Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Triple molybdate scheelite-type upconversion phosphor NaCaLa(MoO4)3:Er3+/Yb3+: structural and spectroscopic properties†
Chang Sung
Lim
a,
Aleksandr S.
Aleksandrovsky
bc,
Maxim S.
Molokeev
de,
Aleksandr S.
Oreshonkov
fg,
Denis A.
Ikonnikov
g and
Victor V.
Atuchin
*hijk
aDepartment of Advanced Materials Science & Engineering, Hanseo University, Seosan 356-706, Republic of Korea
bLaboratory of Coherent Optics, Kirensky Institute of Physics, Federal Research Center KSC SB RAS, Krasnoyarsk 660036, Russia
cLaboratory for Nonlinear Optics and Spectroscopy, Siberian Federal University, Krasnoyarsk 660079, Russia
dLaboratory of Crystal Physics, Kirensky Institute of Physics, Federal Research Center KSC SB RAS, Krasnoyarsk 660036, Russia
eDepartment of Physics, Far Eastern State Transport University, Khabarovsk 680021, Russia
fLaboratory of Molecular Spectroscopy, Kirensky Institute of Physics, Federal Research Center KSC SB RAS, Krasnoyarsk 660036, Russia
gDepartment of Photonics and Laser Technologies, Siberian Federal University, Krasnoyarsk 660079, Russia
hLaboratory of Optical Materials and Structures, Institute of Semiconductor Physics, SB RAS, Novosibirsk 630090, Russia. E-mail: atuchin@isp.nsc.ru; Fax: +7 (383) 3332771; Tel: +7 (383) 3308889
iFunctional Electronics Laboratory, Tomsk State University, Tomsk 634050, Russia
jLaboratory of Semiconductor and Dielectric Materials, Novosibirsk State University, Novosibirsk 630090, Russia
kInstitute of Chemistry, Tyumen State University, Tyumen 525003, Russia
First published on 31st August 2016
Abstract
Triple molybdate NaCaLa(1−x−y)(MoO4)3:xEr3+,yYb3+ (x = y = 0, x = 0.05 and y = 0.45, x = 0.1 and y = 0.2, x = 0.2 and y = 0) phosphors were successfully synthesized for the first time by the microwave sol–gel method. Well-crystallized particles formed after heat treatment at 900 °C for 16 h showed a fine and homogeneous morphology with particle sizes of 2–3 μm. The structures were refined by the Rietveld method in the space group I41/a. The optical properties were examined comparatively using photoluminescence emission and Raman spectroscopy. Under excitation at 980 nm, the NaCaLa0.7(MoO4)3:0.1Er3+,0.2Yb3+ and NaCaLa0.5(MoO4)3:0.05Er3+,0.45Yb3+ particles exhibited a strong 525 nm emission band, a weaker 550 nm emission band in the green region, and three weak 655 nm, 490 nm and 410 nm emission bands in the red, blue and violet regions. The pump power dependence and Commission Internationale de L'Eclairage chromaticity of the upconversion emission intensity were evaluated in detail.
1. Introduction
In recent years, rare-earth doped oxide-based upconversion (UC) particles have become of extensive interest due to their stable luminescent properties and potential applications in photonic products such as lasers, three-dimensional displays, light-emitting devices, solar cells and biological luminescent imaging media.1–3 Previously, scheelite-type binary molybdates were reported in terms of new structures, including structure-modulation effects, promising spectroscopic characteristics and excellent upconversion (UC) photoluminescence properties.4–9 In particular, the rare-earth binary NaLn(MoO4)2 (Ln = La3+, Gd3+, Y3+) compounds possess the tetragonal phase with the space group I41/a, and belong to the family of sheelite-type structures.10 The trivalent rare-earth ions in the tetragonal phase can be partially substituted by laser-active Er3+, Ho3+, Tm3+ and Yb3+ ions. These ions are efficiently doped into the crystal lattice of the tetragonal binary molybdates due to the similar radii of the trivalent rare earth ions, which results in the excellent UC photoluminescence properties.4–9,11–14Among rare-earth ions, the Er3+ ion is suitable for the infrared to visible light conversion through the UC process due to its appropriate electronic energy level configuration. The Yb3+ ion, used as a sensitizer, can be dramatically excited by incident light source energy. This energy is transferred to the activator from which radiation can be emitted. The Er3+ ion activator is an efficient luminescence center of the UC particles, while the sensitizer enhances the UC luminescence efficiency. Er3+ and Yb3+ ion co-doping can remarkably enhance the UC efficiency for the shift from the infrared to visible light due to the efficiency of the energy transfer from Yb3+ to Er3+.15–17
For the preparation of the double molybdate NaLn(MoO4)2, several processes have been developed via specific preparation processes, including solid-state reactions,4,8,18–21 the sol–gel method,22,23 Czochralski growth,24–27 the hydrothermal method,28–32 the microwave assisted hydrothermal method33 and pulsed laser deposition.34 Nevertheless, it is necessary to create new triple molybdate compounds for the observation of the UC photoluminescence in the materials and to search for features such as a well-defined morphology and stable UC luminescent properties. However, so far, triple molybdates with the general composition NaRLn(MoO4)3 (R = Ca2+, Sr2+ and Ba2+, while Ln is a rare-earth element) have not been reported. Compared to the common technological methods, microwave synthesis has its advantages of a very short reaction time, small particle size, narrow particle size distribution, and high final polycrystalline sample purity.35,36 The microwave heating is delivered to the material surface by radiant and/or convection heating and the heat energy is transferred to the bulk of the material via conduction.37–39 It is an inexpensive method that provides high-homogeneity powder products and it is easy to scale up the process. Thus, the microwave method is considered a viable alternative approach for the quick synthesis of high-quality luminescent materials. In this concept, this method is optimal for the synthesis of complex oxide compounds.
In the present study, triple molybdate NaCaLa(1−x−y)(MoO4)3:xEr3+,yYb3+ (NCLM:xEr3+,yYb3+) phosphors with correct doping concentrations of Er3+ and Yb3+ (x = y = 0, x = 0.05 and y = 0.45, x = 0.1 and y = 0.2, x = 0.2 and y = 0) were successfully prepared by the microwave sol–gel method followed by heat treatment in air. The synthesized particles were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS). Their optical properties were examined comparatively using photoluminescence (PL) emission and Raman spectroscopy. The pump power dependence and Commission Internationale de L'Eclairage (CIE) chromaticity parameters of the UC emission were evaluated in detail.
2. Experimental methods
In this study, precise amounts of the raw materials, the products of Sigma-Aldrich (USA) for Ca(NO3)2·4H2O (99%), Na2MoO4·2H2O (99%), La(NO3)3·6H2O (99%), Er(NO3)3·5H2O (99.9%), Yb(NO3)3·5H2O (99.9%), Alfa Aesar (USA) for (NH4)6Mo7O24·4H2O (99%), Daejung Chemicals (Korea) for citric acid (99.5%), NH3·H2O (A.R.), ethylene glycol (A.R.) and distilled water, were used to prepare NaCaLa(MoO4)3, NaCaLa0.8(MoO4)3:0.2Er3+, NaCaLa0.7(MoO4)3:0.1Er3+,0.2Yb3+, and NaCaLa0.5(MoO4)3:0.05Er3+,0.45Yb3+ compositions. The reagents were taken in accordance with the nominal composition of the designed compounds. For the preparation of the compounds, initially 0.4 mol% Ca(NO3)2, 0.2 mol% Na2MoO4·2H2O and 0.143 mol% (NH4)6Mo7O24·4H2O were dissolved in 20 mL of ethylene glycol and 80 mL of 5 M NH3·H2O under vigorous stirring and heating. Subsequently, 0.4 mol% (1 − x − y)La(NO3)3·6H2O with 0.4 mol% xEr(NO3)3·5H2O and 0.4 mol% yYb(NO3)3·5H2O (x = 0.05 and y = 0.45, x = 0.1 and y = 0.2, x = 0.2 and y = 0) were dissolved in 100 mL of distilled water under vigorous stirring and heating. At this stage, citric acid was employed with the molar ratio of citric acid to metal ions of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Then, the two kinds of transparent solutions were co-mixed together under vigorous stirring under heating at 80–100 °C. Finally, the mixed solutions appeared highly transparent and were treated by adjusting to pH = 7–8 using the addition of citric acid or NH3·H2O. The co-mixed and adjusted solutions were transferred into an oven for microwave irradiation. Microwave operations for 30 min were conducted by precise controlling. The frequency was 2.45 GHz and the maximum output-power was 1250 W. After the microwave process, the samples were treated in an ultrasonicator for 10 min, and transferred into a dry oven. The drying conditions were at 120 °C and dried gels in black color were obtained. For the crystallization of the compounds, the black dried gels were heat-treated at 900 °C for 16 h. Finally, white pure NaCaLa(MoO4)3 and pink particles for the Er/Yb-doped compositions were obtained. The chemical compositions of the final powder products were confirmed using EDS measurements.
