 Open Access Article
Open Access ArticleSelf-assembly of highly luminescent heteronuclear coordination cages†
Andrea
Schmidt
ab,
Manuela
Hollering
a,
Jiaying
Han
c,
Angela
Casini
*bcd and
Fritz E.
Kühn
*a
aMolecular Catalysis, Catalysis Research Center and Department of Chemistry, Technische Universität München, Lichtenbergstr. 4, 85747 Garching bei München, Germany. E-mail: fritz.kuehn@ch.tum.de
bMedicinal and Bioinorganic Chemistry, School of Chemistry, Cardiff University, Park Place, CF103AT Cardiff, UK. E-mail: casinia@cardiff.ac.uk
cGroningen Research Institute of Pharmacy, University of Groningen, Antonius Deusinglaan 1, 9713 AV Groningen, The Netherlands
dInstitute of Advanced Study, Technische Universität München, Lichtenbergstr. 2a, 85748 Garching, Germany
First published on 12th July 2016
Abstract
Exo-functionalized Pd2L4 cage compounds with attached Ru(II) pyridine complexes were prepared via coordination-driven self-assembly. Unlike most of the previously reported palladium(II) cages, one of these metallocages exhibits an exceptionally high quantum yield of 66%. The presented approach is promising to obtain luminescent coordination complexes for various applications.
Metal-mediated self-assembly is a useful tool to design discrete two- and three-dimensional supramolecular coordination complexes (SCCs) with precise geometries and cavities.1 These metal-based entities have attracted much attention for a variety of applications in molecular recognition,2 catalysis3 and medicinal applications4 due to their interesting chemical–physical properties and guest-binding abilities. Especially, the development of luminescent SCCs for potential applications in chemosensing,5 material science6,7 and biological imaging8,9 has gained increasing attention during the last years,10 although it is still less explored. Despite the existence of some highly fluorescent coordination complexes,6,11 the majority of metal-based self-assemblies are little- or non-emissive due to the quenching effect of heavy metal ions.12
An interesting research field of SCCs is the self-assembly of M2L4 (M = metal, L = ligand) cages because of their simple and highly symmetric structures.13 In addition, the cages’ properties can be easily altered by functionalizing the ligand framework.14 Emissive properties of M2L4 metallocages have been discussed, yet examples of highly emissive Pd2L4 cages are rare.15 The incorporation of luminescent groups, such as anthracene16,17 and ruthenium pyridine complexes,18 into the ligand framework resulted in palladium cages displaying low emission so far.
Nevertheless, these results generate an increasing interest in tailored design of highly luminescent coordination cages. In this work, an approach is presented to increase the photo-physical properties of palladium cages by separating the luminescent tag from the emissive ligand coordinated to palladium ions. Inspired by previous investigations,17 two Pd2L4 cage compounds ligated by bis(pyridyl) systems coupled to ruthenium complexes were synthesized and their photo-physical properties were investigated. A comparison is made between the Ru terpyridine ligand L1 having no spacer and the ruthenium bipyridine ligand L2 featuring an alkyl bridge as spacer between two emissive moieties.
First, the rigid bis(pyridyl) ligands L1 and L2 coupled to Ru(II) terpyridine and Ru(II) bipyridine, respectively, were synthesized via an amide bond formation (Scheme 1).
 | ||
| Scheme 1 Coupling of the ligand L-NH2 with Ru(II) complexes R1/R2 using the reagent CMPI, followed by precipitation with NaBF4 to obtain Ru(II)-based ligands L1/L2. | ||
The amine-based ligand L-NH2 was coupled to [Ru(terpy)(terpy-4-COOH)](PF6)2R1 and [Ru(bipy)2(bipy-4′-CH3-4-(CH2)2-COOH)](PF6)2R2 using the coupling reagent 2-chloro-1-methylpyridinium iodide (CMPI) and DMAP as a base. After purification by column chromatography, the Ru(II) complexes L1 and L2 were precipitated by NaBF4 in 68% yield as red solid and in 56% yield as orange solid, respectively. The complexes were characterized by 1H, 13C, 11B, 19F, and DOSY NMR spectroscopy, ESI-MS and X-ray crystallography (for details see ESI†).
