Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Single ion magnets based on lanthanoid polyoxomolybdate complexes†
José J.
Baldoví‡
a,
Yan
Duan‡
 a,
Carlos
Bustos
bc,
Salvador
Cardona-Serra
d,
Pierre
Gouzerh
c,
Richard
Villanneau
c,
Geoffrey
Gontard
c,
Juan M.
Clemente-Juan
a,
Alejandro
Gaita-Ariño
*a,
Carlos
Giménez-Saiz
a,
Carlos
Bustos
bc,
Salvador
Cardona-Serra
d,
Pierre
Gouzerh
c,
Richard
Villanneau
c,
Geoffrey
Gontard
c,
Juan M.
Clemente-Juan
a,
Alejandro
Gaita-Ariño
*a,
Carlos
Giménez-Saiz
 *a,
Anna
Proust
*c and
Eugenio
Coronado
*a
*a,
Anna
Proust
*c and
Eugenio
Coronado
*a
aInstituto de Ciencia Molecular (ICMol), Universidad de Valencia, C/Catedrático José Beltran, 2, E-46980 Paterna, Spain. E-mail: alejandro.gaita@uv.es; carlos.giménez@uv.es; eugenio.coronado@uv.es
bFacultad de Ciencia, Instituto de Química, Campus Isla Teja, Universidad Austral de Chile, Valdivia, Chile
cSorbonne Universites, UPMC-Paris 06, UMR 8232, Institut Parisien de Chimie Moléculaire, 4 Place Jussieu, F-75005 Paris, France. E-mail: anna.proust@upmc.fr
dTrinity College Dublin, College Green, Dublin 2, Ireland
First published on 25th August 2016
Abstract
Polyoxometalate (POM) chemistry has recently offered excellent examples of single ion magnets (SIMs) and molecular spin qubits. Compared with conventional coordination compounds, POMs provide rigid and highly symmetric coordination sites. However, all POM-based SIMs reported to date exhibit a very limited range of possibilities for chemical processability. We present herein two new families of POM-based SIMs which are soluble in organic solvents: [Ln(β-Mo8O26)2]5− {LnIII = Tb, Dy, Ho, Er, Tm and Yb} and the functionalised POMs [Ln{Mo5O13(OMe)4NNC6H4-p-NO2}2]3− {LnIII = Tb, Dy, Ho, Er, Yb and Nd}. In addition, these two families represent the first SIMs based on polyoxomolybdates. A magneto-structural analysis of these families is presented, which is based on an effective crystal field model, and compared with the results reported in analogous lanthanoid SIMs based on polyoxotungstates.
Introduction
The main goal of spintronics is the active manipulation of the electron spin degrees of freedom in solid-state systems for carrying information.1,2 Spintronic systems have experienced a rapid development and currently are used in a range of applications, including read-heads devices and non-volatile magnetic memories (MRAM).3,4 Extraordinary potential for these systems is expected for the fabrication of spin-transfer nano-oscillators (STNOs)5 and quantum computers.6–8 The progress of molecular electronics and molecular magnetism has led to the emergence of a new field known as molecular spintronics,9,10 which combines the ideas and concepts of spintronics with the singular possibilities offered by molecular electronics and molecular magnetism to develop a second generation of spintronic devices.11–14A particularly challenging area within this field is that of single-molecule spintronics, which intends to use individual molecules as main components of spintronic devices. In this context, single-molecule magnets (SMMs)15 have been proposed as promising candidates.16–18 These systems, which represent the limit of miniaturization of nanomagnets, are between the most complex magnetic entities, exhibiting slow relaxation of the magnetization19 and magnetic hysteresis20 at liquid-helium temperatures. Moreover, they may also present quantum phenomena from purely molecular origin.21–24 In this context, SMMs based on mononuclear lanthanide complexes deserve a special attention.25,26 The first example of this class of molecular nanomagnets, also known as single-ion magnets (SIMs), was reported by Ishikawa and co-workers in the series with general formula [LnPc2]−, where lanthanoid ions are sandwiched between two phthalocyaninato moieties displaying a square-antiprismatic D4d symmetry.27 Derivatives closely related to this family include the oxidised terbium phthalocyaninato complex [TbPc2]0. This system, besides its “double-decker” structure which favours adsorption on surfaces, is electrically neutral. This feature facilitates its sublimation under UHV conditions. The processability of lanthanoid phthalocyaninato complexes has allowed a series of breakthroughs including the realization of molecular/supramolecular spin valves28,29 and the electrical control of nuclear spin qubits.30,31 Indeed, sublimable lanthanoid complexes have demonstrated a great potential in molecular spintronics. Among the few sublimable lanthanoid-based systems reported so far, we can highlight the trinuclear Tb3+ complex Tb3(OQ)9 (OQ = quinolinato), where spin-polarised hopping transport has been realised.32 It has also been proposed as a model system for quantum error correction in quantum computing.33