1. Then, the two kinds of transparent solutions were co-mixed together under vigorous stirring under heating at 80–100 °C. Finally, the mixed solutions appeared highly transparent and were treated by adjusting to pH = 7–8 using the addition of citric acid or NH3·H2O. The co-mixed and adjusted solutions were transferred into an oven for microwave irradiation. Microwave operations for 30 min were conducted by precise controlling. The frequency was 2.45 GHz and the maximum output-power was 1250 W. After the microwave process, the samples were treated in an ultrasonicator for 10 min, and transferred into a dry oven. The drying conditions were at 120 °C and dried gels in black color were obtained. For the crystallization of the compounds, the black dried gels were heat-treated at 900 °C for 16 h. Finally, white pure NaCaLa(MoO4)3 and pink particles for the Er/Yb-doped compositions were obtained. The chemical compositions of the final powder products were confirmed using EDS measurements.
The powder diffraction data of the synthesized particles for Rietveld analysis were collected over the range of 2θ = 5–90° at room temperature with a D/MAX 2200 (Rigaku, Japan) diffractometer (Cu-Kα radiation, θ–2θ geometry). The step size of 2θ was 0.02°, and the counting time was 5 s per step. The microstructure and surface morphology of the synthesized particles were examined using SEM (JSM-5600, JEOL, Japan). PL spectra were recorded using a spectrophotometer (Perkin Elmer LS55, UK) at room temperature. The pump power dependence of the UC emission intensity was measured at working power from 20 to 110 mW levels. Raman spectroscopy measurements were performed using a LabRam Aramis (Horiba Jobin-Yvon, France) with the spectral resolution of 2 cm−1. The 514.5 nm line of an Ar ion laser was used as an excitation source; the power on the samples was kept at the 0.5 mW level to avoid decomposition of the sample.
3. Results and discussion
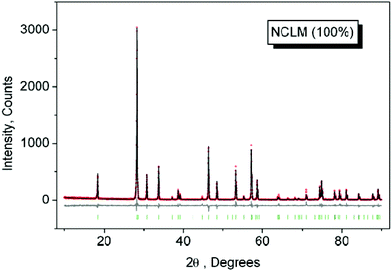
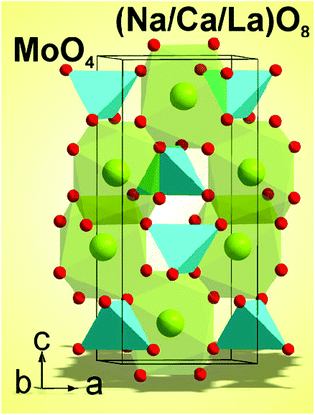
The XRD patterns recorded from the synthesized molybdates are shown in Fig. 1 and 1S–3S.† In general, the patterns of solutions NCLM:xEr3+,yYb3+ are similar. The difference profile plot of NCLM is shown in Fig. 1. The difference profile plots of NCLM:xEr3+,yYb3 are very similar to that of NCLM, as is evident from a comparison of Fig. 1 and 1S–3S.†Rietveld refinement was performed using the TOPAS 4.2 package.40 All diffraction peaks were indexed using the tetragonal unit cell in the space group I41/a with parameters close to those of CaMoO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 and Na0.5La0.5MoO4.42 Therefore, these crystal structures were taken as a starting model for the Rietveld refinement. The site of the Ca or (Na/La) ion was taken as occupied by Ca, Na, La, Er, and Yb ions at fixed occupations according to the nominal compositions. The refinement was stable and gave low R-factors (Table 1, Fig. 1 and 1S–3S†). The obtained atomic coordinates and the main bond lengths can be found in Tables 1S and 2S,† respectively. In the compounds under consideration, Z = 4 and, from the structural point of view,43 the total chemical formula calculated by summing all elements in the unit cell Na4/3Ca4/3La4(1−x−y)/3(MoO4)4:4x/3Er3+,4y/3Yb3+ can be generalized as Na1/3Ca1/3La(1−x−y)/3MoO4:(x/3)Er3+,(y/3)Yb3+, which clearly emphasizes the relationship of the solid solutions to the CaMoO4 structural family.41 As an example, the structure of NCLM is shown in Fig. 2.
41 and Na0.5La0.5MoO4.42 Therefore, these crystal structures were taken as a starting model for the Rietveld refinement. The site of the Ca or (Na/La) ion was taken as occupied by Ca, Na, La, Er, and Yb ions at fixed occupations according to the nominal compositions. The refinement was stable and gave low R-factors (Table 1, Fig. 1 and 1S–3S†). The obtained atomic coordinates and the main bond lengths can be found in Tables 1S and 2S,† respectively. In the compounds under consideration, Z = 4 and, from the structural point of view,43 the total chemical formula calculated by summing all elements in the unit cell Na4/3Ca4/3La4(1−x−y)/3(MoO4)4:4x/3Er3+,4y/3Yb3+ can be generalized as Na1/3Ca1/3La(1−x−y)/3MoO4:(x/3)Er3+,(y/3)Yb3+, which clearly emphasizes the relationship of the solid solutions to the CaMoO4 structural family.41 As an example, the structure of NCLM is shown in Fig. 2.
| Compound | NCLM | NCLM:0.2Er3+ | NCLM:0.1Er3+,0.2Yb3+ | NCLM:0.05Er3+,0.45Yb3+ |
|---|---|---|---|---|
| x | 0 | 0.2 | 0.1 | 0.05 |
| y | 0 | 0 | 0.2 | 0.45 |
| Sp. gr. | I41/a | I41/a | I41/a | I41/a |
| a, Å | 5.2991 (1) | 5.2806 (2) | 5.2675 (1) | 5.2421 (1) |
| c, Å | 11.6223 (3) | 11.5726 (3) | 11.5377 (3) | 11.4678 (2) |
| V, Å3 | 326.37 (2) | 322.70 (1) | 320.13 (2) | 315.17 (1) |
| Z | 4 | 4 | 4 | 4 |
| 2θ-interval, ° | 5–90 | 5–90 | 5–90 | 5–90 |
| No. of reflections | 70 | 70 | 70 | 70 |
| No. of refined parameters | 7 | 7 | 7 | 7 |
| R wp, % | 19.46 | 16.75 | 15.65 | 15.06 |
| R p, % | 13.39 | 11.30 | 10.31 | 9.97 |
| R exp, % | 16.22 | 14.87 | 13.61 | 13.49 |
| χ 2 | 1.20 | 1.13 | 1.15 | 1.12 |
| R B, % | 5.29 | 3.15 | 2.09 | 1.65 |
The linear cell volume increase with the averaged increase of ion radii IR(Na/Ca/La/Er/Yb), as shown in Fig. 3, proves the chemical compositions of the synthesized samples.44 On the basis of the structural results, it can be reasonably supposed that different triple molybdates with the general composition NaRLn(MoO4)3 should crystallize in tetragonal structures close to that of CaMoO4 and the general formula should be rewritten as Na1/3R1/3Ln1/3MoO4. Further details of the crystal structure may be obtained from Fachinformationszentrum Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany (fax: (+49)7247-808-666; e-mail: crystdata@fiz-karlsruhe.de; http://www.fiz-karlsruhe.de/request_for_deposited_data.html) on quoting the deposition numbers: CSD 431015–431018.