The coordination cages C1/C2 were self-assembled by mixing the bidentate Ru(II)-based ligands L1/L2 and the palladium precursor [Pd(NCCH3)4](BF4)2 in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand
1 ligand![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal ratio in DMSO at room temperature for one hour (Scheme 2). Additionally, the self-assembly of the previously described cage C-NH2
metal ratio in DMSO at room temperature for one hour (Scheme 2). Additionally, the self-assembly of the previously described cage C-NH2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 is depicted in Scheme 2, in order to evaluate the synthesis and photo-physical properties of the cage compounds C1 and C2 compared to the amine-based cage. Notably, the bulky ruthenium complexes have no effect on the self-assembly reaction.
8 is depicted in Scheme 2, in order to evaluate the synthesis and photo-physical properties of the cage compounds C1 and C2 compared to the amine-based cage. Notably, the bulky ruthenium complexes have no effect on the self-assembly reaction.
 | ||
| Scheme 2 Synthesis of the palladium(II) cages C-NH2,8C1 and C2via self-assembly using the bidentate ligands L-NH2, L1 and L2 and the precursor [Pd(NCCH3)4](BF4)2. | ||
1H NMR spectroscopy confirms the formation of the cage compounds. In 1H NMR spectra (Fig. 1), the pyridyl protons Ha–Hd are significantly downfield shifted, particularly the signals of Ha and Hb experienced a shift of ca. 0.9 ppm. The terpyridine and bipyridine proton resonances of the attached ruthenium complexes are not influenced by the Pd–N coordination.
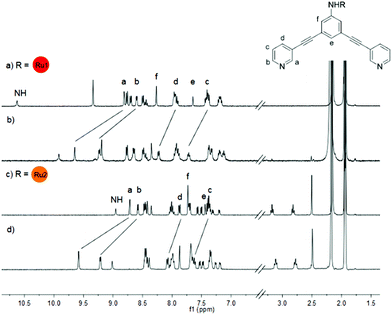 | ||
| Fig. 1 Stacked 1H NMR spectra (400 MHz, CD3CN) of ligand L1 (a), cage C1 (b), ligand L2 (c) and cage C2 (d). | ||
Additional proof of the successful self-assembly in solution is given by diffusion-disordered NMR spectroscopy (DOSY), since all proton signals of the cages reveal the same diffusion coefficient. The diffusion coefficients (D) of the ligands L1 and L2 and of the cages C1 and C2 in acetonitrile are approximately 6.9 × 10−10 m2 s−1 and 3.3 × 10−10 m2 s−1, respectively (see Table S1, ESI†). Thus, the ratios of Dligand/Dcage are approximately 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, being in accordance with reported Pd2L4 systems.8,19 The hydrodynamic radii rs of C1 and C2 have been calculated to be 1.5 nm.
1, being in accordance with reported Pd2L4 systems.8,19 The hydrodynamic radii rs of C1 and C2 have been calculated to be 1.5 nm.
The molecular composition of the Pd2L4 cages C1 and C2 is further evidenced by ESI mass spectrometry showing isotopically resolved peaks for [C-nBF4−]n+ (n = 4–6). For example, the ESI-MS analysis of cage C2 reveals peaks at m/z = 744.3, 910.6 and 1160.3, which can be assigned to [C2-6BF4−]6+, [C2-5BF4−]5+ and [C2-4BF4−]4+, respectively.
In order to predict the shape and size of the cages, a geometry optimization was performed using semi-empirical methods (PM6). Exemplarily, the molecular model of C2 is depicted in Fig. 2. The optimized structure of C2 exhibit a Pd⋯Pd distance of 1.1 nm, a distance between the opposing inner C-atoms of 1.2 nm and a span of 5.0 nm. The calculated shape and size is in agreement with previously reported Pd2L4 cages.8,17 Suitable single crystals of the metallocages for X-ray diffraction could not be obtained.
Both palladium(II) cages are stable under air and light in solution and in solid state. The compounds are soluble in acetonitrile, DMF and DMSO.
