Open Access Article
Open Access ArticleSmall, beautiful and magnetically exotic: {V4W2}- and {V4W4}-type polyoxometalates†
Maren
Rasmussen
a,
Christian
Näther
a,
Jan
van Leusen
b,
Ulrike
Warzok
c,
Christoph A.
Schalley
c,
Paul
Kögerler
 *b and
Wolfgang
Bensch
*a
*b and
Wolfgang
Bensch
*a
aInstitut für Anorganische Chemie, Christian-Albrechts-Universität zu Kiel, 24118 Kiel, Germany. E-mail: wbensch@ac.uni-kiel.de
bInstitut für Anorganische Chemie, RWTH Aachen University, 52074 Aachen, Germany. E-mail: paul.koegerler@ac.rwth-aachen.de
cInstitut für Chemie und Biochemie der Freien Universität, 14195 Berlin, Germany
First published on 10th June 2016
Abstract
Minimal-nuclearity vanadato-tungstate clusters in [{VIV(dien)}4WVI2O14]·4H2O (1) and [{VIV(dien)}4WVI4O20]·6H2O (2) feature cores of edge-sharing WO6 octahedra, surrounded by a ring of four vanadyl groups. Surprisingly, the V(IV) centers in both 1 and 2 are ferromagnetically coupled, in contrast to all other known vanadato-polyoxotungstates featuring the ubiquituos V–O–W–O–V exchange pathways.
The chemistry of mixed-metal polyoxometalates has witnessed an impressive development during the last few decades, with synthetic and structural aspects, properties and possible applications summarized in several review articles.1 The first mixed V–W polyanions were reported already in the 19th century;2 efficient synthesis protocols were developed for the Lindqvist-type polyanions [VxW6−xO19]−2−x (x = 1, 2),3 the solution stability of which is strongly pH dependent.4 The chemistry of mixed tungstato-vanadate compounds was further developed, resulting primarily in several compounds containing {VxW6−x} (x = 1–3) Lindqvist anions, where V and W atoms usually are disordered over all six metal sites.5 Few other small W/V complexes are known with N- and O-donor ligand environments: in [L′O(H2O)VIV(μ-O)WVIO2L]2+ (L = 1,4,7-triazacyclononane, L′ = 1,4,7-trimethyl-L), VN3O2 and WN3O2 moieties are μ-oxo-bridged,6 in [V2O2(μ-OMe)2(μ-WO4)2(4,4′-di-tert-butyl-2,2′-bipyridine)2], two VN2O3 units are bridged by two WO4 groups.7
After identifying a {V13W4}-type extended Keggin structure under solvothermal conditions at high pH (ca. 12) in the presence of tris(2-aminoethyl)amine (tren),8 we now were able to isolate [(V(dien))4W2O14]·4H2O (1) and [(V(dien))4W4O20]·6H2O (2) (dien = diethylenetriamine, C4H13N3) under similar conditions, where a higher reactant V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W ratio (1
W ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 vs. 1
3 vs. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
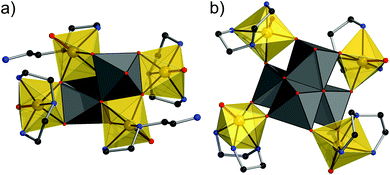
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) appears to favor a smaller W nuclearity.‡ The crystal structures feature rare VN2O4 and VN3O3 moieties interconnected by edge-sharing WO6 octahedra (Fig. 1).
4) appears to favor a smaller W nuclearity.‡ The crystal structures feature rare VN2O4 and VN3O3 moieties interconnected by edge-sharing WO6 octahedra (Fig. 1).