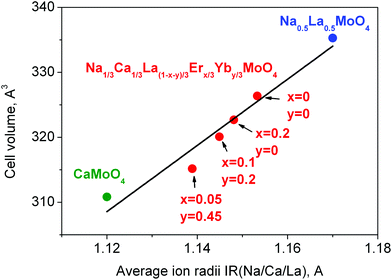 | ||
| Fig. 3 Cell volume per averaged ion radii IR(Na/Ca/La/Er/Yb) of CaMoO4 (green),41 NCLM:xEr3+,yYb3+ (red) and Na0.5La0.5MoO4 (blue)42 compounds. | ||
Thus, post heat-treatment plays an important role in molybdate gel crystallization. To reach a high-quality crystalline state, samples need to be heat treated at 900 °C for 16 h. It should be pointed out that this temperature is optimal for molybdate treatment in air and, earlier, other simple and complex molybdates were formed at close temperatures.6,7,9,37–39,45–47 Besides excellent crystallinity, the selected synthesis route provides a uniform particle morphology. The SEM images of the synthesized (a) NCLM:0.2Er3+ and (b) NCLM:0.05Er3+,0.45Yb3+ particles are shown in Fig. 4. The as-synthesized samples are well formed with a fine and homogeneous morphology and a particle size of 2–3 μm. The samples show no discrepancy in the aspect of morphological features, and closely agglomerated particles were induced by active grain interdiffusion.48–50 It should be noted that doping concentrations of Er3+ and Yb3+ have no effects on the particle morphology. The recorded EDS patterns and quantitative compositions of the NCLM:0.1Er3+,0.2Yb3+ sample are shown in Fig. 4S and Table 3S.† Only constituent elements are found in the samples and the quantitative compositions are in good accord with nominal compositions. Thus, the microwave sol–gel method of triple molybdate preparation provides energy uniformly over the material bulk, and fine particles with controlled morphology can be fabricated in a short time. The method is an inexpensive way to fabricate highly homogeneous powder products with an easy scale-up and is a viable alternative for the rapid synthesis of UC particles. This suggests that the microwave sol–gel route is suitable for the creation of homogeneous NCLM:xEr3+,yYb3+ crystallites and can be successfully applied to other molybdates from this crystal family.
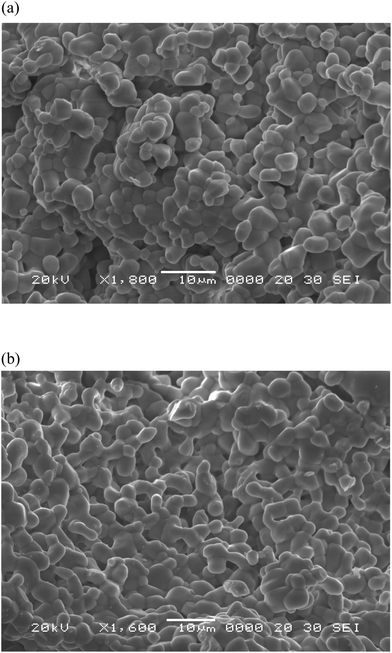 | ||
| Fig. 4 Scanning electron microscopy images of the synthesized (a) NCLM:0.2Er3+ and (b) NCLM:0.05Er3+,0.45Yb3+ particles. | ||
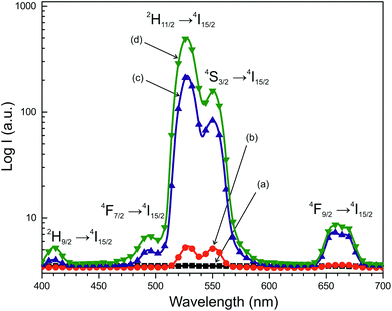
The UC photoluminescence emission spectra of the as-prepared NCLM, NCLM:0.2Er3+, NCLM:0.1Er3+,0.2Yb3+ and NCLM:0.05Er3+,0.45Yb3+ particles excited at 980 nm at room temperature are shown in Fig. 5. The UC NCLM:0.1Er3+,0.2Yb3+ and NCLM:0.05Er3+,0.45Yb3+ particles exhibited a strong 525 nm emission band, a weaker 550 nm emission band in the green region and three very weak emission bands: at 655 nm in the red region, at 490 nm in the blue region, and at 410 nm in the violet region. The strong 525 nm emission band and the weak 550 nm emission band in the green region correspond to the 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions, respectively, while the very weak 655 nm emission band in the red region corresponds to the 4F9/2 → 4I15/2 transition. Another very weak band at 410 nm is due to the 2H9/2 → 4I15/2 transition, while a 490 nm peak is due to the 4F9/2 → 4I15/2 transition. It must be noted that in sample (d), the shortest-wavelength band at 410 nm is only two times smaller than the red one, despite the fact that a three-stage process is necessary to excite the 2H9/2 level. The mechanism of excitation of this level might be the energy transfer from the Yb excited state to the Er 4F9/2 level, since the energy difference between 4F9/2 and 2H9/2 is close to the energy of the excited Yb ion. However, since the 4F9/2 population is likely to be low, the most probable excitation channel is the population of a pair of high-lying levels 4G9/2 and 2K15/2 from the well-populated 4S3/2 level through the energy transfer from the Yb ion, with the subsequent decay of these high-lying levels to the 2H9/2 one.
The UC intensities of NCLM were not detected. The UC intensities of NCLM:0.2Er3+ are well above the detection limit; however, they are one or two orders of magnitude smaller than those of the Yb-doped samples and, hence, the UC luminescence for an Er-doped sample is not well seen in Fig. 5. The UC luminescence is observed from all levels: 2H11/2, 4S3/2 and even from 4F9/2. The intensity ratio of green and red lines is 1.4 for sample (b), 30 for sample (c) and 56 for sample (d). At the same time, the ratio of UC green band peak values for samples (d) and (b) is 92. The latter effect admits that the absorption coefficient of erbium at 980 nm is much smaller than that of ytterbium in the matrix under study. The variation of the green-to-red ratio with the ytterbium content increase is rather common and was observed earlier for several hosts.15–17,37–39
The Er3+ ion activator is the luminescence center of these UC particles and the sensitizer Yb3+ effectively enhances the UC luminescence intensity because of efficient energy transfer from Yb3+ to Er3+. The concentration quenching effect can be explained by the energy transfer between the nearest Er3+ and Yb3+ ions. On increasing the Er3+ and Yb3+ ion concentrations, the distance between Er3+ and Yb3+ ions decreases, which can promote a non-radiative energy transfer, such as an exchange interaction or multipole–multipole interactions.51 As shown in Fig. 5, the higher intensity of (d) NCLM:0.05Er3+,0.45Yb3+ is caused by the ratio of Yb3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+ = 9
Er3+ = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, while the lower intensity of (c) NCLM:0.1Er3+,0.2Yb3+ is caused by the ratio of Yb3+
1, while the lower intensity of (c) NCLM:0.1Er3+,0.2Yb3+ is caused by the ratio of Yb3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+ = 2
Er3+ = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Thus, the preferable Yb3+
1. Thus, the preferable Yb3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+ = 9
Er3+ = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio is induced by the concentration quenching effect of Er3+ ions. Therefore, the higher content of the Yb3+ ions used as a sensitizer and the lower content of the Er3+ ions close to the preferable ratio of Yb3+
1 ratio is induced by the concentration quenching effect of Er3+ ions. Therefore, the higher content of the Yb3+ ions used as a sensitizer and the lower content of the Er3+ ions close to the preferable ratio of Yb3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+ = 9
Er3+ = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 can remarkably enhance the UC luminescence through the efficient energy transfer. The ratio of the 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transition intensities may be influenced not only by the change in radiation probabilities from starting levels, but also by the probabilities of the non-radiative relaxation from the UC-populated 4F7/2 level. Because the lifetime of the 4F7/2 level is comparatively short, the excited Er3+ ions decay non-radiatively to the 2H11/2 level with a higher probability, as compared to the 4S3/2 level, in the case of the NCLM host matrix.