In order to assess the photo-physical properties of the metallocages with attached ruthenium(II) moieties, UV-Vis, excitation and emission spectroscopy were carried out on the Ru(II) complexes R1/R2, the ligands L1/L2/L-NH2 and the cages C1/C2/C-NH2. The absorption and emission spectra of the compounds are depicted in Fig. 3 and 4, while the photo-physical parameters are presented in Table 1.
 | ||
| Fig. 3 UV-Vis spectra of ligands and cage compounds in DMSO (c = 10−5–10−6 M). Insets: Photographs of DMSO solutions of the cages. | ||
 | ||
| Fig. 4 Emission spectra of ligands and cage compounds in DMSO (c = 10−5 M, λex = 260 nm). Insets: Photographs of solutions of the cages in DMSO under UV light irradiation (λex = 365 nm). | ||
| Compound | λ max (abs) [nm] | ε max [L mol−1 cm−1] | λ max (em) [nm] | Φ [%] |
|---|---|---|---|---|
| R1 | 278, 317, 492 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
— | — |
| R2 | 292, 456 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
645 | 12 |
| L-NH2 | 293, 305, 360 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
430 | 52 |
| C-NH2 | 293, 371 | 152![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
435 | 17 |
| L1 | 290, 303, 493 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
— | — |
| C1 | 289, 311, 494 | 232![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
— | — |
| L2 | 293, 461 | 134![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
640 | 88 |
| C2 | 293, 462 | 523![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
640 | 66 |
The absorption spectra of the metallocages are dominated by strong π–π* transitions of the highly conjugated ligands showing bands in the range of 250–350 nm. The UV-Vis spectra of the cages with conjugated ruthenium complexes exhibit an additional band in the vis region, C1 (red solution) at 495 nm and C2 (orange solution) at 460 nm. Overall, the cage compounds feature an approximately four-times higher extinction coefficient compared to their corresponding ligands resulting from the M2L4 composition.
The metallocages reveal interesting emissive properties, showing that the luminescence can be increased or decreased by altering the molecular structure of the ligand framework. Recently, we investigated the photo-physical properties of bis(pyridyl) ligands coupled to naphthalene and anthracene moieties via an amide bond.17 These systems possess less emissive properties due to a disruption of the chromophoric system in the excited state by bending the amide bond.
As expected, ligand L1 and the respective cage C1 are not luminescent, although the amine ligand L-NH2 is highly emissive by itself. Notably, the red solution of R1 is not luminescent at room temperature being in accordance with reports on similar ruthenium(II) terpyridine complexes.20
To avoid the predicted torsion of the amide bond, a spacer, namely an alkyl bridge, was inserted between the bis(pyridyl) ligand and the ruthenium moiety. Upon irradiation at 260 nm, ligand L2 emits strong orange luminescence showing a broad band in the emission spectrum at λmax = 640 nm with an exceptional high quantum yield of 88%. However, by irradiation at lower energies at 460 or 495 nm the quantum yield is significantly reduced to 6 and 4%, respectively. The amine ligand L-NH2 shows blue fluorescence at λmax = 430 nm with a quantum yield of 52%. Interestingly, cage C2 exhibits one of the highest quantum yields (Φ = 66%) at λmax = 640 nm reported for supramolecular coordination complexes.6,11a,b The coordination cage C-NH2 features a fluorescence quantum yield of 17%. In agreement with previous reports, in both cases C2 and C-NH2 the luminescence is significantly reduced by coordination of the ligand to palladium ions. Notably, cage C2 displays a higher emission compared to the amine-based cage, while cage C1 exhibits lower luminescence.
In summary, two palladium(II) coordination cages coupled to ruthenium(II) pyridine complexes via an amide bond have been synthesized by self-assembly. In order to obtain bright luminescence, the ruthenium complex was separated from the coordinating bis(pyridyl) ligand using an alkyl spacer. The photo-physical properties of the Pd2L4 cage coupled to a ruthenium complex with and without spacer were compared. Remarkably, the palladium cage without spacer is non-emissive, while the other one features a quite high quantum yield of 66%, making it one of the highest luminescent metallosupramolecular complexes known to date. The applied approach is promising to further design highly emissive metallocages for potential applications as biological labels and chemosensors, among others.