Compound 1 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Table S1†) with all atoms located on general positions. A W2O10 core composed of two edge-sharing WO6 octahedra connects to two VON2 moieties (vanadyl-bidentate diene complexes) via three μ-O sites, and edge-sharing to two VON3 units (vanadyl-tridentate fac-dien complexes). The four V sites form a planar rhomboid (V⋯V: 3.78 Å and 5.38 Å, V–V–V: 70.8°). The N⋯N distances in the VN3O3 octahedron are 2.732, 2.714, and 3.278 Å, and the N–N–N angle amounts to 74°. Vanadium dien complexes are rare, with only two corresponding entries, all of tridentate fac conformation, in the CSD.9 In 1, V–N bonds in VN2O4 and VN3O3 (2.116(4)–2.289(4) Å) exhibit a slight elongation of one V–N bond (V1–N2, Fig. S1†), caused by the trans effect. The V–O bonds (1.620(3)–2.219(3) Å) show the typical short vanadyl V
(Table S1†) with all atoms located on general positions. A W2O10 core composed of two edge-sharing WO6 octahedra connects to two VON2 moieties (vanadyl-bidentate diene complexes) via three μ-O sites, and edge-sharing to two VON3 units (vanadyl-tridentate fac-dien complexes). The four V sites form a planar rhomboid (V⋯V: 3.78 Å and 5.38 Å, V–V–V: 70.8°). The N⋯N distances in the VN3O3 octahedron are 2.732, 2.714, and 3.278 Å, and the N–N–N angle amounts to 74°. Vanadium dien complexes are rare, with only two corresponding entries, all of tridentate fac conformation, in the CSD.9 In 1, V–N bonds in VN2O4 and VN3O3 (2.116(4)–2.289(4) Å) exhibit a slight elongation of one V–N bond (V1–N2, Fig. S1†), caused by the trans effect. The V–O bonds (1.620(3)–2.219(3) Å) show the typical short vanadyl V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds (1.633(4) and 1.620(3) Å). A database analysis (CSD) of compounds containing octahedral VN2O4 or VN3O3 units yielded a slightly smaller mean value around 1.600 Å. The W–O bonds fall into four groups: 1.752(3) Å (Oterm), 1.824(3)–1.910(3) Å (μ2-O), 2.052(3) Å (μ3-OWV2), and 2.356(3) Å (μ3-OW2V), all typical for polyoxotungstates. In 1, the [(V(dien))4W2O14] complexes are arranged in stacks along [100] and [001], and the inter-cluster voids are occupied by crystal water molecules. Intra-cluster N–H⋯O and extensive 3D inter-cluster H bonding interactions stabilize the structure. O6term is involved in three relatively strong H bonding contacts, which may explain the longer V
O bonds (1.633(4) and 1.620(3) Å). A database analysis (CSD) of compounds containing octahedral VN2O4 or VN3O3 units yielded a slightly smaller mean value around 1.600 Å. The W–O bonds fall into four groups: 1.752(3) Å (Oterm), 1.824(3)–1.910(3) Å (μ2-O), 2.052(3) Å (μ3-OWV2), and 2.356(3) Å (μ3-OW2V), all typical for polyoxotungstates. In 1, the [(V(dien))4W2O14] complexes are arranged in stacks along [100] and [001], and the inter-cluster voids are occupied by crystal water molecules. Intra-cluster N–H⋯O and extensive 3D inter-cluster H bonding interactions stabilize the structure. O6term is involved in three relatively strong H bonding contacts, which may explain the longer V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, while O7term has only one such contact (Table S2†). Bond valence sum (BVS) calculations yield values of 4.06/4.09 for V1/V2 and 5.93 for the unique W atom, in line with the formal oxidation states V4+ and W6+ in 1.
O bond, while O7term has only one such contact (Table S2†). Bond valence sum (BVS) calculations yield values of 4.06/4.09 for V1/V2 and 5.93 for the unique W atom, in line with the formal oxidation states V4+ and W6+ in 1.
Compound 2 crystallizes in the monoclinic space group P21/n (Table S1†) with all unique atoms being located on general positions. Here, the cluster core consists of four edge-sharing WO6 octahedra, forming a distorted W4O4 cubane. Four independent vanadyl groups each bind to a tridentate dien ligand in fac conformation and to two O atoms of neighboring WO6 octahedra, resulting in distorted VN3O3 octahedral environments, with two shorter (2.127(7)–2.175(7) Å) and one longer (2.263(5)–2.318(7) Å) V–N bond, the latter trans to the terminal vanadyl O site. The resulting V4 structure is an approximately planar square (V⋯V: 5.94–6.22 Å, root mean square deviation from ideal plane: 0.276 Å). The V–O bonds are similar to those in 1 with one short (1.610(6)–1.628(6) Å, V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oterm) and two longer bonds. The W–O bonds exhibit an identical pattern as in 1. BVS values (V: 3.97–4.17; W: 5.95–6.09) support the proposed oxidation states.
Oterm) and two longer bonds. The W–O bonds exhibit an identical pattern as in 1. BVS values (V: 3.97–4.17; W: 5.95–6.09) support the proposed oxidation states.
In 2, the charge-neutral clusters are arranged in the (010) plane generating channels along [010]. A similar arrangement is observed in the (100) plane, and a second channel type runs along [100]. As in 1, neighbored clusters are interlinked by N–H⋯O interactions, in addition to extensive H bonding to the crystal water molecules present in these channels.