1 can remarkably enhance the UC luminescence through the efficient energy transfer. The ratio of the 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transition intensities may be influenced not only by the change in radiation probabilities from starting levels, but also by the probabilities of the non-radiative relaxation from the UC-populated 4F7/2 level. Because the lifetime of the 4F7/2 level is comparatively short, the excited Er3+ ions decay non-radiatively to the 2H11/2 level with a higher probability, as compared to the 4S3/2 level, in the case of the NCLM host matrix.
The logarithmic scale dependences of the UC emission intensities at 525, 550 and 655 nm on the working pump power over the range of 20–110 mW in the NCLM:0.05Er3+,0.45Yb3+ sample are shown in Fig. 6. In the UC process, the UC emission intensity I is proportional to the slope value n of the irradiation pumping power P. The maximum value of n is the number of pumping photons required to reach the starting energy level in the UC ion and produce UC emission:52
| I ∝ Pn | (1) |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I ∝ n I ∝ n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P | (2) |
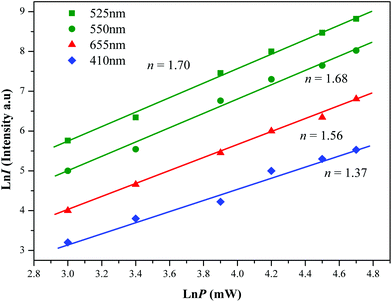 | ||
| Fig. 6 The logarithmic scale dependence of the upconversion emission intensity on the pump power in the range of 20–110 mW at 525, 550, 655 and 410 nm in the NCLM:0.05Er3+,0.45Yb3+ sample. | ||
The slopes n = 1.70 and 1.68 for green emission at 525 and 550 nm, and n = 1.56 for red emission at 655 nm, respectively, are evident from Fig. 5. These results show that the UC mechanism of the green and red emissions can be explained by a two-photon UC process in the Er3+/Yb3+ co-doped phosphors.53–55
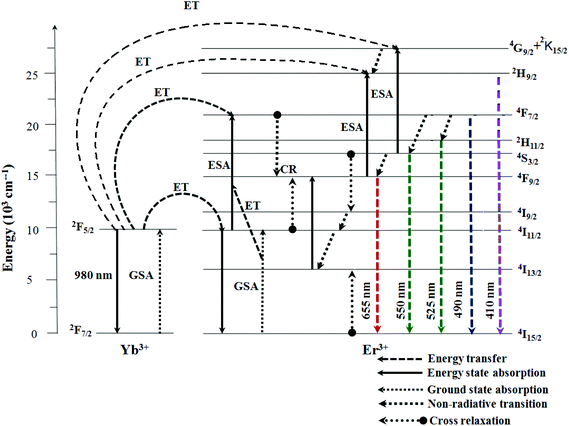
Based on the results of the analysis of pump power dependence, the schematic energy level diagrams of Er3+ ions (activator) and Yb3+ ions (sensitizer) in the NCLM:xEr3+,yYb3+ samples and the UC mechanisms, accounting for the green and red emissions excited by the 980 nm laser wavelength, are shown in Fig. 7. The UC emissions are generated via multiple processes of ground state absorption (GSA), energy transfer upconversion (ETU), excited state absorption (ESA) and cross relaxation (CR). Under excitation at 980 nm, the Er3+ and Yb3+ ions are initially excited from the ground state to the excited state through the ground state absorption (GSA) process (Er3+: 4I15/2 → 4I11/2, Yb3+: 2F7/2 → 2F5/2) and ETU processes of 4I15/2 (Er3+) + 2F5/2 (Yb3+) → 4I11/2 (Er3+) + 2F7/2 (Yb3+), which are responsible for the population at the 4I11/2 level in the Er3+ ion. For the green emissions, the energy transition from the 4I11/2 level to the 4F7/2 level of Er3+ is involved in three possible processes:53–55 (1) ESA: 4I11/2 (Er3+) + a photon (980 nm) → 4F7/2, (2) ETU: 2I11/2 (Er3+) + 2F5/2 (Yb3+) → 4F7/2 (Er3+) + 2F7/2 (Yb3+) and (3) ETU: 4I11/2 (Er3+) + 4I11/2 (Er3+) → 4F7/2 (Er3+) + 4I15/2 (Er3+). These three possible processes can populate the 4F7/2 level from the 4I11/2 level in Er3+ and, then, the 4F7/2 level relaxes rapidly and non-radiatively to the next lower 2H11/2 and 4S3/2 levels in Er3+ because of the short lifetime of the 4F7/2 level. As a result, the radiative transitions of 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 processes can generate the green emission at 525 and 550 nm. The strong suppression of red UC luminescence in the host under study is similar to the effect in the earlier studied CaGd2(MoO4)4:Er,Yb and CaGd2(WO4)4:Er,Yb systems and differentiates this host class from a number of others. For the red emission, the 4F9/2 level population is generated by non-radiative relaxation either (1) from the 4S3/2 to the 4F9/2 level or (2) from I11/2 to I13/2, and then pumping to 4F9/2, as well as by cross relaxation (CR) via either (3) the 4F7/2 + 4I11/2 → 4F9/2 + 4F9/2 transition51–53 or (4) 4S3/2 + 4I15/2 = 4I9/2 + 4I13/2, and then pumping to 4F9/2.56 Finally, the 4F9/2 level relaxes radiatively to the ground state at the 4I15/2 level, and releases the red emission at 655 nm.37–39 The radiation-free transitions (1) and (2) must not strongly vary from one oxide host to another and, then, they cannot provide such a significant difference between the red and green luminescence in the selected group of hosts. Consequently, processes (1) and (2) must be deduced to play a negligible role in the host class under study. So, intense red luminescence at the Er/Yb ratio of 3/8 is most likely, due to one of the mentioned cross-relaxation channels.54 The (3) 4F7/2 + 4I11/2 = 4F9/2 + 4F9/2 cross-relaxation will be very weak since 4F7/2 has a comparatively short lifetime and its radiationless depopulation to 4S3/2 and 2H11/2 levels is rather fast, which may explain the weak red luminescence in our host. For the (4) population channel, the weak red UC luminescence is explainable by the suggestion that, in our host, detuning between the energy of the 4S3/2 state and the sum of the energies of 4I9/2 and 4I13/2 states is not so favorable.54 Moreover, as Yb3+ concentration increases, the green emission dramatically increases, compared to the red emission. The strong 525 nm and 550 nm emission lines in the green region, as shown in Fig. 5, are assigned to the 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions of the Er3+ ions, respectively, while the weak 655 nm emission band in the red region is assigned to the 4F9/2 → 4I15/2 transition.
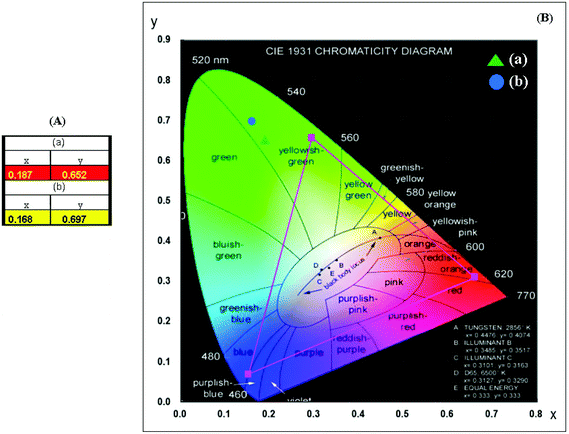
The chromaticity coordinates calculated for (a) NCLM:0.1Er3+,0.2Yb3+ and (b) NCLM:0.05Er3+,0.45Yb3+ particles and the related CIE chromaticity diagram are shown in Fig. 8. The legend of Fig. 8(B) shows the chromaticity points for samples (a) and (b). When the Er3+/Yb3+ concentration ratio is varied, the chromaticity coordinate values (x, y) change. As shown in Fig. 8(A), the calculated chromaticity coordinates x = 0.227 and y = 0.686 for (a) NCLM:0.1Er3+,0.2Yb3+ and x = 0.206 and y = 0.727 for (b) NCLM:0.05Er3+,0.45Yb3+ correspond to the yellowish-green sector in the CIE diagram.