A second series of SIMs is that provided by polyoxometalate (POM) chemistry,34 a class of systems that has produced a number of conventional SMMs.35,36 In this case, mononuclear lanthanoid complexes encapsulated by POMs have produced key examples of spin qubits for quantum computing. Indeed, extended quantum coherence was recently achieved on concentrated samples of the holmium derivative of the series [Ln(W5O18)2]9− (in short LnW10) through the use of atomic clock transitions.37 Employing a different strategy, a large number of coherent manipulations was realised in the Gd3+ derivative38 of the [LnP5W30O110]12− series (in short LnW30).39 Within the same POM family, current rectification was achieved in a single molecule diode of DyW30.40 Still, the incorporation of these molecular inorganic polyanions onto surfaces or its anchoring to electrodes have been limited by their poor solubility in organic solvents and by their difficult functionalization with organic ligands.
Here, we have prepared two families of POM-based mononuclear lanthanide complexes with a rigid square antiprism structure, analogous to that of LnW10, which overcome these processing limitations. The first family is formulated as [Ln(β-Mo8O26)2]5− (in short, LnMo16), {LnIII = Tb, Dy, Ho, Er, Tm and Yb} and consists of a lanthanide ion trapped by two [β-Mo8O26]4− moieties.41 The second family is formulated as [Ln{Mo5O13(OMe)4NNC6H4-p-NO2}2]3− (in short, LnMo10), {LnIII = Tb, Dy, Ho, Er, Yb and Nd}. In this cases the lanthanide ion is trapped by a functionalised POM based on a lacunary Lindqvist-type pentamolybdate.42
Results and discussion
Solubility and stability
Crystal structures
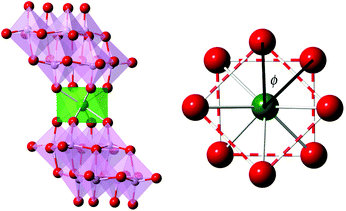 | ||
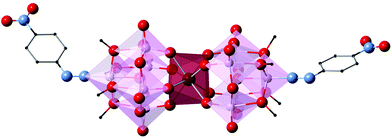
| Fig. 1 (Left) Polyhedral and ball-and-stick representation of LnMo16 series and (right) projection of the coordination sphere showing the square-antiprismatic coordination site. | ||
| LnMo16 | LnMo10 | |||||
|---|---|---|---|---|---|---|
d
pp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (Å) a (Å) |
d
in![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (Å) b (Å) |
φ (°) |
d
pp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (Å) a (Å) |
d
in![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (Å) b (Å) |
φ (°) | |
| a d pp defines the average distance between two oxygen based square planes. b d in is the average O–O distance within the oxygen-based square planes. c φ is defined as the relative orientation between the two squares defined by the coordinating oxygen atoms. | ||||||
| Tb | 2.604(13) | 2.853(15) | 40.2(5) | 2.661(8) | 2.816(8) | 44.1(2) |
| Dy | 2.552(18) | 2.844(18) | 44.1(5) | 2.640(6) | 2.797(6) | 44.2(2) |
| Ho | 2.553(10) | 2.825(10) | 44.3(3) | 2.638(5) | 2.786(5) | 39.2(7) |
| Er | 2.551(11) | 2.819(11) | 44.2(3) | 2.614(6) | 2.783(6) | 40.0(6) |
| Tm | 2.506(14) | 2.818(14) | 44.1(4) | — | — | — |
| Yb | 2.506(14) | 2.796(14) | 44.3(4) | 2.585(6) | 2.759(6) | 39.3(7) |
Magnetic properties
The most satisfactory agreement was obtained when the radial displacement (Dr) equals 0.72 Å and the effective charge (Zi) is 0.253. If we compare the obtained parameters with the ones extracted in the families LnW10 and [Ln(β2-SiW11O39)2]13− (in short: LnW22),45 the radial displacement is smaller in this case. This means that the effective point charge needs a smaller covalent correction to produce an adequate relation between the crystal-field parameters that describe the experimental data. This difference can be related to the different Pauling electronegativity of the Mo and W atoms (2.16 and 2.36 respectively).46 The larger difference in electronegativity between Mo (2.16) and O (3.44) enhances the ionic character of the Mo–O bonding and also the coordination bond Ln–O.