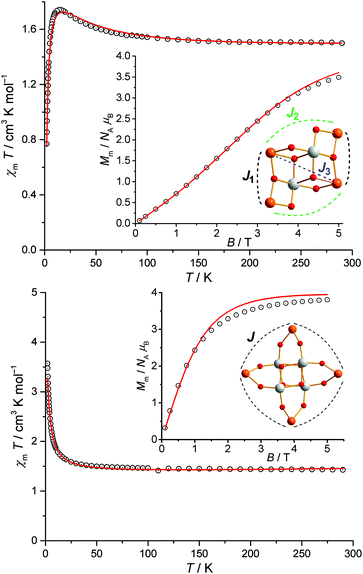
The magnetic properties of 1 and 2 are represented in Fig. 2 as χmT vs. T and Mmvs. B plots. For 1, the ambient temperature (290 K) value of χmT is 1.50 cm3 K mol−1 at 0.1 T. This value lies within the range 1.36–1.53 cm3 K mol−1 expected for four non-interacting VIV centers. Upon cooling χmT continuously increases up to a maximum of 1.74 cm3 K mol−1 at 14 K, and subsequently drops off sharply down to 0.77 cm3 K mol−1 at 2.0 K. At 2.0 K, the molar magnetization Mm as a function of the applied field B shows an inflection point at ca. 2.5 T revealing the presence of minor antiferromagnetic exchange interactions (the inflection point here indicates a change of the total spin ground state). Modeling the magnetic properties of 1 utilized the computational framework CONDON, employing a “full model” Hamiltonian,10 and assumed four identical V(IV) centers in a C4v-symmetric ligand field, reflecting the pronounced tetragonal distortion typical for vanadyl groups. Five Heisenberg-type exchange interaction pathways between nearest-neighbor V(IV) sites (Fig. 2, inset) are characterized by three independent exchange parameters J1 (V–O–V and V–O–WVI–O–V), J2 (V–O–WVI–O–V) and J3 (2 × V–O–WVI–O–V). The O–WVI–O bridges here efficiently mediate the coupling via the extended, unoccupied W 6d orbitals. For fitting purposes, the standard spin–orbit coupling constant ζ3d = 248 cm−1 is taken as a constant,11 and all 10 states of a 3d1 electron configuration are accounted for in the calculation of single ion (vanadyl) effects and Heisenberg exchange interactions (“−2J” notation), i.e. considering in total 104 states. Finally, we consider the mean-field approach for potential inter-molecular interactions in the solid-state lattice. The least-squares fit (relative root mean squared error, SQ = 1.7%) yields the ligand field parameters (Wybourne notation) B20 = 4230 cm−1, B40 = 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 cm−1, B44 = 31
250 cm−1, B44 = 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 310 cm−1, the exchange interaction parameters J1 = +15.6 cm−1, J2 = –3.7 cm−1, J3 = +5.9 cm−1, and the mean-field interaction parameter zJ′ = +0.1 cm−1. The ligand field parameters Bkq describe a ligand field characterized by strong tetragonal distortion generating a well-isolated Kramer's ground state doublet separated from the first excited state by more than 4000 cm−1, reconfirming the almost spin-like behavior of the vanadyl groups. The exchange interaction parameters show predominant ferromagnetic exchange, and the additional antiferromagnetic exchange pathways yields a ground state characterized by Stotal = 0, slightly separated (approx. 2 cm−1) from the first excited Stotal = 1 state, translating into Mm ≈ 2.0NAμB as reflected by the inflection point in the Mmvs. B curve. Inter-cluster interactions are almost negligible.
310 cm−1, the exchange interaction parameters J1 = +15.6 cm−1, J2 = –3.7 cm−1, J3 = +5.9 cm−1, and the mean-field interaction parameter zJ′ = +0.1 cm−1. The ligand field parameters Bkq describe a ligand field characterized by strong tetragonal distortion generating a well-isolated Kramer's ground state doublet separated from the first excited state by more than 4000 cm−1, reconfirming the almost spin-like behavior of the vanadyl groups. The exchange interaction parameters show predominant ferromagnetic exchange, and the additional antiferromagnetic exchange pathways yields a ground state characterized by Stotal = 0, slightly separated (approx. 2 cm−1) from the first excited Stotal = 1 state, translating into Mm ≈ 2.0NAμB as reflected by the inflection point in the Mmvs. B curve. Inter-cluster interactions are almost negligible.