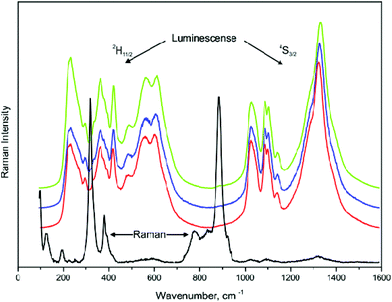
The Raman spectra of the synthesized pure NCLM, NCLM:0.2Er3+, NCLM:0.1Er3+,0.2Yb3+ and NCLM:0.05Er3+,0.45Yb3+ particles are shown in Fig. 9. As to pure NCLM, the well-resolved sharp peaks clearly indicate a highly crystalline state of the synthesized particles. The symmetry and frequencies of all the observed modes in the Raman spectrum of pure NCLM in comparison with the Raman spectrum of isostructural CaMoO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57,58 are presented in Table 2. The decomposition of spectral regions corresponding to the bending and stretching vibrations of MoO4 tetrahedra is shown in Fig. 10.
57,58 are presented in Table 2. The decomposition of spectral regions corresponding to the bending and stretching vibrations of MoO4 tetrahedra is shown in Fig. 10.
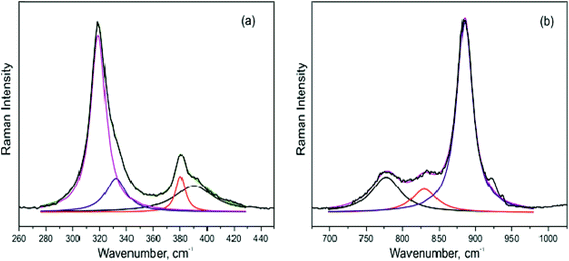 | ||
| Fig. 10 The decomposition of Raman spectra of NCLM in the regions of (a) bending and (b) stretching vibrations of MoO4 tetrahedra. | ||
| Number | Symmetry type | Exp. | Calc. | Exp. | ||||
|---|---|---|---|---|---|---|---|---|
| (a) | (b) | (c) | (d) | CaMoO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57,58 57,58 |
CaLa2(MoO4)4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
|||
| 1 | Ag | 885 | 881 | 907 | 874 | 895 | 877 | 906 |
| 2 | Bg | 829 | 822 | 849 | 826 | 841 | 845 | 828 |
| 3 | Eg | 777 | 785 | 804 | 779 | 798 | 792 | 762 |
| 4 | Bg | 390 | 398 | 420 | 424 | 430 | 402 | 393 |
| 5 | Eg | 380 | 371 | 378 | 384 | 388 | 391 | 377 |
| 6 | Bg | 332 | 330 | 328 | 315 | 328 | 327 | 333 |
| 7 | Ag | 318 | 326 | 348 | 343 | 347 | 321 | 317 |
| 8 | Eg | 255 | 241 | 257 | 267 | 272 | 267 | 251 |
| 9 | Bg | 228 | 231 | 219 | 229 | 229 | 214 | |
| 10 | Ag | 195 | 202 | 193 | 200 | 202 | 204 | 193 |
| 11 | Eg | 190 | 183 | 186 | 192 | 196 | 189 | |
| 12 | Bg | 141 | 142 | 149 | 151 | 152 | 143 | |
| 13 | Eg | 125 | 127 | 131 | 134 | 137 | 111 | |
| 14 | Bg | 96 | 96 | 90 | 95 | 95 | ||
| 15 | Eg | 79 | 80 | 83 | 85 | |||
| 16 | Bg | 21 | 20 | 21.4 | 21.4 | |||
| 17 | Eg | 18 | 18 | 18.8 | 19.4 | |||
| 18 | Bg | 18 | 19.7 | 19.6 | ||||
| 19 | Eg | 16 | 17.2 | 17.7 | ||||
| 20 | Bg | 19.1 | 19.1 | |||||
| 21 | Eg | 16.8 | 17.4 | |||||
To perform the lattice dynamics (LD) simulation of the investigated compounds, the program package LADY was used.59 The atomic vibration frequencies were obtained using a modified random-element-isodisplacement model.60 Only the pair-wise interactions and bond-stretching force constants A are considered. A depends on rij, and the A(rij) dependences are the same for all atom pairs – A = λ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(–rij/ρ), where rij is the interatomic distance, and λ and ρ are the parameters characterizing the selected pair interaction. To find model parameters, a special optimization program was written and tested for several compounds from different chemical classes.7,47,61–66 The initial parameter values were accepted as random ones and lattice stability conditions were taken into account. The resulting model parameters were obtained by minimization of residual difference values of the simulated and experimental Raman frequencies of pure NCLM using the Fletcher–Reeves method.67 In the case of suspension of the Fletcher–Reeves algorithm because of incompatible model parameters, the initial model parameters were set randomly again. To obtain a satisfactory agreement between experimental and calculated results, O–O interatomic interactions within MoO4 tetrahedral groups and O–O intermolecular interactions between neighboring MoO4 groups should be described within different force constants, and the cation–cation interactions can be neglected.68,69 The model parameters obtained for pure NCLM are shown in Table 4S.† The simulations of NCLM:0.2Er3+, NCLM:0.1Er3+,0.2Yb3+, and NCLM:0.05Er3+,0.45Yb3+ were carried out using the same model parameters. Uniform values of Er–O, Yb–O and La–O force constants were selected. A comparison of the observed and calculated Raman modes of pure NCLM can be found in Table 2. Calculations predict noticeable shifts of Raman frequencies in doped samples; the extent of these shifts is in general agreement with the variation of Mo–O length according to the empirical formula of Hardcastle.70 However, the Raman signals measured for Er-doped compositions cannot be directly compared to the simulated results because of strong Er3+ luminescence, and the experimental check will be the subject of a separate study.
exp(–rij/ρ), where rij is the interatomic distance, and λ and ρ are the parameters characterizing the selected pair interaction. To find model parameters, a special optimization program was written and tested for several compounds from different chemical classes.7,47,61–66 The initial parameter values were accepted as random ones and lattice stability conditions were taken into account. The resulting model parameters were obtained by minimization of residual difference values of the simulated and experimental Raman frequencies of pure NCLM using the Fletcher–Reeves method.67 In the case of suspension of the Fletcher–Reeves algorithm because of incompatible model parameters, the initial model parameters were set randomly again. To obtain a satisfactory agreement between experimental and calculated results, O–O interatomic interactions within MoO4 tetrahedral groups and O–O intermolecular interactions between neighboring MoO4 groups should be described within different force constants, and the cation–cation interactions can be neglected.68,69 The model parameters obtained for pure NCLM are shown in Table 4S.† The simulations of NCLM:0.2Er3+, NCLM:0.1Er3+,0.2Yb3+, and NCLM:0.05Er3+,0.45Yb3+ were carried out using the same model parameters. Uniform values of Er–O, Yb–O and La–O force constants were selected. A comparison of the observed and calculated Raman modes of pure NCLM can be found in Table 2. Calculations predict noticeable shifts of Raman frequencies in doped samples; the extent of these shifts is in general agreement with the variation of Mo–O length according to the empirical formula of Hardcastle.70 However, the Raman signals measured for Er-doped compositions cannot be directly compared to the simulated results because of strong Er3+ luminescence, and the experimental check will be the subject of a separate study.