A. S. and M. H. are grateful for the financial support of the TUM Graduate School of Chemistry. A. C. acknowledges support from Cardiff University and the August–Wilhelm Scheer Visiting Professorship at the Technical University of Munich. Authors acknowledge the support of the Technische Universität München – Institute for Advanced Study, funded by the German Excellence Initiative and the European Union Seventh Framework Program under grant agreement no. 291763. Authors thank the Chinese Scholarship Council for a PhD fellowship to J. H. Authors thank Christian Jandl for measuring the emission spectra. Dr. Alexander Pöthig's support with crystallographic data is greatly appreciated.
Notes and references
- (a) H. Amouri, C. Desmarets and J. Moussa, Chem. Rev., 2012, 112, 2015–2041 CrossRef CAS PubMed; (b) T. R. Cook and P. J. Stang, Chem. Rev., 2015, 115, 7001–7045 CrossRef CAS PubMed; (c) T. R. Cook, Y.-R. Zheng and P. J. Stang, Chem. Rev., 2013, 113, 734–777 CrossRef CAS PubMed.
- (a) E. Persch, O. Dumele and F. Diederich, Angew. Chem., Int. Ed., 2015, 54, 3290–3327 CrossRef CAS PubMed; (b) A. M. Castilla, W. J. Ramsay and J. R. Nitschke, Acc. Chem. Res., 2014, 47, 2063–2073 CrossRef CAS PubMed; (c) M. Han, D. M. Engelhard and G. H. Clever, Chem. Soc. Rev., 2014, 43, 1848–1860 RSC; (d) M. D. Pluth and K. N. Raymond, Chem. Soc. Rev., 2007, 36, 161–171 RSC.
- (a) S. H. A. M. Leenders, R. Gramage-Doria, B. de Bruin and J. N. H. Reek, Chem. Soc. Rev., 2015, 44, 433–448 RSC; (b) M. D. Pluth, R. G. Bergman and K. N. Raymond, Acc. Chem. Res., 2009, 42, 1650–1659 CrossRef CAS PubMed; (c) D. M. Vriezema, M. Comellas Aragonès, J. A. A. W. Elemans, J. J. L. M. Cornelissen, A. E. Rowan and R. J. M. Nolte, Chem. Rev., 2005, 105, 1445–1490 CrossRef CAS PubMed.
- (a) R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Chem. Rev., 2011, 111, 6810–6918 CrossRef CAS PubMed; (b) T. R. Cook, V. Vajpayee, M. H. Lee, P. J. Stang and K.-W. Chi, Acc. Chem. Res., 2013, 46, 2464–2474 CrossRef CAS PubMed; (c) H. Vardhan, M. Yusubov and F. Verpoort, Coord. Chem. Rev., 2016, 306, 171–194 CrossRef CAS.
- (a) A. Kumar, S.-S. Sun and A. J. Lees, Coord. Chem. Rev., 2008, 252, 922–939 CrossRef CAS; (b) J. A. Thomas, Dalton Trans., 2011, 40, 12005–12016 RSC.
- X. Yan, T. R. Cook, P. Wang, F. Huang and P. J. Stang, Nat. Chem., 2015, 7, 342–348 CrossRef CAS PubMed.
- X. Yan, M. Wang, T. R. Cook, M. Zhang, M. L. Saha, Z. Zhou, X. Li, F. Huang and P. J. Stang, J. Am. Chem. Soc., 2016, 138, 4580–4588 CrossRef CAS PubMed.
- A. Schmidt, V. Molano, M. Hollering, A. Pöthig, A. Casini and F. E. Kühn, Chem. – Eur. J., 2016, 22, 2253–2256 CrossRef CAS PubMed.
- J. Wang, C. He, P. Wu, J. Wang and C. Duan, J. Am. Chem. Soc., 2011, 133, 12402–12405 CrossRef CAS PubMed.
- (a) L. Xu, Y.-X. Wang and H.-B. Yang, Dalton Trans., 2015, 44, 867–890 RSC; (b) P. Thanasekaran, C.-H. Lee and K.-L. Lu, Coord. Chem. Rev., 2014, 280, 96–175 CrossRef CAS.