The low-field χmT value of 2 at 290 K of 1.45 cm3 K mol−1 falls into the expected range for four non-interacting VIV centers. Upon cooling χmT increases sharply below ca. 50 K, reaching 3.57 cm3 K mol−1 at 2.0 K. At 2.0 K, Mm is linear in B up to 1 Tesla, and indicates saturation for fields larger than 5 T at approximately Mm = 4NAμB, i.e. pointing to an Stotal = 2 ground state, i.e. in line with dominant ferromagnetic exchange interactions in 2. In analogy to the analysis of 1 except for the coupling scheme (four V–O–W–O–V pathways characterized by a single exchange energy J), the least-squares fit (SQ = 3.2%) yields B20 = 120 cm−1, B40 = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 630 cm−1, B44 = 29
630 cm−1, B44 = 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 460 cm−1, J = +2.7 cm−1, and the mean-field interaction parameter zJ′ = +0.1 cm−1. As for 1, the ligand field parameters here correspond to a strong tetragonal distortion of the V ligand field, generating a well-isolated (ca. 6000 cm−1) Kramer's ground state doublet. Note that the common V coordination geometry in 2 is significantly different from 1 (two slightly different site geometries), resulting in different ligand field parameters. The positive J reveals small ferromagnetic nearest-neighbor coupling in 2. The ground state of 2 amounts to Stotal = 2, consistent with the observed saturation value of Mm ≈ 4.0NAμB. As for 1, inter-cluster coupling in 2 is almost negligible.
460 cm−1, J = +2.7 cm−1, and the mean-field interaction parameter zJ′ = +0.1 cm−1. As for 1, the ligand field parameters here correspond to a strong tetragonal distortion of the V ligand field, generating a well-isolated (ca. 6000 cm−1) Kramer's ground state doublet. Note that the common V coordination geometry in 2 is significantly different from 1 (two slightly different site geometries), resulting in different ligand field parameters. The positive J reveals small ferromagnetic nearest-neighbor coupling in 2. The ground state of 2 amounts to Stotal = 2, consistent with the observed saturation value of Mm ≈ 4.0NAμB. As for 1, inter-cluster coupling in 2 is almost negligible.
Compound 2 is soluble in water (0.24 mmol L−1), while the solubility of 1 is extremely low. Positive-mode electrospray ionization of a 100 μM water solution of 2 results in an ESI mass spectrum exhibiting the intact cluster as the singly and doubly protonated species at m/z = 836 und 1672 (Fig. 3). The base peak of the spectrum can be assigned to [(V(dien))4W4O19]2+ which is most likely formed by elimination of H2O upon protonation of the cluster. Measurements were performed shortly after preparation of the sample solution in degassed H2O as the cluster complex was only stable in solution over a period of 30 minutes.
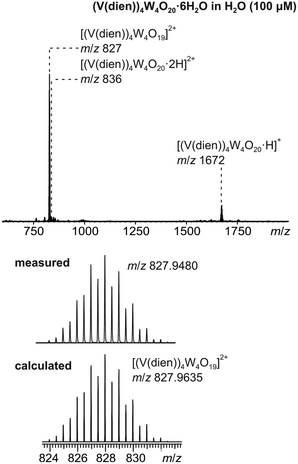 | ||
| Fig. 3 ESI-Q-TOF-HRMS spectrum of compound 2 (100 μM in H2O, top); experimental isotopic pattern of dication at m/z 827 and calculated isotopic pattern of [(V(dien))4W4O19]2+. | ||
In summary, we infer from the two title compounds that the molecular growth of polyoxotungstates at pH ca. 12 appears to be impeded by coordination of VO(dien)2+ groups and the associated decrease in negative molecular charge, effectively stopping at {V4W2} and {V4W4} nuclearities. Comparison to species formed at similar conditions such as the {V13W4}-type polyanion emphasizes the crucial role of the employed polyamines. These clusters are among the smallest known heterometal polyoxometalates and as such demonstrate the utility of polydentate ligands such as dien in the isolation of novel polyoxometalates structures. To our great surprise, the resulting exchange pathway geometries allow for ferromagnetic coupling between neighboring vanadyl groups, in stark contrast to the usually strongly antiferromagnetic coupling present in larger vanadato-polyoxometalates featuring similar VIV–O–MVI–O–VIV motifs such as the {MVI72V30} Keplerate polyanions.12
Notes and references
- (a) O. Oms, A. Dolbecq and P. Mialane, Chem. Soc. Rev., 2012, 41, 7497 RSC; (b) K. Y. Monakhov, W. Bensch and P. Kögerler, Chem. Soc. Rev., 2015, 44, 8443 RSC; (c) A. Proust, R. Thouvenot and P. Gouzerh, Chem. Commun., 2008, 1837 RSC; (d) A. Müller, P. Kögerler and H. Bögge, Struct. Bonding, 2000, 96, 203 CrossRef.
- (a) R. Finkener, Ber. Dtsch. Chem. Ges., 1878, 11, 1638 CrossRef; (b) A. Rosenheim and H. Jahn, Ber. Dtsch. Chem. Ges., 1893, 26, 1191 CrossRef; (c) C. Friedheim, Z. Anorg. Chem., 1894, 6, 11 CrossRef.
- C. M. Flynn and M. T. Pope, Inorg. Chem., 1971, 10, 2524 CrossRef CAS.
- (a) C. M. Flynn and M. T. Pope, Inorg. Chem., 1973, 12, 1626 CrossRef CAS; (b) C. M. Flynn and M. T. Pope, Inorg. Chem., 1971, 10, 2745 CrossRef CAS.
- (a) L. Ouahab, S. Golhen, S. Triki, A. Łapinski, M. Golub and R. Swietlik, J. Cluster Sci., 2002, 13, 267 CrossRef CAS; (b) W. Huang, L. Todaro, L. C. Francesconi and T. Polenova, J. Am. Chem. Soc., 2003, 125, 5928 CrossRef CAS PubMed; (c) H. Driss, R. Thouvenot and M. Debbabi, Polyhedron, 2008, 27, 2059 CrossRef CAS; (d) J.-H. Son and Y.-U. Kwon, Inorg. Chem., 2004, 43, 1929 CrossRef CAS PubMed; (e) P. T. Ma, C. F. Yu, J. W. Zhao, Y. Q. Feng, J. P. Wang and J. Y. Niu, J. Coord. Chem., 2009, 62, 3117 CrossRef CAS; (f) X. Wang, B. Zhou, C. Zhong and M. Ji, Cryst. Res. Technol., 2006, 41, 874 CrossRef CAS; (g) C. Wang, L. Weng, Y. Ren, C. Du, B. Yue, M. Gu and H. He, Z. Anorg. Allg. Chem., 2011, 637, 472 CrossRef CAS; (h) Y. Xu, J.-Q. Xu, G.-Y. Yang, T.-G. Wang, Y. Xing, Y.-H. Lin and H.-Q. Jia, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1998, 54, 563 Search PubMed.
- U. Bossek, P. Knopp, C. Habenicht, K. Wieghardt, B. Nuber and J. Weiss, J. Chem. Soc., Dalton Trans., 1991, 3165 RSC.
- S. Kodama, A. Nomoto, S. Yano, M. Ueshima and A. Ogawa, Inorg. Chem., 2011, 50, 9942 CrossRef CAS PubMed.
- M. Rasmussen, C. Näther, J. van Leusen, P. Kögerler and W. Bensch, Eur. J. Inorg. Chem., 2015, 3285 CrossRef CAS.
- (a) M.-L. Fu, G.-C. Guo, A.-Q. Wu, B. Liu, L.-Z. Cai and J.-S. Huang, Eur. J. Inorg. Chem., 2005, 3104 CrossRef CAS; (b) J. Wang, C. Näther, J. Djamil and W. Bensch, Z. Anorg. Allg. Chem., 2012, 638, 1452 CrossRef CAS.
- (a) J. van Leusen, M. Speldrich, H. Schilder and P. Kögerler, Coord. Chem. Rev., 2015, 289–290, 137 CrossRef CAS; (b) M. Speldrich, H. Schilder, H. Lueken and P. Kögerler, Isr. J. Chem., 2011, 51, 215 CrossRef CAS.
- J. S. Griffith, The Theory of Transition-Metal Ions, Cambridge University Press, Cambridge, 1971 Search PubMed.
- P. Kögerler, B. Tsukerblat and A. Müller, Dalton Trans., 2010, 39, 21 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental, crystallographical and structural details, optical properties and thermal stability data. CCDC 1475726 and 1475727. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt02282k |
| ‡ Reaction of 1 mmol NH4VO3 and 3 mmol WO3·H2O in a mixture of 2 mL concentrated diethylentriamine and 2 mL water in a sealed glass tube at 130 °C afforded green rod-shaped crystals of 1 after 7 d (70% yield based on V). Orange block-shaped crystals of 2 formed under otherwise identical conditions with 1 mmol NH4VO3 and 4 mmol WO3·H2O (60% yield based on V). CCDC 1475726 (1) and 1475727 (2). |
| This journal is © The Royal Society of Chemistry 2016 |