By the group theory analysis, 17 active Raman modes were predicted for the NCLM structure: Γraman = 3Ag + 7Bg + 7Eg; 19 active modes for NCLM:0.2Er3+: Γraman = 3Ag + 8Bg + 8Eg; 21 active modes for NCLM:0.1Er3+,0.2Yb3+ and NCLM:0.05Er3+,0.45Yb3+: Γraman = 3Ag + 9Bg + 9Eg. As can be seen from Table 2, additional modes, in comparison with pure molybdate, should be observed in the Raman spectra of Er,Y-doped compounds in the range below 80 cm−1. The most intensive band of the NCLM Raman spectrum found at 884 cm−1 corresponds to the ν1 symmetric stretching vibration of the MoO4 group. The lines at 829 and 777 cm−1 are related to the ν3 antisymmetric stretching vibrations. The bending ν2 and ν4 vibrations are situated in the wavenumber region of 300–400 cm−1. The shape of the observed normal vibration modes of tetrahedral MoO4 groups has been considered in ref. 71. The translation and rotational vibrations of MoO4 are in the region of 150–260 cm−1. The big cation vibrations are below 150 cm−1. As can be seen from a comparison of Raman spectra of pure NCLM and CaLa2(MoO4)4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 shown in Fig. 5S,† the positions of the bands corresponding to the symmetric stretching of MoO4 are slightly different in these crystals. In this case, according to the LD model presented above, Mo–O bonds should have different lengths in these molybdates and this is confirmed by structural results (Table 2S†). As to Raman spectra of the doped samples recorded under excitation at 514.5 nm, the Raman lines are superimposed by strong Er3+ luminescence lines. To consider the vibrational spectra of these samples, it is topical to use an excitation source with a drastically longer wavelength that should avoid the excitation of Er3+ ion optical transitions.
7 shown in Fig. 5S,† the positions of the bands corresponding to the symmetric stretching of MoO4 are slightly different in these crystals. In this case, according to the LD model presented above, Mo–O bonds should have different lengths in these molybdates and this is confirmed by structural results (Table 2S†). As to Raman spectra of the doped samples recorded under excitation at 514.5 nm, the Raman lines are superimposed by strong Er3+ luminescence lines. To consider the vibrational spectra of these samples, it is topical to use an excitation source with a drastically longer wavelength that should avoid the excitation of Er3+ ion optical transitions.
4. Conclusions
Triple molybdate NCLM:xEr3+,yYb3+ phosphors were successfully synthesized by the microwave sol–gel method. Well-crystallized particles formed after heat-treatment at 900 °C for 16 h showed a fine and homogeneous morphology with particle sizes of 2–3 μm. Under excitation at 980 nm, the UC doped particles exhibited a strong 525 nm emission band and a weak 550 nm emission band in the green region, corresponding to the 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions, and a very weak 655 nm emission band in the red region, corresponding to the 4F9/2 → 4I15/2 transition. The preferable Yb3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+ ratio of 9
Er3+ ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is controlled by the concentration quenching effect in Er3+ ions. The calculated slope value n indicated slopes of n = 1.70 and 1.68 for the green emission at 525 and 550 nm, respectively, and n = 1.56 for the red emission at 655 nm. The calculated chromaticity coordinates of the NCLM:xEr3+,yYb3+ phosphors correspond to the yellowish-green sector in the CIE diagram.
1 is controlled by the concentration quenching effect in Er3+ ions. The calculated slope value n indicated slopes of n = 1.70 and 1.68 for the green emission at 525 and 550 nm, respectively, and n = 1.56 for the red emission at 655 nm. The calculated chromaticity coordinates of the NCLM:xEr3+,yYb3+ phosphors correspond to the yellowish-green sector in the CIE diagram.
Acknowledgements
This research was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2015-058813), by the Russian Foundation for Basic Research (15-52-53080) and by project no. 0358-2015-0012 of SB RAS Program no. II.2P. ASO and VVA are partially supported by the Ministry of Education and Science of the Russian Federation.References
- M. V. DaCosta, S. Doughan, Y. Han and U. J. Krull, Lanthanide upconversion nanoparticles and applications in bioassays and bioimaging: A review, Anal. Chim. Acta, 2014, 832, 1–33 CrossRef CAS PubMed.
- Y. J. Chen, H. M. Zhu, Y. F. Lin, X. H. Gong, Z. D. Luo and Y. D. Huang, Efficient diode-pumped continuous-wave monolithic 1.9 μm micro-laser based on Tm3+:BaGd2(MoO4)4 cleaved plate, Opt. Mater., 2013, 35, 1422–1425 CrossRef CAS.
- C. Zhang, L. D. Sun, Y. W. Zhang and C. H. Yan, Rare earth upconversion nanophosphors: synthesis, functionalization and application as biolabels and energy transfer donors, J. Rare Earths, 2010, 28, 807–819 CrossRef CAS.
- V. V. Atuchin, O. D. Chimitova, T. A. Gavrilova, M. S. Molokeev, S.-J. Kim, N. V. Surovtsev and B. G. Bazarov, Synthesis, structural and vibrational properties of microcrystalline RbNd(MoO4)3, J. Cryst. Growth, 2011, 318, 683–686 CrossRef CAS.
- V. A. Morozov, A. Bertha, K. W. Meert, S. Van Rompaey, D. Batuk, G. T. Martinez, S. Van Aert, P. F. Smet, M. V. Raskina, D. Poelman, A. M. Abakumov and J. Hadermann, Incommensurate modulation and luminescence in the CaGd2(1–x)Eu2x(MoO4)4(1–y)(WO4)4y(0 ≤ x ≤ 1, 0 ≤ y ≤ 1) red phosphors, Chem. Mater., 2013, 25, 4387–4395 CrossRef CAS.
- P. L. Shi, Z. G. Xia, M. S. Molokeev and V. V. Atuchin, Crystal chemistry and luminescence properties of red-emitting CsGd1−xEux(MoO4)2 solid-solution phosphors, Dalton Trans., 2014, 43, 9669–9676 RSC.
- C. S. Lim, A. Aleksandrovsky, M. Molokeev, A. Oreshonkov and V. Atuchin, The modulated structure and frequency upconversion properties of CaLa2(MoO4)4:Ho3+/Yb3+ phosphors prepared by microwave synthesis, Phys. Chem. Chem. Phys., 2015, 17, 19278–19287 RSC.
- V. V. Atuchin, A. S. Aleksandrovsky, O. D. Chimitova, C.-P. Diao, T. A. Gavrilova, V. G. Kesler, M. S. Molokeev, A. S. Krylov, B. G. Bazarov, J. G. Bazarova and Z. S. Lin, Electronic structure of β-RbSm(MoO4)2 and chemical bonding in molybdates, Dalton Trans., 2015, 44, 1805–1815 RSC.
- C. S. Lim, Highly modulated structure and upconversion photoluminescence properties of PbGd2(MoO4)4:Er3+/Yb3+, Mater. Res. Bull., 2016, 75, 211–216 CrossRef CAS.
- O. D. Chimitova, V. V. Atuchin, B. G. Bazarov, M. S. Molokeev and Z. G. Bazarova, The formation and structural parameters of new double molybdates RbLn(MoO4)2 (Ln = Pr, Nd, Sm, Eu), Proc. SPIE-Int. Soc. Opt. Eng., 2013, 8771, 87711A CrossRef.
- C. F. Guo, H. K. Yang and J.-H. Jeong, Preparation and luminescent properties of phosphor MGd2(MoO4)4: Eu3+ (M = Ca, Sr and Ba), J. Lumin., 2010, 130, 1390–1393 CrossRef CAS.
- J. Y. Sun, Y. J. Lan, Z. G. Xia and H. Y. Du, Sol–gel synthesis and green upconversion luminescence in BaGd2(MoO4)4:Yb3+,Er3+ phosphors, Opt. Mater., 2011, 33, 576–581 CrossRef CAS.
- J. S. Liao, D. Zhou, B. Yang, R. Q. Liu, Q. Zhang and Q. H. Zhou, Sol–gel preparation and photoluminescence properties of CaLa2(MoO4)4:Eu3+ phosphors, J. Lumin., 2013, 134, 533–538 CrossRef CAS.
- F. R. Chen, Z. G. Xia, M. S. Molokeev and X. P. Jing, Effects of composition modulation on the luminescence properties of Eu3+ doped Li1−xAgxLu(MoO4)2 solid-solution phosphors, Dalton Trans., 2015, 44, 18078–18089 RSC.
- C. S. Lim, Preparation of CaLa2(MoO4)4:Er3+/Yb3+ phosphors via the microwave-modified sol-gel route and the upconversion of their photoluminescence properties, Mater. Res. Bull., 2014, 60, 537–542 CrossRef CAS.
- C. S. Lim, Upconversion photoluminescence properties of SrY2(MoO4)4:Er3+/Yb3+ phosphors synthesized by a cyclic microwave-modified sol-gel method, Infrared Phys. Technol., 2014, 67, 371–376 CAS.
