 218.7 (t, 2JCP = 35.5 Hz, CO), 30.5, 30.2, 29.9 (overlapping multiplets, 3PCH2CH2CH2), 28.0–26.8 (overlapping signals, CH2), 24.1, 23.7, 23.5 (overlapping multiplets, 3PCH2CH2); 31P{1H} NMR (202 MHz): 41.6 (s).
IR (powder film, cm−1): 3076 (w), 2926 (w), 2853 (w), 1911 (s, νCO), 1691 (s, νNO), 1459 (w), 1409 (w), 1262 (w), 1089 (w), 1019 (w), 799 (w), 756 (w), 718 (w). MS:37 892 (9c-I+, 5%), 864 ([9c-I − CO]+, 25%), 834 ([9c-I − CO − NO]+, 100%), 767 ([9c-I − I]+, 5%), 737 ([9c-I − NO − I]+, 25%), 684 ([9c-I − CO − NO − I − Fe + 2O]+, 100%), 668 ([9c-I − CO − NO − I − Fe + O]+, 15%), 652 ([9c-I − CO − NO − I − Fe]+, 10%).
NMR (CDCl3, δ in ppm): 1H NMR (500 MHz): 2.11–1.97 (m, 12H, CH2), 1.86–1.74 (m, 12H, CH2), 1.61–1.48 (m, 60H, CH2); 13C{1H} NMR (126 MHz): 218.3 (t, 2JCP = 30.1 Hz, CO), 140.7 (t, 2JCP = 40.6 Hz, CN), 30.7 (virtual t, 3JCP, 5JCP′ = 6.9 Hz,26 PCH2CH2CH2), 29.3 (virtual t, 1JCP, 3JCP′ = 13.5 Hz,26 PCH2), 27.9 (s, CH2), 27.8 (s, CH2), 27.0 (s, CH2), 26.7 (s, CH2), 23.8 (s, CH2); 31P{1H} NMR (202 MHz): 58.2 (s).
IR (powder film, cm−1): 2924 (w), 2852 (w), 2098 (w, νCN), 1917 (s, νCO), 1703 (s, νNO), 1458 (w), 1307 (w), 1238 (w), 1089 (w), 985 (w), 759 (w), 736 (w), 721 (w), 619 (s). MS:37 791 (9c-CN+, 15%), 762 ([9c-CN − CO]+, 55%), 737 ([9c-CN − CO − CN]+, 35%), 732 ([9c-CN − NO − CO]+, 100%), 704 ([9c-CN − CO − NO − CN ]+, 5%).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) CH(CH2)6)3P) (7c-Cl).
A Schlenk flask was charged with trans-Fe(CO)(NO)(Cl)(P((CH2)6CH
CH(CH2)6)3P) (7c-Cl).
A Schlenk flask was charged with trans-Fe(CO)(NO)(Cl)(P((CH2)6CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)3)2, (6c-Cl;21 0.127 g, 0.145 mmol), CH2Cl2 (75 mL; the resulting solution is 0.0020 M in 6c-Cl), and Grubbs’ catalyst (0.022 g, 0.026 mmol, 18 mol%), and fitted with a condenser. The solution was refluxed. After 48 h, the solvent was removed by oil pump vacuum. The residue was filtered through neutral alumina (2.5 × 2.5 cm) using 2
CH2)3)2, (6c-Cl;21 0.127 g, 0.145 mmol), CH2Cl2 (75 mL; the resulting solution is 0.0020 M in 6c-Cl), and Grubbs’ catalyst (0.022 g, 0.026 mmol, 18 mol%), and fitted with a condenser. The solution was refluxed. After 48 h, the solvent was removed by oil pump vacuum. The residue was filtered through neutral alumina (2.5 × 2.5 cm) using 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v hexane/CH2Cl2. The solvent was removed from the filtrate by oil pump vacuum to give 7c-Cl as a red-brown waxy solid (0.046 g, 0.054 mmol, 37%, mixture of E/Z isomers).
1 v/v hexane/CH2Cl2. The solvent was removed from the filtrate by oil pump vacuum to give 7c-Cl as a red-brown waxy solid (0.046 g, 0.054 mmol, 37%, mixture of E/Z isomers).