The resulting energy levels for all the series are reported in the ESI (Fig. S10†). A general trend for LnMo16 compared with LnW10 and LnW22 is a slightly larger crystal field splitting. In average, the Ln–O distance is practically identical in both series, e.g. in ErW10 and ErMo16 (2.367(7) Å and 2.367(3) Å). This would mean that polyoxomolybdates tend to produce a slightly stronger ligand field splitting compared with polyoxotungstates at a given metal–ligand distance. For a deeper analysis of the compounds that exhibit SMM properties in both families, the Stark sublevels of the HoMo16 and ErMo16 derivatives are plotted in Fig. 4. Regarding the lower states wave functions, one can observe that in the case of the HoMo16 the ground state is defined by a mixture of 47% of |+4> and 47% of |−4>. In this case, the crystal field operators enable a quantum tunnelling splitting between wave functions with a strong mixture of MJ components, and thus in absence of an external magnetic field no blocking of the magnetic moment is to be expected. Nevertheless when a longitudinal external field is applied, the purity of the MJ = +4 and MJ = −4 is recovered and thus, relaxation via tunnelling is cancelled and a blocking of the magnetization is possible. For ErMo16 the wave function for the ground state appears to be MJ = 0.78|±1/2> + 0.12|±13/2> with two states lying closely above it (at about 1.6 and 3.7 cm−1). Those are described by MJ = 0.78|±15/2> –marked in red in Fig. 4– and MJ = 0.74|±13/2> + 0.15|±15/2> respectively. The low energy difference between the ground and the first excited state (and even the second) is definitively below the precision of the method. Also, thermal effects over the chemical structure, while only slightly affecting the energy level description,47 are expected to become significant. This is expected to affect our modelling of the magnetization. As seen below, it is likely from the dynamic susceptibility behaviour that the Kramers doublet described by 78% of ±15/2 is the actual ground doublet. Within this assumption, the ground wave function would show a high MJ projection compatible with SMM behaviour.
 | ||
| Fig. 4 Energy level scheme and main contributions to the ground state wave function for HoMo16 and ErMo16. | ||
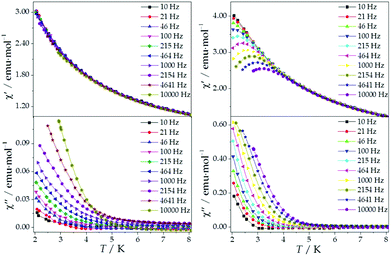
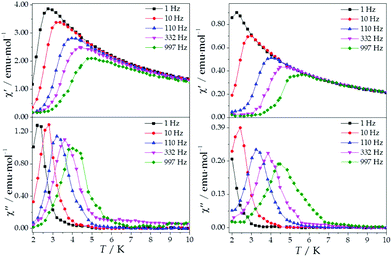
The ac magnetic susceptibilities were collected in the range 2–12 K with an applied alternating field of 3.95 Oe at different frequencies in the range 1–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz. ErMo16 and HoMo16 show frequency dependent out-of-phase signals at very low temperatures. In neither complex a clear peak is observed (Fig. S2 and S3†) even under an external applied field of 1000 Oe. (Fig. 5 and 6) This feature is a characteristic of the fast relaxation process that takes place at low temperatures. In none of the other derivatives an increase in the out-of-phase signal down to 2 K was observed.
000 Hz. ErMo16 and HoMo16 show frequency dependent out-of-phase signals at very low temperatures. In neither complex a clear peak is observed (Fig. S2 and S3†) even under an external applied field of 1000 Oe. (Fig. 5 and 6) This feature is a characteristic of the fast relaxation process that takes place at low temperatures. In none of the other derivatives an increase in the out-of-phase signal down to 2 K was observed.