- J. Y. Sun, W. Zhang, W. H. Zhang and H. Y. Du, Synthesis and two-color emission properties of BaGd2(MoO4)4:Eu3+,Er3+,Yb3+ phosphors, Mater. Res. Bull., 2012, 47, 786–789 CrossRef CAS.
- J. F. Tang, C. H. Cheng, Y. J. Chen and Y. D. Huang, Yellow–green upconversion photoluminescence in Yb3+, Ho3+ co-doped NaLa(MoO4)2 phosphor, J. Alloys Compd., 2014, 609, 268–273 CrossRef CAS.
- W. T. Zhang, J. F. Li, Y. L. Wang, J. P. Long and K. H. Qiu, Synthesis and luminescence properties of NaLa(MoO4)2−xAGx:Eu3+ (AG = SO42−, BO33−) red phosphors for white light emitting diodes, J. Alloys Compd., 2015, 635, 16–20 CrossRef CAS.
- F. W. Mo, L. Zhou, Q. Pang, F. Z. Gong and Z. J. Liang, Potential red-emitting NaGd(MO4)2:R (M = W, Mo, R = Eu3+, Sm3+, Bi3+) phosphors for white light emitting diodes applications, Ceram. Int., 2012, 38, 6289–6294 CrossRef CAS.
- G. H. Li, S. Lan, L. L. Li, M. M. Li, W. W. Bao, H. F. Zou, X. C. Xu and S. C. Gan, Tunable luminescence properties of NaLa(MoO4)2:Ce3+,Tb3+ phosphors for near UV-excited white light-emitting-diodes, J. Alloys Compd., 2012, 513, 145–149 CrossRef CAS.
- J. S. Liao, H. Z. Huang, H. Y. You, X. Qiu, Y. Li, B. Qiu and H.-R. Wen, Photoluminescence properties of NaGd(MoO4)2:Eu3+ nanophosphors prepared by sol–gel method, Mater. Res. Bull., 2010, 45, 1145–1149 CrossRef CAS.
- F.-B. Cao, L.-S. Li, Y.-W. Tian and X.-R. Wu, Sol–gel synthesis of red-emitting [Na0.6La0.8−xEux]2(MoO4)3 phosphors and improvement of its luminescent properties by the co-doping method, Opt. Laser Technol., 2014, 55, 6–10 CrossRef CAS.
- G. M. Kuz'micheva, D. A. Lis, K. A. Subbotin, V. B. Rybakov and E. V. Zharikov, Growth and structural X-ray investigations of scheelite-like single crystals Er, Ce:NaLa(MoO4)2 and Yb:NaGd(WO4)2, J. Cryst. Growth, 2005, 275, e1835–e1842 CrossRef.
- X. A. Lu, Z. N. You, J. F. Li, Z. J. Zhu, G. H. Jia, B. C. Wu and C. Y. Tu, Optical absorption and spectroscopic characteristics of Tm3+ ions doped NaY(MoO4)2 crystal, J. Alloys Compd., 2008, 458, 462–466 CrossRef CAS.
- X. Z. Li, Z. B. Lin, L. Z. Zhang and G. F. Wang, Growth, thermal and spectral properties of Nd3+-doped NaGd(MoO4)2 crystal, J. Cryst. Growth, 2006, 290, 670–673 CrossRef CAS.
- Y. K. Voron'ko, K. A. Subbotin, V. E. Shukshin, D. A. Lis, S. N. Ushakov, A. V. Popov and E. V. Zharikov, Growth and spectroscopic investigations of Yb3+-doped NaGd(MoO4)2 and NaLa(MoO4)2 - new promising laser crystals, Opt. Mater., 2009, 29, 246–252 CrossRef.
- H. Lin, X. H. Yan and X. F. Wang, Controllable synthesis and down-conversion properties of flower-like NaY(MoO4)2 microcrystals via polyvinylpyrrolidone-mediated, J. Solid State Chem., 2013, 204, 266–271 CrossRef CAS.
- G. H. Li, L. L. Li, M. M. Li, W. W. Bao, Y. H. Song, S. C. Gan, H. F. Zou and X. C. Xu, Hydrothermal synthesis and luminescent properties of NaLa(MoO4)2:Eu3+,Tb3+ phosphors, J. Alloys Compd., 2013, 550, 1–8 CrossRef CAS.
- Y. Huang, L. Q. Zhou, L. Yang and Z. W. Tang, Self-assembled 3D flower-like NaY(MoO4)2:Eu3+ microarchitectures: Hydrothermal synthesis, formation mechanism and luminescence properties, Opt. Mater., 2011, 33, 777–782 CrossRef CAS.
- L. L. Li, W. W. Zi, G. H. Li, S. Lan, G. J. Ji, S. C. Gan, H. F. Zou and X. C. Xu, Hydrothermal synthesis and luminescent properties of NaLa(MoO4)2:Dy3+ phosphor, J. Solid State Chem., 2012, 191, 175–180 CrossRef CAS.
- Y. Tian, B. J. Chen, B. N. Tian, J. S. Sun, X. P. Li, J. S. Zhang, L. H. Cheng, H. Y. Zhong, H. Zhong, Q. G. Meng and R. N. Hua, Ionic liquid-assisted hydrothermal synthesis of dendrite-like NaY(MoO4)2:Tb3+ phosphor, Physica B, 2012, 407, 2556–2559 CrossRef CAS.
- J. C. Zhang, X. F. Wang, X. H. Zhang, X. D. Zhao, X. Y. Liu and L. P. Peng, Microwave synthesis of NaLa(MoO4)2 microcrystals and their near-infrared luminescent properties with lanthanide ion doping (Er3+, Nd3+, Yb3+), Inorg. Chem. Commun., 2011, 14, 1723–1727 CrossRef CAS.
- S. W. Park, B. K. Moon, B. C. Choi, J. H. Jeong, J. S. Bae and K. H. Kim, Red photoluminescence of pulsed laser deposited Eu:NaY(MoO4)2 thin film phosphors on sapphire substrates, Curr. Appl. Phys., 2012, 12, S150–S155 CrossRef.
- K. I. Rybakov, E. A. Olevsky and E. V. Krikun, Microwave sintering: Fundamentals and modeling, J. Am. Ceram. Soc., 2013, 96, 1003–1020 CrossRef CAS.
- H. J. Kitchen, S. R. Vallance, J. I. Kennedy, N. Tapia-Ruiz, L. Carassiti, A. Harrison, A. G. Whittaker, T. D. Drysdale, S. W. Kingman and D. H. Gregory, Modern microwave methods in solid-state inorganic materials chemistry: From fundamentals to manufacturing, Chem. Rev., 2014, 114, 1170–1206 CrossRef CAS PubMed.
- C. S. Lim, Cyclic MAM synthesis and upconversion photoluminescence properties of CaMoO4:Er3+/Yb3+ particles, Mater. Res. Bull., 2012, 47, 4220–4225 CrossRef CAS.
- C. S. Lim, Preparation of PbLa2(MoO4)2:Er3+/Yb3+ particles via microwave sol-gel route and upconversion photoluminescence properties, Ceram. Int., 2015, 41, 12464–12470 CrossRef CAS.
- C. S. Lim, V. Atuchin, A. Aleksandrovsky, M. Molokeev and A. Oreshonkov, Microwave sol-gel synthesis of CaGd2(MoO4)2:Er3+/Yb3+ phosphors and their upconversion photoluminescence properties, J. Am. Ceram. Soc., 2015, 98, 3223–3230 CrossRef CAS.
- Bruker AXS TOPAS V4: General profile and structure analysis software for powder diffraction data – User's Manual, Bruker AXS, Karlsruhe, Germany, 2008 Search PubMed.
- R. M. Hazen, L. W. Finger and J. W. E. Mariathasan, High-pressure crystal chemistry of scheelite-type tungstates and molybdates, J. Phys. Chem. Solids, 1985, 46, 253–263 CrossRef CAS.
- S. B. Stevens, C. A. Morrison, T. H. Allik, A. L. Rheingold and B. S. Haggerty, NaLa(MoO4)2 as a laser host material, Phys. Rev. B: Condens. Matter, 1991, 43, 7386–7394 CrossRef CAS.