- (a) P. D. Frischmann, V. Kunz and F. Würthner, Angew. Chem., Int. Ed., 2015, 54, 7285–7289 CrossRef CAS PubMed; (b) Z. Li, N. Kishi, K. Hasegawa, M. Akita and M. Yoshizawa, Chem. Commun., 2011, 47, 8605–8607 RSC; (c) M. Wang, V. Vajpayee, S. Shanmugaraju, Y.-R. Zheng, Z. Zhao, H. Kim, P. S. Mukherjee, K.-W. Chi and P. J. Stang, Inorg. Chem., 2011, 50, 1506–1512 CrossRef CAS PubMed; (d) M. Yamashina, M. M. Sartin, Y. Sei, M. Akita, S. Takeuchi, T. Tahara and M. Yoshizawa, J. Am. Chem. Soc., 2015, 137, 9266–9269 CrossRef CAS PubMed.
- (a) M. Fujita, M. Tominaga, A. Hori and B. Therrien, Acc. Chem. Res., 2005, 38, 369–378 CrossRef CAS PubMed; (b) B. J. Holliday and C. A. Mirkin, Angew. Chem., Int. Ed., 2001, 40, 2022–2043 CrossRef CAS; (c) J. K. Klosterman, Y. Yamauchi and M. Fujita, Chem. Soc. Rev., 2009, 38, 1714–1725 RSC.
- A. Schmidt, A. Casini and F. E. Kühn, Coord. Chem. Rev., 2014, 275, 19–36 CrossRef CAS.
- (a) S. Freye, J. Hey, A. Torras-Galán, D. Stalke, R. Herbst-Irmer, M. John and G. H. Clever, Angew. Chem., Int. Ed., 2012, 51, 2191–2194 CrossRef CAS PubMed; (b) S. Freye, R. Michel, D. Stalke, M. Pawliczek, H. Frauendorf and G. H. Clever, J. Am. Chem. Soc., 2013, 135, 8476–8479 CrossRef CAS PubMed; (c) J. E. M. Lewis, E. L. Gavey, S. A. Cameron and J. D. Crowley, Chem. Sci., 2012, 3, 778–784 RSC; (d) J. E. M. Lewis, C. J. McAdam, M. G. Gardiner and J. D. Crowley, Chem. Commun., 2013, 49, 3398–3400 RSC; (e) S. Löffler, J. Lübben, L. Krause, D. Stalke, B. Dittrich and G. H. Clever, J. Am. Chem. Soc., 2015, 137, 1060–1063 CrossRef PubMed.
- (a) A. M. Johnson, O. Moshe, A. S. Gamboa, B. W. Langloss, J. F. K. Limtiaco, C. K. Larive and R. J. Hooley, Inorg. Chem., 2011, 50, 9430–9442 CrossRef CAS PubMed; (b) J. E. M. Lewis, A. B. S. Elliott, C. J. McAdam, K. C. Gordon and J. D. Crowley, Chem. Sci., 2014, 5, 1833 RSC.
- Z. Li, N. Kishi, K. Yoza, M. Akita and M. Yoshizawa, Chem. – Eur. J., 2012, 18, 8358–8365 CrossRef CAS PubMed.
- A. Schmidt, M. Hollering, M. Drees, A. Casini and F. E. Kühn, Dalton Trans., 2016, 45, 8556–8565 RSC.
- A. B. S. Elliott, J. E. M. Lewis, H. van der Salm, C. J. McAdam, J. D. Crowley and K. C. Gordon, Inorg. Chem., 2016, 55, 3440–3447 CrossRef CAS PubMed.
- (a) Y. Cohen, L. Avram and L. Frish, Angew. Chem., Int. Ed., 2005, 44, 520–554 CrossRef CAS PubMed; (b) D. Li, G. Kagan, R. Hopson and P. G. Williard, J. Am. Chem. Soc., 2009, 131, 5627–5634 CrossRef CAS PubMed.
- (a) E. Jakubikova, W. Chen, D. M. Dattelbaum, F. N. Rein, R. C. Rocha, R. L. Martin and E. R. Batista, Inorg. Chem., 2009, 48, 10720–10725 CrossRef CAS PubMed; (b) C. Kreitner and K. Heinze, Dalton Trans., 2016, 45, 5640–5658 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, NMR spectra and crystallographic details. CCDC 1484108. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt02708c |
| This journal is © The Royal Society of Chemistry 2016 |