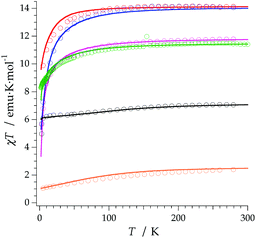
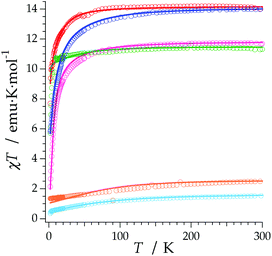
The REC model permits to characterize the magnetic and spectroscopic properties of a family of complexes using a range of approaches. An extreme limit is the rough prediction with no free parameters,48 which allows the discovery of new SIMs. On the opposite extreme, one can perform an individual fit for each metal, which describes the observables more accurately but has a more reduced ability to extrapolate the results to related derivatives. In this case, we have decided to employ an intermediate path. Owing to the chemical similarity of the two families of polyoxomolybdates presented in this work, especially in the coordination environment, the product of the parameters Dr = 0.72 Å and Zi = 0.253 obtained in the study of LnMo16 was used to reduce the number of independent REC parameters for each metal when modelling the magnetic properties of LnMo10. The magnetic susceptibility curves of this series were fitted individually keeping the relation f = Dr·Zr constant, allowing just one free parameter per compound. The results are plotted in Fig. 6, where we can observe the excellent agreement between the model and the experiment. The sharp decrease that appears in ErMo10 below 10 K is reminiscent of the behavior previously observed at the same temperature range in other complexes,49,50 such as [Er(COT)2]−, [Er(COT′′)2]− and Er(Cp*COT), where COT = cyclooctatetraenyl dianion, COT′′ = 1,4-bis-(trimethylsilyl) cyclooctatetraenyl dianion and Cp* = pentamethylcyclopentadienide. As in the present case, in those examples the ground doublet can be characterized as an Ising state (MJ = ±15/2), and this may favour a stronger dipolar interaction at low temperature. The estimation of the energy levels (Fig. S8†) and wave functions of this new family of polyoxomolybdates are reported in the ESI.†
An analysis of the frequency dependence of the χ′′ peaks through Arrhenius plots (Fig. S6†) has permitted to estimate the magnetization-relaxation parameters. Compared with analogous polyoxotungstates, polyoxomolybdates display poorer behaviour as SIMs. Out of the whole series, Dy3+, Er3+ and Yb3+ complexes can be characterised as SIMs (displaying clear peaks above 2 K) and only under an external dc field H = 1000 G. Under these conditions, a naïve fitting of the ac data of the Dy3+, Yb3+ derivatives assuming an Orbach process would result in a barrier height (Ueff/kB) of 38.5 K with a preexponential factor (τ0) of 6.6 × 10−8 s for the Dy3+ case and 23.3 K with τ0 = 6.7 × 10−6 s for the Yb3+ case, compared with the archetypical ErW10 (Ueff/kB) = 55.2 K and τ0 = 1.6 × 10−8 s. These plots do not contain enough information to independently fit a Raman process, but this should not be taken as a proof of an Orbach mechanism, especially lacking spectroscopic indication of levels at these energies. This is even more marked for the Er3+ derivative, which displays only two clear χ′′ peaks, precluding an Arrhenius fit.
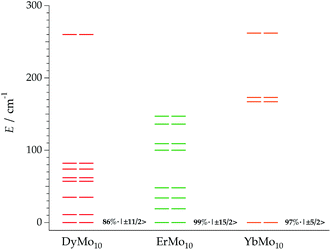
The calculated energy level schemes for DyMo10, ErMo10 and YbMo10 are represented in Fig. 8. According to the individual magnetic susceptibility data fits based on the predictions of the REC model, DyMo10 possesses a ground state dominated by MJ = ±11/2 (86%) with an excited state of MJ = 0.92|±9/2>, which is placed at about 11 cm−1 above it. Such scheme is compatible with the observed SMM behaviour. One can notice that in this example all the MJ states are bunched below 100 cm−1 with the exception of the highest MJ = 15/2 which is located at 260 cm−1. In ErMo10, the ground state is characterised by 99% of MJ = 15/2 and the first excited Kramers doublet is placed at about 19 cm−1. In the case of YbMo10, the level scheme also supports the relationship between the ground-state wave function (described by MJ = 97% of |±5/2>) and the SMM behaviour. The rest of the energy levels appear at about 167 cm−1 above it, starting with the MJ = 0.69|±3/2>. The absence of mixing provides a quasi-pure MJ function in the ground state without a clear path to invert its moment, thus the magnetic moment may be blocked at low temperatures. Here one again needs to highlight the risk of an Orbach-only interpretation in absence of richer experimental information.