- http://www.iucr.org/__data/iucr/cifdic_html/1/cif_core.dic/Cchemical_formula.html .
- R. D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Cryst., 1976, 32, 751–767 CrossRef.
- V. V. Atuchin, V. G. Grossman, S. V. Adichtchev, N. V. Surovtsev, T. A. Gavrilova and B. G. Bazarov, Structural and vibrational properties of microcrystalline TlM(MoO4)2 (M = Nd, Pr) molybdates, Opt. Mater., 2012, 34, 812–816 CrossRef CAS.
- V. V. Atuchin, A. S. Aleksandrovsky, O. D. Chimitova, A. S. Krylov, M. S. Molokeev, B. G. Bazarov, J. G. Bazarova and Z. Xia, Synthesis and spectroscopic properties of multiferroic β′-Tb2(MoO4)3, Opt. Mater., 2014, 36, 1631–1635 CrossRef CAS.
- V. V. Atuchin, A. S. Aleksandrovsky, O. D. Chimitova, T. A. Gavrilova, A. S. Krylov, M. S. Molokeev, A. S. Oreshonkov, B. G. Bazarov and J. G. Bazarova, Synthesis and spectroscopic properties of monoclinic α-Eu2(MoO4)3, J. Phys. Chem., 2014, C 118, 15404–15411 Search PubMed.
- V. V. Atuchin, T. A. Gavrilova, J.-C. Grivel and V. G. Kesler, Electronic structure of layered titanate Nd2Ti2O7, Surf. Sci., 2008, 602, 3095–3099 CrossRef CAS.
- V. V. Atuchin, S. V. Adichtchev, B. G. Bazarov, Zh. G. Bazarova, T. A. Gavrilova, V. G. Grossman, V. G. Kesler, G. S. Meng, Z. S. Lin and N. V. Surovtsev, Electronic structure and vibrational properties of KRbAl2B2O7, Mater. Res. Bull., 2013, 48, 929–934 CrossRef CAS.
- V. Atuchin, L. Zhu, S. H. Lee, D. H. Kim and C. S. Lim, Microwave-assisted solvothermal synthesis of Sr3V2O8 nanoparticles and their spectroscopic properties, Asian J. Chem., 2014, 26(5), 1290–1292 CAS.
- F. Auzel, G. Baldacchini, L. Laversenne and G. Boulon, Radiation trapping and self-quenching analysis in Yb3+, Er3+, and Ho3+ doped Y2O3, Opt. Mater., 2003, 24, 103–109 CrossRef CAS.
- M. Pollnau, D. R. Gamelin, S. R. Lüthi and H. U. Güdel, Power dependece of upconversion luminescence in lanthanide and transition-metal-ion systems, Phys. Rev. B: Condens. Matter, 2000, 61, 3337–3346 CrossRef CAS.
- H. Y. Du, Y. J. Lan, Z. G. Xia and J. Y. Sun, Synthesis and upconversion luminescence properties of Yb3+/Er3+ codoped BaGd2(MoO4)4 powder, Mater. Res. Bull., 2009, 44, 1660–1662 CrossRef CAS.
- B. Li, B. Joshi, Y. K. Kshetri, R. Adhikari, R. Narro-Gracia and S. W. Lee, Upconversion luminescence properties of Er3+/Yb3+ in transparent a-Sialon ceramics, Opt. Mater., 2015, 39, 239–246 CrossRef CAS.
- J. H. Chung, J.-I. Lee, S.-L. Ryu and J. H. Ryu, Visible green upconversion luminescence of Er3+/Yb3+/Li+ co-doped CaWO4 particles, Ceram. Int., 2013, 39, S369–S372 CrossRef CAS.
- J. Castañeda, M. A. Meneses-Nava, O. Barbosa-García, E. de la Rosa-Cruz and J. F. Mosiño, The red emission in two and three steps up-conversion process in a doped erbium SiO2–TiO2 sol–gel powder, J. Lumin., 2003, 102–103, 504–509 CrossRef.
- E. Sarantopoulou, C. Raptis, S. Ves, D. Christofilos and G. A. Kourouklis, Temperature and pressure dependence of Raman-active phonons of CaMoO4: an anharmonicity study, J. Phys.: Condens. Matter, 2002, 14, 8925–8938 CrossRef CAS.
- P. G. Zverev, Vibronic relaxation of Raman modes in CaMoO4 and PbMoO4 molecular ionic crystals, Phys. Status Solidi C, 2004, 1, 3101–3105 CrossRef CAS.
- M. B. Smirnov and V. Yu. Kazimirov, LADY: software for lattice dynamics simulations, JINR Communications, 2001, E 14-2001-159 Search PubMed.
- I. F. Chang and S. S. Mitra, Application of a modified random-element-isodisplacement model to long-wavelength optic phonons of mixed crystals, Phys. Rev., 1968, 172, 924–933 CrossRef CAS.
- A. N. Vtyurin, A. S. Krylov, S. N. Krylova, S. V. Goryainov, V. N. Voronov and A. S. Oreshonkov, Hydrostatic pressure-induced phase transitions in Rb2KInF6 and Rb2KScF6 crystals: Raman spectra and lattice dynamics simulations, Ferroelectrics, 2012, 440, 100–104 CrossRef CAS.
- Y. V. Gerasimova, A. S. Oreshonkov, A. N. Vtyurin, A. A. Ivanenko, L. I. Isaenko, A. A. Ershov and E. I. Pogoreltsev, Infrared absorption investigation of the role of octahedral groups upon the phase transition in the Rb2KMoO3F3 crystal, Phys. Solid State, 2013, 55, 2331–2334 CrossRef CAS.
- A. S. Krylov, A. N. Vtyurin, A. S. Oreshonkov, V. N. Voronov and S. N. Krylova, Structural transformations in a single-crystal Rb2NaYF6: Raman scattering study, J. Raman Spectrosc., 2013, 44, 763–769 CrossRef CAS.
- Z. G. Xia, M. S. Molokeev, A. S. Oreshonkov, V. V. Atuchin, R.-S. Liu and C. Dong, Crystal and local structure refinement in Ca2Al3O6F explored by X-ray diffraction and Raman spectroscopy, Phys. Chem. Chem. Phys., 2014, 16, 5952–5957 RSC.
- A. A. Savina, V. V. Atuchin, S. F. Solodovnikov, Z. A. Solodovnikova, A. S. Krylov, E. A. Maximovskiy, M. S. Molokeev, A. S. Oreshonkov, A. M. Pugachev and E. G. Khaikina, Synthesis, structural and spectroscopic properties of acentric triple molybdate Cs2NaBi(MoO4)3, J. Solid State Chem., 2015, 225, 53–58 CrossRef CAS.
- C. S. Lim, A. Aleksandrovsky, M. Molokeev, A. Oreshonkov and V. Atuchin, Microwave sol-gel synthesis and upconversion photoluminescence properties of CaGd2(WO4)4:Er3+/Yb3+ phosphors with incommensurately modulated structure, J. Solid State Chem., 2015, 228, 160–166 CrossRef CAS.
- R. Fletcher, Practical Methods of Optimization, Wiley, Chichesteretc., 2nd edn, 1987 Search PubMed.
- A. Senyshyn, H. Kraus, V. B. Mikhailik and V. Yakovyna, Lattice dynamics and thermal properties of CaWO4, Phys. Rev. B: Condens. Matter, 2004, 70, 214306 CrossRef.
- A. Senyshyn, H. Kraus, V. B. Mikhailik, L. Vasylechko and M. Knapp, Thermal properties of CaMoO4: Lattice dynamics and synchrotron powder diffraction studies, Phys. Rev. B: Condens. Matter, 2006, 73, 014104 CrossRef.
- F. D. Hardcastle and I. E. Wachs, Determination of molybdenum-oxygen bond distances and bond orders by Raman spectroscopy, J. Raman Spectrosc., 1990, 21, 683–691 CrossRef CAS.
- K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Wiley, New Yorketc., 6th edn, 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6dt02378a |
| This journal is © The Royal Society of Chemistry 2016 |