NMR (CDCl3, δ in ppm): 1H (500 MHz) 5.48–5.20 (m, 6H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 2.04–1.93 (m, 12H, CH2), 1.88–1.64 (m, 12H, CH2), 1.62–1.47 (m, 12H, CH2), 1.41–1.28 (m, 36H, CH2); 31P{1H} (202 MHz) 50.4 (s, 77%), 49.8 (s, 23%).
), 2.04–1.93 (m, 12H, CH2), 1.88–1.64 (m, 12H, CH2), 1.62–1.47 (m, 12H, CH2), 1.41–1.28 (m, 36H, CH2); 31P{1H} (202 MHz) 50.4 (s, 77%), 49.8 (s, 23%).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) CH(CH2)6)3P) (7c-Br).
A Schlenk flask was charged with trans-Fe(CO)(NO)(Br)(P((CH2)6CH
CH(CH2)6)3P) (7c-Br).
A Schlenk flask was charged with trans-Fe(CO)(NO)(Br)(P((CH2)6CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)3)2, (6c-Br;21 0.157 g, 0.170 mmol), CH2Cl2 (85 mL; the resulting solution is 0.0020 M in 6c-Br), and Grubbs’ catalyst (0.025 g, 0.031 mmol, 18 mol%), and fitted with a condenser. The solution was refluxed. After 48 h, the solvent was removed by oil pump vacuum. The residue was filtered through neutral alumina (2.5 × 2.5 cm) using 1
CH2)3)2, (6c-Br;21 0.157 g, 0.170 mmol), CH2Cl2 (85 mL; the resulting solution is 0.0020 M in 6c-Br), and Grubbs’ catalyst (0.025 g, 0.031 mmol, 18 mol%), and fitted with a condenser. The solution was refluxed. After 48 h, the solvent was removed by oil pump vacuum. The residue was filtered through neutral alumina (2.5 × 2.5 cm) using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v hexane/CH2Cl2. The solvent was removed from the filtrate by oil pump vacuum to give 7c-Br as a red-brown waxy solid (0.047 g, 0.053 mmol, 31%, mixture of E/Z isomers).
1 v/v hexane/CH2Cl2. The solvent was removed from the filtrate by oil pump vacuum to give 7c-Br as a red-brown waxy solid (0.047 g, 0.053 mmol, 31%, mixture of E/Z isomers).
NMR (CDCl3, δ in ppm): 1H (500 MHz) 5.43–5.15 (m, 6H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 2.10–1.90 (m, 12H, CH2), 1.91–1.66 (m, 12H, CH2), 1.64–1.45 (m, 12H, CH2), 1.42–1.27 (m, 36H, CH2); 31P{1H} (202 MHz) 50.4 (s, 63%), 49.8 (s, 15%), 47.8 (s, 22%).
), 2.10–1.90 (m, 12H, CH2), 1.91–1.66 (m, 12H, CH2), 1.64–1.45 (m, 12H, CH2), 1.42–1.27 (m, 36H, CH2); 31P{1H} (202 MHz) 50.4 (s, 63%), 49.8 (s, 15%), 47.8 (s, 22%).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) CH(CH2)6)3P) (7c-I).
A Schlenk flask was charged with trans-Fe(CO)(NO)(I)(P((CH2)6CH
CH(CH2)6)3P) (7c-I).
A Schlenk flask was charged with trans-Fe(CO)(NO)(I)(P((CH2)6CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)3)2, (6c-I;21 0.232 g, 0.239 mmol), CH2Cl2 (120 mL; the resulting solution is 0.0020 M in 6c-I), and Grubbs’ catalyst (0.035 g, 0.043 mmol, 18 mol%), and fitted with a condenser. The solution was refluxed. After 19 h, additional catalyst was added (0.012 g, 0.014 mmol, 6 mol%). After another 24 h, the solvent was removed by oil pump vacuum. The residue was filtered through neutral alumina (2.5 × 2.5 cm) using CH2Cl2. The solvent was removed from the filtrate by oil pump vacuum to give 7c-I as red-brown waxy solid (0.091 g, 0.096 mmol, 40%, mixture of E/Z isomers).