Magneto-structural correlations
The optimization of f-element single-molecule magnets is still an open problem.26 Thus, at this point, it is worth to briefly compare the results of a given lanthanide ion as a function of the different ligand fields created in the different families of POMs showing an approximate D4d symmetry. For that, we will focus in the erbium analogue because its crystal structure has been determined for ErW10, ErW22, ErMo16 and ErMo10. Regarding the lowest energy levels, we found a ground state characterised by ±13/2 in both polyoxotungstates, and a first excited state dominated by ±1/2 (placed at about 16 cm−1 in ErW10 and 11 cm−1 in ErW22). In the case of ErMo10, the ground doublet is apparently dominated by ±15/2 (99%) and the first excited state (located at about 19 cm−1) presents 85% of ±1/2, according to our theoretical calculations based on available susceptibility data. The explanation of such a different energy scheme can be attributed to differences in the first coordination sphere, as well as differences in the effective charges and distances of the oxygen donor atoms. Thus, while in the two polyoxotungstate series the interplanar distances, dpp, are equal to 2.46 Å in ErW10 and 2.48 Å in ErW22, in the ErMo10 this distance increases to a value of 2.61 Å. ErMo16 represents an intermediate situation, having dpp = 2.55 Å and a skew angle comparable to that of ErW10 (44.2°). In this case, these inexpensive calculations indicate that there are three Kramers doublets very close in energy, two of them dominated by high MJ values and one dominated by ±1/2, being difficult to determine the true nature of the ground Kramers doublet.Conclusions
In the last years, a sufficiently large set of lanthanoid-based SIMs has already been obtained. This has provided a good basis for relating their magnetic properties with the nature and structure of the coordination site around the lanthanide ion. A subsequent development that has been recently initiated is that related with the improvement in the chemical processability of these magnetic molecules in order to incorporate them into functional devices.The present work contributes towards these two goals. Thus, we have used a robust family of ligands based on POMs to prepare new SIMs exhibiting SMM properties, which can be modelled using an effective crystal field approach previously developed for this kind of ligands. The key novelty has been the unprecedented use of polyoxomolybdates instead of polyoxotungstates for obtaining SIMs. While the chemistry of polyoxomolybdates is more challenging, this result has opened the possibility of making these magnetic molecules soluble in organic solvents, thus facilitating their processing and incorporation into devices. This contrasts with the SIMs based on polyoxotungstate ligands, which are commonly only soluble in water. Furthermore, we have also shown that polyoxomolybdate-based SIMs can be functionalised with organic groups, facilitating their processability -e.g. grafting onto surfaces/electrodes – and the incorporation of a second property coming from the organic ligand. Last but not least, the enhanced reduction potential of polyoxomolybdates may also be of interest to incorporate delocalised electrons into the mixed-valence POM framework, thus increasing the interest of these magnetic molecules in spintronics.51–54
Experimental methods
All reagents and solvents used were commercially available. Acetonitrile was distilled under argon before use. The precursor for the LnMo16 series: [TBA]4[β-Mo8O26], where TBA = (n-C4H9)4N was prepared according to a previously described literature procedure.55The precursor [TBA]3[Mo6O18NN-C6H4-p-NO2] of the LnMo10 series was prepared as described elsewhere.56 The subsequent synthesis of the ligand [TBA]2[Mo5O13(MeO)4(NNC6H4-p-NO2){Na(MeOH)}] was slightly modified from the initial report (see below).42
