The control of the electronic structure of dinuclear copper complexes of redox-active tetrakisguanidine ligands by the environment†
Abstract
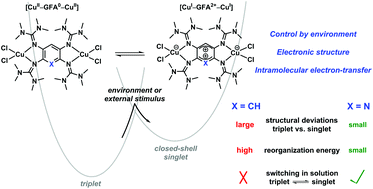
The electronic structures of dinuclear copper complexes of the general formula [GFA(CuX2)2], where X = Br or Cl and GFA denotes a redox-active bridging Guanidino-Functionalized Aromatic ligand, were analysed and compared. The diamagnetic complexes [GFA(CuBr2)2] can all be described as dinuclear CuI complexes with bridging GFA2+ dicationic ligand units exhibiting a [CuI–GFA2+–CuI] electronic structure. The electronic structure prevails in the solid state and in all applicable organic solvents. The situation changes completely for the [GFA(CuCl2)2] complexes. They are paramagnetic in the solid state, where they are adequately described as dinuclear CuII complexes with neutral bridging GFA ligand units ([CuII–GFA–CuII]). In solution, they exist either as [CuII–GFA–CuII] or as valence-tautomeric [CuI–GFA2+–CuI] complexes, depending on the polarity of the solvent. Only in the case of GFA = 2,3,5,6-tetrakis(tetramethylguanidino)pyridine and in acetone as solvent, the two valence tautomers are in a temperature-dependent equilibrium. Quantum chemical computations show that the structural difference between the two valence tautomeric forms is smaller for this complex than for the others, explaining the low energy barrier for the intramolecular electron transfer in accordance with Marcus theory.
- This article is part of the themed collection: Reactions Facilitated by Ligand Design

 Please wait while we load your content...
Please wait while we load your content...