CH2)3)2, (6c-I;21 0.232 g, 0.239 mmol), CH2Cl2 (120 mL; the resulting solution is 0.0020 M in 6c-I), and Grubbs’ catalyst (0.035 g, 0.043 mmol, 18 mol%), and fitted with a condenser. The solution was refluxed. After 19 h, additional catalyst was added (0.012 g, 0.014 mmol, 6 mol%). After another 24 h, the solvent was removed by oil pump vacuum. The residue was filtered through neutral alumina (2.5 × 2.5 cm) using CH2Cl2. The solvent was removed from the filtrate by oil pump vacuum to give 7c-I as red-brown waxy solid (0.091 g, 0.096 mmol, 40%, mixture of E/Z isomers).
NMR (CDCl3, δ in ppm): 1H (500 MHz) 5.47–5.20 (m, 6H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 2.12–1.47 (m, 36H, CH2), 1.46–1.28 (m, 36H, CH2); 31P{1H} (202 MHz) 44.9 (s, 63%), 42.0 (s, 15%).
), 2.12–1.47 (m, 36H, CH2), 1.46–1.28 (m, 36H, CH2); 31P{1H} (202 MHz) 44.9 (s, 63%), 42.0 (s, 15%).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) CH(CH2)6)3P) (7c-CN).
A Schlenk flask was charged with trans-Fe(CO)(NO)(CN)(P((CH2)6CH
CH(CH2)6)3P) (7c-CN).
A Schlenk flask was charged with trans-Fe(CO)(NO)(CN)(P((CH2)6CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)3)2, (6c-CN;21 0.162 g, 0.187 mmol), CH2Cl2 (95 mL; the resulting solution is 0.0020 M in 6c-CN), and Grubbs’ catalyst (0.028 g, 0.034 mmol, 18 mol%), and fitted with a condenser. The solution was refluxed. After 48 h, the solvent was removed by oil pump vacuum. The residue was filtered through neutral alumina (2.5 × 2.5 cm) with CH2Cl2. The solvent was removed from the filtrate by oil pump vacuum to give 7c-CN as a red-brown waxy solid (0.038 g, 0.045 mmol, 24%, mixture of E/Z isomers).
CH2)3)2, (6c-CN;21 0.162 g, 0.187 mmol), CH2Cl2 (95 mL; the resulting solution is 0.0020 M in 6c-CN), and Grubbs’ catalyst (0.028 g, 0.034 mmol, 18 mol%), and fitted with a condenser. The solution was refluxed. After 48 h, the solvent was removed by oil pump vacuum. The residue was filtered through neutral alumina (2.5 × 2.5 cm) with CH2Cl2. The solvent was removed from the filtrate by oil pump vacuum to give 7c-CN as a red-brown waxy solid (0.038 g, 0.045 mmol, 24%, mixture of E/Z isomers).
NMR (CDCl3, δ in ppm): 1H (500 MHz) 5.41–5.17 (m, 6H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 2.08–1.90 (m, 12H, CH2), 1.89–1.60 (m, 12H, CH2), 1.61–1.46 (m, 12H, CH2) 1.46–1.26 (m, 36H, CH2); 31P{1H} (202 MHz) 60.2 (s, 75%), 59.6 (s, 15%), 59.4 (s, 22%).
), 2.08–1.90 (m, 12H, CH2), 1.89–1.60 (m, 12H, CH2), 1.61–1.46 (m, 12H, CH2) 1.46–1.26 (m, 36H, CH2); 31P{1H} (202 MHz) 60.2 (s, 75%), 59.6 (s, 15%), 59.4 (s, 22%).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) CH(CH2)6)3P)]+ BF4− (8c+ BF4−).
A Schlenk flask was charged with trans-[Fe(CO)2(NO)(P((CH2)6CH
CH(CH2)6)3P)]+ BF4− (8c+ BF4−).
A Schlenk flask was charged with trans-[Fe(CO)2(NO)(P((CH2)6CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)3)2]+ BF4− (5c+ BF4−;21 0.151 g, 0.159 mmol), CH2Cl2 (80 mL; the resulting solution is 0.0020 M in 5c+ BF4−), and Grubbs’ catalyst (0.024 g, 0.029 mmol, 18 mol%), and fitted with a condenser. The solution was refluxed. After 48 h, the solvent was removed by oil pump vacuum. The residue was filtered through celite (2.5 × 2.5 cm) with CH2Cl2. The solvent was removed from the filtrate by oil pump vacuum and the residue was triturated under ether. The ether was decanted and the residue dried by oil pump vacuum to give 8c+ BF4− as a red-brown waxy solid (0.083 g, 0.091 mmol, 57%, mixture of E/Z isomers).
CH2)3)2]+ BF4− (5c+ BF4−;21 0.151 g, 0.159 mmol), CH2Cl2 (80 mL; the resulting solution is 0.0020 M in 5c+ BF4−), and Grubbs’ catalyst (0.024 g, 0.029 mmol, 18 mol%), and fitted with a condenser. The solution was refluxed. After 48 h, the solvent was removed by oil pump vacuum. The residue was filtered through celite (2.5 × 2.5 cm) with CH2Cl2. The solvent was removed from the filtrate by oil pump vacuum and the residue was triturated under ether. The ether was decanted and the residue dried by oil pump vacuum to give 8c+ BF4− as a red-brown waxy solid (0.083 g, 0.091 mmol, 57%, mixture of E/Z isomers).
NMR (CDCl3, δ in ppm): 1H (500 MHz) 5.48–5.11 (m, 6H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 2.36–2.13 (m, 12H, CH2), 2.09–1.80 (m, 12H, CH2), 1.70–1.25 (m, 48H, CH2); 31P{1H} (202 MHz) 60.9 (s, 82%), 60.1 (s, 18%).
), 2.36–2.13 (m, 12H, CH2), 2.09–1.80 (m, 12H, CH2), 1.70–1.25 (m, 48H, CH2); 31P{1H} (202 MHz) 60.9 (s, 82%), 60.1 (s, 18%).
Crystallography
A concentrated CH2Cl2 solution of 9c-Cl was layered with MeOH and kept at room temperature. After 4 d, data were collected on one of the orange prisms as outlined in Table 2. Analogous procedures gave deep red prisms of 9c-Br and pale orange prisms of 9c-CN, which were similarly analyzed.Cell parameters were obtained from 10 frames using a 10° scan and refined with the following numbers of reflections: 9c-Cl, 5268; 9c-Br, 280; 9c-CN, 5177. Lorentz, polarization, and absorption corrections were applied.38 Space groups were determined from systematic absences and subsequent least-squares refinement. The structures were solved by direct methods. The parameters were refined with all data by full-matrix-least-squares on F2 using SHELXL-97.39 Non-hydrogen atoms were refined with anisotropic thermal parameters. The hydrogen atoms were fixed in idealized positions using a riding model. Scattering factors were taken from the literature.40 In the structure of 9c-Cl, the Cl, NO, and CO ligands were disordered over three positions (33% occupancy). For refinement, the CO and NO positions were arbitrarily assigned in accord with the overall symmetry. In the structure of 9c-Br, the bromine atom was disordered over three positions (33% occupancy). The NO and CO ligands were also disordered over three positions, and furthermore could not be distinguished. For refinement, they were assigned 50% occupancy within each of the three conformations. In the structure of 9c-CN, the CN, NO, and CO ligands were equally disordered over three positions. Refinements were carried out with fixed occupancy factors for each position (2/3 C + 1/3 N for the ligating atom and 2/3 O + 1/3 N for the non-ligating atom).
Acknowledgements
The authors thank the US National Science Foundation (CHE-0719267, CHE-1153085, CHE-156601) and Deutsche Forschungsgemeinschaft (DFG, GL 300/9-1) for support, and Mr. Andreas Ehnbom for preliminary dipole moment computations.Notes and references
- See http://www.gyroscopes.org/uses.asp. The authors are unaware of any books or peer reviewed articles that match the diversity of examples on the gyroscope.org web site, which is maintained by G. Turner.
- (a) T. Shima, F. Hampel and J. A. Gladysz, Angew. Chem., Int. Ed., 2004, 43, 5537–5540 ( Angew. Chem. , 2004 , 116 , 5653–5656 ) CrossRef CAS PubMed; (b) G. M. Lang, T. Shima, L. Wang, K. J. Cluff, F. Hampel, J. Blumel and J. A. Gladysz, J. Am. Chem. Soc., 2016, 138, 7649–7663 CrossRef CAS PubMed.