Synthesis of LnMo16 series
The salts [TBA]5[Ln(β-Mo8O26)2] (LnIII = Tb, Dy, Ho, Er, Tm, and Yb) were prepared following a slight modification of a previously described procedure.43 A solution of lanthanide salt Ln(NO3)3 (0.200 mmol) in 5 mL of acetonitrile was added drop-wise to a solution of [TBA]4[β-Mo8O26] (0.376 mmol) in 25 mL of acetonitrile under stirring. Then, 50 mL of diethyl ether were added carefully, in such a way that two layers were formed. After three days of slow diffusion, block-shaped crystals formed and were collected by filtration, washed with a small amount of acetonitrile, and dried under vacuum overnight (see ESI† for details of the elemental analysis and yield IR spectra).Synthesis of LnMo10 series
Magnetic measurements
Magnetic static and dynamic measurements of samples of LnMo16 and LnMo10 were performed by compacted powder molded from ground crystalline samples. Variable-temperature susceptibility measurements were carried out under a magnetic field of 1000 Oe in the temperature range 2–300 K on a magnetometer equipped with a SQUID sensor (Quantum Design MPMS-XL-5). The data were corrected for diamagnetic contribution from eicosane and for the diamagnetic contributions of the polyanions as deduced by using the Pascal's constant tables. Isothermal magnetization measurements at low temperature (2 K) were performed up to a field of 5 T in the same apparatus (see ESI†).Ligand field calculations
Acknowledgements
The present work has been funded by the EU (ERC Consolidator Grant DECRESIM, COST Actions CM1203 “Polyoxometalate Chemistry for Molecular Nanoscience, PoCheMon” and CA15128 Molecular Spintronics (MOLSPIN)), the Spanish MINECO (grant MAT2014-56143-R, CTQ2014-52758-P and Excellence Unit María de Maeztu MDM-2015-0538 granted to ICMol), and the Generalitat Valenciana (Prometeo Programme of Excellence). A. G.-A. acknowledges funding by the MINECO (Ramón y Cajal contract). E. C. and J. J. B. acknowledge the Blaise Pascal International Chair for financial support. S. C.-S. acknowledges the Generalitat Valenciana for a VALi+D postdoctoral grant. We thank J. M. Martínez-Agudo for performing the magnetic measurements.Notes and references
- S. A. Wolf, D. D. Awschalom, R. A. Buhrman, J. M. Daughton, S. Von Molnár, M. L. Roukes, A. Y. Chtchelkanova and D. M. Treger, Science, 2001, 294, 1488–1495 CrossRef CAS PubMed.
- M. E. Flatté, Nature, 2009, 462, 419–420 CrossRef PubMed.
- J. Akerman, Science, 2005, 308, 508–510 CrossRef CAS PubMed.
- I. L. Prejbeanu, M. Kerekes, R. C. Sousa, H. Sibuet, O. Redon, B. Dieny and J. P. Nozières, J. Phys.: Condens. Matter, 2007, 19, 165218 CrossRef.
- B. Georges, J. Grollier, M. Darques, V. Cros, C. Deranlot, B. Marcilhac, G. Faini and a. Fert, Phys. Rev. Lett., 2008, 101, 4–7 CrossRef PubMed.
- J. R. Petta, A. C. A. Johnson, J. Taylor, E. A. Laird, A. Yacoby, M. D. Lukin, C. M. Marcus, M. Hanson and A. Gossard, Science, 2005, 309, 2180–2184 CrossRef CAS PubMed.
- J. F. Gregg, Nat. Mater., 2007, 6, 798–799 CrossRef CAS PubMed.
- K. L. Wang, I. Ovchinnikov, F. Xiu, A. Khitun and M. Bao, J. Nanosci. Nanotechnol., 2011, 11, 306–313 CrossRef CAS PubMed.
- A. R. Rocha, V. M. García-Suárez, S. W. Bailey, C. J. Lambert, J. Ferrer and S. Sanvito, Nat. Mater., 2005, 4, 335–339 CrossRef CAS PubMed.
- S. Sanvito, Nat. Phys., 2010, 6, 562–564 CrossRef CAS.
- J. Camarero and E. Coronado, J. Mater. Chem., 2009, 19, 1678–1684 RSC.
- F. Prins, M. Monrabal-Capilla, E. A. Osorio, E. Coronado and H. S. J. Van Der Zant, Adv. Mater., 2011, 23, 1545–1549 CrossRef CAS PubMed.
- J. Dugay, M. Giménez-Marqués, T. Kozlova, H. W. Zandbergen, E. Coronado and H. S. J. Van Der Zant, Adv. Mater., 2015, 27, 1288–1293 CrossRef CAS PubMed.
- A. Holovchenko, J. Dugay, M. Giménez-Marqués, R. Torres-Cavanillas, E. Coronado and H. S. J. van der Zant, Adv. Mater., 2016 DOI:10.1002/adma.201600890.