- (a) A. J. Nawara, T. Shima, F. Hampel and J. A. Gladysz, J. Am. Chem. Soc., 2006, 128, 4962–4963 CrossRef CAS PubMed; (b) A. J. Nawara-Hultzsch, M. Stollenz, M. Barbasiewicz, S. Szafert, T. Lis, F. Hampel, N. Bhuvanesh and J. A. Gladysz, Chem. – Eur. J., 2014, 20, 4617–4637 CrossRef CAS PubMed; (c) D. Taher, A. J. Nawara-Hultzsch, N. Bhuvanesh, F. Hampel and J. A. Gladysz, J. Organomet. Chem., 2016 DOI:10.1016/j.jorganchem.2016.03.022 , in press (available on the web).
- L. Wang, F. Hampel and J. A. Gladysz, Angew. Chem., Int. Ed., 2006, 45, 4372–4375 ( Angew. Chem. , 2006 , 118 , 4479–4482 ) CrossRef CAS PubMed.
- (a) L. Wang, T. Shima, F. Hampel and J. A. Gladysz, Chem. Commun., 2006, 4075–4077 RSC; (b) A. L. Estrada, T. Jia, N. Bhuvanesh, J. Blümel and J. A. Gladysz, Eur. J. Inorg. Chem., 2015, 5318–5321 CrossRef CAS.
- G. D. Heß, F. Hampel and J. A. Gladysz, Organometallics, 2007, 26, 5129–5131 CrossRef.
- K. Skopek and J. A. Gladysz, J. Organomet. Chem., 2008, 693, 857–866 CrossRef CAS.
- (a) T. Fiedler, N. Bhuvanesh, F. Hampel, J. H. Reibenspies and J. A. Gladysz, Dalton Trans., 2016, 45, 7131–7147 RSC; (b) T. Fiedler, L. Chen, N. D. Wagner, D. R. Russell and J. A. Gladysz, Organometallics, 2016, 35, 2071–2075 CrossRef CAS.
- G. M. Lang, N. Bhuvanesh, J. H. Reibenspies and J. A. Gladysz, Organometallics, 2016, 35, 2873–2889 CrossRef CAS.
- http://www.unisys.com/about-us/company-history .
- http://findingaids.hagley.org/xtf/view?docId=ead/1915.xml .
- (a) T.-A. V. Khuong, J. E. Nuñez, C. E. Godinez and M. A. Garcia-Garibay, Acc. Chem. Res., 2006, 39, 413–422 CrossRef CAS PubMed; (b) C. S. Vogelsberg and M. A. Garcia-Garibay, Chem. Soc. Rev., 2012, 41, 1892–1910 RSC; (c) S. Pérez-Estrada, B. Rodríguez-Molina, L. Xiao, R. Santillan, G. Jiménez-Osés, K. N. Houk and M. A. Garcia-Garibay, J. Am. Chem. Soc., 2015, 137, 2175–2178 CrossRef PubMed; (d) R. Acros-Ramos, B. Rodríguez-Molina, E. Gonzalez-Rodriguez, P. I. Ramirez-Montes, M. E. Ochoa, R. Santillan, N. Farfán and M. A. Garcia-Garibay, RSC Adv., 2015, 5, 55201–55208 RSC; (e) X. Jiang, Z. J. O'Brien, S. Yang, L. H. Lai, J. Buenaflor, C. Tan, S. Khan, K. N. Houk and M. A. Garcia-Garibay, J. Am. Chem. Soc., 2016, 138, 4650–4656 CrossRef CAS PubMed.
- (a) W. Setaka and K. Yamaguchi, J. Am. Chem. Soc., 2013, 135, 14560–14563 CrossRef CAS PubMed and earlier work cited therein. (b) W. Setaka, K. Inoue, S. Higa, S. Yoshigai, H. Kono and K. Yamaguchi, J. Org. Chem., 2014, 79, 8288–8295 CrossRef CAS PubMed; (c) H. Shionari, Y. Inagaki, K. Yamaguchi and W. Setaka, Org. Biomol. Chem., 2015, 13, 10511–10516 RSC; (d) Y. Nishiyama, Y. Inagaki, K. Yamaguchi and W. Setaka, J. Org. Chem., 2015, 80, 9959–9966 CrossRef CAS PubMed.
- T. Fiedler and J. A. Gladysz, Multifold Ring Closing Olefin Metatheses in Syntheses of Organometallic Molecules with Unusual Connectivities, in Olefin Metathesis: Theory and Practice, ed. K. Grela, Wiley/VCH, Weinheim, 2013, pp. 311–328 Search PubMed.
- See also: E. Prack, C. A. O'Keefe, J. K. Moore, A. Lai, A. J. Lough, P. M. Macdonald, M. S. Conradi, R. W. Schurko and U. Fekl, J. Am. Chem. Soc., 2015, 137, 13464–13467 CrossRef CAS PubMed.
- Since fictional characters can often be useful in outreach lectures, we note that the presence of gyro stabilizers in Iron Man's flying suit was first described in Iron Man, April 1976, Vol. 1, #85 (see https://www.quora.com/How-do-Tony-Starks-Iron-Man-suits-generate-lift-for-horizontal-flight). Were Tony Stark a real person, he would have assuredly wanted his gyroscopes to be molecular and based upon iron.
- (a) E. Butikov, Eur. J. Phys., 2006, 27, 1071–1081 CrossRef; (b) D. Kleppner and R. J. Kolenkow, An Introduction to Mechanics, Cambridge University Press, Cambridge, 2010, ch. 7.3–7.5 Search PubMed.
- G. S. Kottas, L. I. Clarke, D. Horinek and J. Michl, Chem. Rev., 2005, 105, 1281–1376 CrossRef CAS PubMed.
- (a) V. Bermudez, N. Capron, T. Gase, F. G. Gatti, F. Kajzar, D. A. Leigh, F. Zerbetto and S. Zhang, Nature, 2000, 406, 608–611 CrossRef CAS PubMed; (b) R. D. Horansky, L. I. Clarke, J. C. Price, T.-A. V. Khuong, P. D. Jarowski and M. A. Garcia-Garibay, Phys. Rev. B: Condens. Matter, 2005, 72, 014302 CrossRef; (c) R. D. Horansky, L. I. Clarke, E. B. Winston, J. C. Price, S. D. Karlen, P. D. Jarowski, R. Santillan and M. A. Garcia-Garibay, Phys. Rev. B: Condens. Matter, 2006, 74, 054306 CrossRef; (d) P. Dhar, C. D. Swayne, T. M. Fischer, T. Kline and A. Sen, Nano Lett., 2007, 7, 1010–1012 CrossRef CAS PubMed; (e) J. J. Arcenegui, P. García-Sánchez, H. Morgan and A. Ramos, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2013, 88, 033025-1–033025-8 Search PubMed; (f) I. Neumann, K. E. Gottschalk and R. D. Astumian, ACS Nano, 2012, 6, 5242–5248 CrossRef PubMed.
- (a) Z. Dominguez, T.-A. Khuong, H. Dang, C. N. Sanrame, J. E. Nuñez and M. A. Garcia-Garibay, J. Am. Chem. Soc., 2003, 125, 8827–8837 CrossRef CAS PubMed; (b) T.-A. Khuong, G. Zepeda, R. Ruiz, S. I. Khan and M. A. Garcia-Garibay, Cryst. Growth Des., 2004, 4, 15–18 CrossRef CAS; (c) B. Rodriguez-Molina, M. E. Ochoa, N. Farfán, R. Santillan and M. A. Garcia-Garibay, J. Org. Chem., 2009, 74, 8554–8565 CrossRef CAS PubMed.
- G. M. Lang, D. Skaper, T. Shima, M. Otto, L. Wang and J. A. Gladysz, Aust. J. Chem., 2015, 68, 1342–1351 CrossRef CAS.
- G. Dolcetti, L. Busetto and A. Palazzi, Inorg. Chem., 1974, 13, 222–225 CrossRef CAS.
- W. E. Carroll, A. F. Deeney and F. J. Lalor, J. Chem. Soc., Dalton Trans., 1974, 1430–1433 RSC.
- (a) M. Jänicke, H.-U. Hund and H. Berke, Chem. Ber., 1991, 124, 719–723 CrossRef; (b) In Tables 3 and 4 in this paper, the labels a and c (denoting the phosphorus donor ligand) for compounds 5 and 7 are reversed.
- See also: J. L. A. Roustan, J. Y. Merour and A. Forgues, J. Organomet. Chem., 1980, 186, C23–C26 CrossRef CAS.
- W. H. Hersh, J. Chem. Educ., 1997, 74, 1485–1488 CrossRef CAS . The J values given represent the apparent coupling between adjacent peaks of the triplet.
- Selected spectra are provided elsewhere: G. M. Lang, Doctoral thesis, Texas A&M University, 2016, ch. 5.
- (a) B. Cordero, V. Gómez, A. E. Platero-Prats, M. Réves, J. Echeverría, E. Cremades, F. Barragán and S. Alvarez, Dalton Trans., 2008, 2832 RSC; (b) A. Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS; (c) M. Mantina, A. C. Chamberlin, R. Valero, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. A, 2009, 113, 5806–5812 CrossRef CAS PubMed.
- (a) Fe(CO)2(I)(PEt3)2: Fe–I 2.610(1) Å. H. Kandler, C. Gauss, W. Bidell, S. Rosenberger, T. Bürgi, I. L. Eremenko, D. Veghini, O. Orama, P. Burger and H. Berke, Chem. – Eur. J., 1995, 1, 541–548 CrossRef CAS; (b) Fe(CO)2(I)(Me)(P(Me)3)2: Fe–I 2.695(1) Å. G. Bellachioma, G. Cardaci, A. Macchioni, G. Reichenbach, E. Foresti and P. Sabatino, J. Organomet. Chem., 1997, 531, 227–235 CrossRef CAS; (c) Fe(CO)2(I)(Ph)(P(Me)3)2: Fe–I 2.61(3) Å. C. Venturi, G. Bellachioma, G. Cardaci and A. Macchioni, Inorg. Chim. Acta, 2004, 357, 3712–3720 CrossRef CAS; (d) The average of all of the FeCO distances in the preceding compounds is 4.43 Å.
- In evaluating the viability of dynamic processes in organic molecules from space filling models, it is generally found that the spatial contributions of the hydrogen atoms can be neglected.
- Given the fluxionality of the methylene chains in solution, another approximation would be to take the average of the six distances.
- P. H. M. Budzelaar, gNMR: NMR Simulation Program, Version 5.0.6.0, Adept Scientific, Luton, UK, 2006 Search PubMed.
- B. L. Hawkins, W. Bremser, S. Borcić and J. D. Roberts, J. Am. Chem. Soc., 1971, 93, 4472–4479 CrossRef.
- A reviewer has inquired about the magnitude of the dipole moments, and interactions in the solid state. First, preliminary DFT calculations on the model compounds trans-Fe(CO)(NO)(X)(PMe3)2, indicate values of 1.38, 2.00, and 2.68 D for X = Cl, Br, and CN, respectively. The dipoles approximately bisect the OC–Fe–NO angles, with the negative ends extending between the two oxygen atoms. Second, there is an extensive literature regarding dipolar interactions between rotators in crystals and ordered arrays.18 For another lead reference, see: J. J. de Jonge, M. A. Ratner and S. W. de Leeuw, J. Phys. Chem. C, 2007, 111, 3770–3777 CAS.
- H. J. Breunig, T. Borrmann, E. Lork, O. Moldovan, C. I. Rat and R. P. Wagner, J. Organomet. Chem., 2009, 694, 427–432 CrossRef CAS.
- A reviewer has inquired whether the activation parameters determined for rotation of trivalent iron rotators in solution (Table 4) have any relationship to those in the crystal lattice. Naturally, different values would be expected. However, considering the homology of many of the crystal structures, it is possible that they might scale in a linear relationship.
- FAB, 3-NBA, m/z (relative intensity, %); the most intense peak of the isotope envelope is given.
- (a) B. V. Nonius, Collect, data collection software, 1998 Search PubMed; (b) Z. Otwinowski and W. Minor, Scalepack, data processing software, Methods Enzymol., 1997, 276, 307 CAS (Macromolecular Crystallography, Part A).
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- D. T. Cromer and J. T. Waber, in International Tables for X-ray Crystallography, ed. J. A. Ibers and W. C. Hamilton, Kynoch, Birmingham, England, 1974 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional synthetic procedures, NMR data and Eyring plots. CCDC 1499242–1499244. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt03258c |
| This journal is © The Royal Society of Chemistry 2016 |