- R. Sessoli, H. L. Tsai, A. R. Schake, S. Wang, J. B. Vincent, K. Folting, D. Gatteschi, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1993, 115, 1804–1816 CrossRef CAS.
- L. Bogani and W. Wernsdorfer, Nat. Mater., 2008, 7, 179–186 CrossRef CAS PubMed.
- W. Wernsdorfer, Int. J. Nanotechnol., 2010, 7, 497–522 CrossRef CAS.
- E. Cremades, C. D. Pemmaraju, S. Sanvito and E. Ruiz, Nanoscale, 2013, 5, 4751–4757 RSC.
- L. Thomas, F. Lionti, R. Ballou, D. Gatteschi, R. Sessoli and B. Barbara, Nature, 1996, 383, 145–147 CrossRef CAS.
- C. Paulsen, J.-G. Park, B. Barbara, R. Sessoli and A. Caneschi, J. Magn. Magn. Mater., 1995, 140–144, 1891–1892 CrossRef CAS.
- A. Ardavan and S. J. Blundell, J. Mater. Chem., 2009, 19, 1754–1760 RSC.
- F. Troiani and M. Affronte, Chem. Soc. Rev., 2011, 40, 3119–3129 RSC.
- P. C. E. Stamp and A. Gaita-Ariño, J. Mater. Chem., 2008, 19, 1718–1730 RSC.
- J. Lehmann, A. Gaita-Arino, E. Coronado and D. Loss, Nat. Nanotechnol., 2007, 2, 312–317 CrossRef CAS PubMed.
- D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Chem. Rev., 2013, 113, 5110–5148 CrossRef CAS PubMed.
- S. T. Liddle and J. van Slageren, Chem. Soc. Rev., 2015, 44, 6655–6669 RSC.
- N. Ishikawa, M. Sugita, T. Ishikawa, S. Y. Koshihara and Y. Kaizu, J. Am. Chem. Soc., 2003, 125, 8694–8695 CrossRef CAS PubMed.
- M. Urdampilleta, S. Klyatskaya, J.-P. Cleuziou, M. Ruben and W. Wernsdorfer, Nat. Mater., 2011, 10, 502–506 CrossRef CAS PubMed.
- M. Urdampilleta, S. Klayatskaya, M. Ruben and W. Wernsdorfer, ACS Nano, 2015, 9, 4458–4464 CrossRef CAS PubMed.
- S. Thiele, F. Balestro, R. Ballou, S. Klyatskaya, M. Ruben and W. Wernsdorfer, Science, 2014, 344, 1135–1138 CrossRef CAS PubMed.
- C. Wäckerlin, F. Donati, A. Singha, R. Baltic, S. Rusponi, K. Diller, F. Patthey, M. Pivetta, Y. Lan, S. Klyatskaya, M. Ruben, H. Brune and J. Dreiser, Adv. Mater., 2016, 28, 5195–5199 CrossRef PubMed.
- A. Bedoya-Pinto, H. Prima-García, F. Casanova, E. Coronado and L. E. Hueso, Adv. Electron. Mater., 2015, 1500065 CrossRef.
- J. J. Baldoví, S. Cardona-Serra, J. M. Clemente Juan, L. Escalera-Moreno, A. Gaita-Ariño and G. Mínguez Espallargas, Europhys. Lett., 2015, 110, 33001 CrossRef.
- J. M. Clemente-Juan, E. Coronado and A. Gaita-Ariño, Chem. Soc. Rev., 2012, 41, 7464–7478 RSC.
- N. V. Izarova and P. Kögerler, Polyoxometalate-based single-molecule magnets, Trends in Polyoxometalates Research, 2015 Search PubMed.
- M. Vonci and C. Boskovic, Aust. J. Chem., 2014, 44, 6655–6669 Search PubMed.
- M. Shiddiq, D. Komijani, Y. Duan, A. Gaita-Ariño, E. Coronado and S. Hill, Nature, 2016, 531, 348–351 CrossRef CAS PubMed.
- J. J. Baldoví, S. Cardona-Serra, J. M. Clemente-Juan, E. Coronado, A. Gaita-Ariño and H. Prima-García, Chem. Commun., 2013, 49, 8922–8924 RSC.
- S. Cardona-Serra, J. M. Clemente-Juan, E. Coronado, A. Gaita-Ariño, A. Camón, M. Evangelisti, F. Luis, M. J. Martínez-Pérez and J. Sesé, J. Am. Chem. Soc., 2012, 134, 14982–14990 CrossRef CAS PubMed.
- S. Sherif, G. Rubio-Bollinger, E. Pinilla-Cienfuegos, E. Coronado, J. C. Cuevas and N. Agraït, Nanotechnology, 2015, 26, 291001 CrossRef PubMed.
- A. Kitamura, T. Ozeki and A. Yagasaki, Inorg. Chem., 1997, 36, 4275–4279 CrossRef CAS.
- C. Bustos, D. M.-L. Carey, K. Boubekeur, R. Thouvenot, A. Proust and P. Gouzerh, Inorg. Chim. Acta, 2010, 363, 4262–4268 CrossRef CAS.
- R. Villanneau, A. Proust, F. Robert and P. Gouzerh, Chem. – Eur. J., 2003, 9, 1982–1990 CrossRef CAS PubMed.
- R. Villanneau, A. Proust, F. Robert and P. Gouzerh, J. Chem. Soc., Dalt. Trans., 1999, 421–426 RSC.
- J. J. Baldoví, J. M. Clemente Juan, E. Coronado, Y. Duan, A. Gaita-Ariño and C. Giménez-Saiz, Inorg. Chem., 2014, 53, 9976–9980 CrossRef PubMed.
- J. J. Baldoví, A. Gaita-Ariño and E. Coronado, Dalton Trans., 2015, 44, 12535–12538 RSC.
- K. Qian, J. J. Baldoví, S.-D. Jiang, A. Gaita-Ariño, Y.-Q. Zhang, J. Overgaard, B.-W. Wang, E. Coronado and S. Gao, Chem. Sci., 2015, 6, 4587–4593 RSC.
- J. J. Baldoví, Y. Duan, R. Morales, A. Gaita-Ariño, E. Ruiz and E. Coronado, Chem. – Eur. J., 2016 DOI:10.1002/chem.201601741.
- K. R. Meihaus and J. R. Long, J. Am. Chem. Soc., 2013, 135, 17952–17957 CrossRef CAS PubMed.
- S. K. Singh, T. Gupta and G. Rajaraman, Inorg. Chem., 2014, 53, 10835–10845 CrossRef CAS PubMed.
- S. Cardona-Serra, J. M. Clemente-Juan, A. Gaita-Ariño, N. Suaud, O. Svoboda and E. Coronado, Chem. Commun., 2013, 49, 9621–9623 RSC.
- S. Cardona-Serra, J. M. Clemente-Juan, E. Coronado, A. Gaita-Ariño, N. Suaud, O. Svoboda, R. Bastardis, N. Guihéry and J. J. Palacios, Chem. – Eur. J., 2015, 21, 763–769 CrossRef CAS PubMed.
- C. Bosch-Serrano, J. M. Clemente-Juan, E. Coronado, A. Gaita-Ariño, A. Palii and B. Tsukerblat, ChemPhysChem, 2012, 13, 2662–2665 CrossRef CAS PubMed.
- C. Bosch-Serrano, J. M. Clemente-Juan, E. Coronado, A. Gaita-Ariño, A. Palii and B. Tsukerblat, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 024432 CrossRef.
- W. Klemperer, Inorg. Synth., 2007, 27, 74–85 CrossRef.
- C. Bustos, B. Hasenknopf, R. Thouvenot, J. Vaissermann, A. Proust and P. Gouzerh, Eur. J. Inorg. Chem., 2003, 15, 2757–2766 CrossRef.
- J. J. Baldoví, J. J. Borrás-Almenar, J. M. Clemente-Juan, E. Coronado and A. Gaita-Ariño, Dalton Trans., 2012, 41, 13705 RSC.
- J. J. Baldoví, S. Cardona-Serra, J. M. Clemente-Juan, E. Coronado, A. Gaita-Ariño and A. Palii, J. Comput. Chem., 2013, 34, 1961–1967 CrossRef.
- J. J. Baldoví, J. M. Clemente-Juan, E. Coronado, A. Gaita-Ariño and A. Palii, J. Comput. Chem., 2014, 35, 1930–1934 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Elemental analysis results; IR spectra; crystal structure determination details; magnetic properties. CCDC 1446092–1446097 (for LnMo16) and 1482838–1482842 (for LnMo10). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt02258h |
| ‡ Both authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |