Open Access Article
Open Access ArticleVersatile coordination of a reactive P,N-ligand toward boron, aluminum and gallium and interconversion reactivity†
M.
Devillard
a,
C.
Alvarez Lamsfus
b,
V.
Vreeken
a,
L.
Maron
b and
J. I.
van der Vlugt
*a
aHomogeneous, Bioinspired and Supramolecular, van 't Hoff Institute for Molecular Sciences, University of Amsterdam, Science Park 904, 1098 XH Amsterdam, The Netherlands. E-mail: j.i.vandervlugt@uva.nl
bLaboratoire de Physique et Chimie des Nanoobjets, Université de Toulouse, INSA-UPS-CNRS (UMR 5215) 135, avenue de Rangueil, 31077 Toulouse cedex 4, France
First published on 9th June 2016
Abstract
The synthesis and reactivity of the first Group 13 complexes bearing a dearomatized phosphino-amido ligand L are reported, i.e. alane AlEt2(L) 1, gallane GaCl2(L) 2 and borane B(Cl)(Ph)(L) 3. The three complexes react very differently with Group 13 trihalogenides, providing access to zwitterionic anti-3·GaCl3 and the unique bis(metalloid) 5·BCl2, with the boron center part of a highly unusual anionic four-membered ring (charge on C) and Ga bound to P. The coordination chemistry and the various transformations are supported by DFT calculations, X-ray crystallography and multinuclear NMR spectroscopic data.
Introduction
The synthetic chemistry and reactivity of Group 13 complexes has recently attracted much attention, both with respect to the preparation of unsaturated derivatives1 and for application in bond activation processes and homogeneous catalysis,2 often in combination with a Lewis base as a “Frustrated Lewis Pair” (FLP).3 At the same time, the bioinspired strategy to combine proton-responsive (‘reactive’) ligands and (transition) metal centers has become prominent, resulting in novel coordination chemistry with applications in small molecule activation and metal–ligand bifunctional catalysis.4One particular strategy that has come to the forefront in this context is the use of lutidine- and picoline-based P,N donor ligands that can undergo reversible dearomatization.5 Many transition metals coordinate strongly to both phosphorus and nitrogen in these ligands,6 and deprotonation of the ligand backbone merely generates a nucleophilic carbon center that does not engage in any direct interaction with the metal coordination sphere. No examples of transmetallation on complexes bearing lutidine- or picoline-derived P,N-ligands are known, to the best of our knowledge.7 The coordination chemistry of Group 13 metals and metalloids such as boron and aluminum is unexplored with this type of reactive ligand scaffold. Hence, the fundamental reactivity of complexes bearing a dearomatized P,N ligand is currently unknown but it could be envisioned that other ligand coordination modes may be accessible with these elements.
To address both challenges, we decided to exploit the reversible dearomatization chemistry of P,N-ligands to generate the first examples of Group 13 species with a dearomatized pyridine ligand (see Scheme 1). This approach has allowed access to highly reactive aluminum, gallium and boron compounds stabilized by an anionic P,N-scaffold and to study their versatile reactivity with G13 trihalogenides.
 | ||
| Scheme 1 Dearomatized Group 13 complexes of (phosphinomethyl)pyridine ligands described in this work, their reactivity with Group 13 trihalogenides and follow-up transformations. | ||
Results and discussion
Reaction of the known bidentate P,N-ligand 2-(diphenylphosphinomethyl)-6-methyl-pyridine LH with one molar equivalent of n-BuLi followed by electrophilic trapping with chlorodiethylaluminum (Scheme 2) resulted in the high yield synthesis of complex AlEt2(L) 1 as a red oil, which was characterized by multinuclear NMR spectroscopy. Contrary to what was previously observed for the anion of lutidine,8 this reaction selectively generates an N-Group 13 element bond, with dearomatization of the N-heterocycle, rather than a C-Group 13 bond. The 1H NMR spectrum contained three different resonances in the olefinic region (δ 5.28, 6.07 and 6.42) and a broad signal at δ 3.46 attributed to the methine proton of the![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–PPh2 arm. Only one resonance was observed for the two tert-butyl groups connected to phosphorus and both Et-groups at aluminum are also equivalent, confirming the Cs symmetry of the molecule. This compound features a clear P → Al interaction, as evidenced by the broad 31P NMR signal at δ −0.9 (Δδ −36.2 relative to LH) and the 27Al NMR resonance at δ 162, which is characteristic of a four-coordinated amido-alane.9 Strikingly, the analogous reaction of LH with AlCl3 failed to generate any well-defined product.
CH–PPh2 arm. Only one resonance was observed for the two tert-butyl groups connected to phosphorus and both Et-groups at aluminum are also equivalent, confirming the Cs symmetry of the molecule. This compound features a clear P → Al interaction, as evidenced by the broad 31P NMR signal at δ −0.9 (Δδ −36.2 relative to LH) and the 27Al NMR resonance at δ 162, which is characteristic of a four-coordinated amido-alane.9 Strikingly, the analogous reaction of LH with AlCl3 failed to generate any well-defined product.
 | ||
| Scheme 2 Preparation of the phosphorus-stabilized amidoalane 1 and subsequent transmetallation to form the parent gallane 2. | ||
To get insight into the geometric and electronic features of 1, we optimized its structure using DFT calculations (Fig. 1). The ligand framework adopts an almost planar configuration, with the phosphine and alane fragments slightly out of the N-heterocycle plane (∠P2–C4–C6–C7 177.8°, ∠Al1–N1–C6–C7 174.6°). The N-heterocycle shows clear alternation of C–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. The HOMO is mainly located on the C1 carbon (64.1%), suggesting that this alane complex with an anionic P,N ligand would likely react with electrophiles at the methine site, as previously established for e.g. MeOTf, CO2 and nitrile electrophiles with either Cu and Ru complexes bearing dearomatized P,N pincers.10 However, common inorganic Lewis acids have not been employed as electrophiles, to the best of our knowledge.
C bonds. The HOMO is mainly located on the C1 carbon (64.1%), suggesting that this alane complex with an anionic P,N ligand would likely react with electrophiles at the methine site, as previously established for e.g. MeOTf, CO2 and nitrile electrophiles with either Cu and Ru complexes bearing dearomatized P,N pincers.10 However, common inorganic Lewis acids have not been employed as electrophiles, to the best of our knowledge.
 | ||
| Fig. 1 (Top) HOMO and (bottom) LUMO frontier orbital plots (top and side views) for the phosphorus-stabilized amidoalane 1. | ||
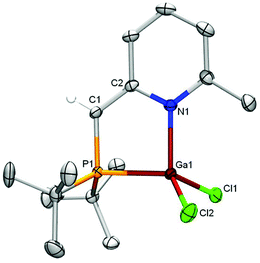
We thus explored its reactivity toward gallium trichloride as a prototypical Lewis acid (Scheme 2). After addition of GaCl3 to a solution of 1 in toluene at room temperature and work-up, the 31P NMR spectrum contained only a very broad resonance at δ −3.1, while the 1H NMR spectrum still showed the characteristic pattern for a dearomatized P,N backbone, but was lacking any signals for an AlEt2 fragment. These spectroscopic data indicate a formal transmetallation from Al to Ga to generate GaCl2(L) 2, which was detected by HR-MS data (FD+, m/z = 390.04338), and AlClEt2. The structure of this gallane complex 2 was confirmed by X-ray structure determination on air-sensitive orange crystals of GaCl2(L), obtained from a diethyl ether-toluene solution at r.t. (Fig. 2). In the crystal structure, the geometry around gallium is strongly pyramidal due to the interaction with the phosphine donor (sum of angles 331.9°). The N-heterocycle shows alternation of single and double C–C bonds, while the external C–C double bond lies in-between (C1–C2 = 1.394(2) Å) and the C1 carbon is clearly sp2 hybridized.
The intriguing conversion of 1 into 2 may be envisioned to involve initial formation of a bis-metalloid intermediate 1·GaCl3 with a C–Ga bond and a formally cationic Al-center, but this proposed intermediate could not be detected using 1, despite various attempts. We hypothesized that such a bis-metalloid structure could be more readily accessible with B instead of Al, as boron cations are known to be stable in tetracoordinated environments (commonly termed boroniums).11 The boron analogue of 1 could be accessed via deprotonation of LH and subsequent addition of Cl2BPh (Scheme 3). Complex B(Cl)(Ph)(L) 3 was obtained in 53% yield as a very air-sensitive red oil that was completely characterized by multinuclear NMR and HR-MS (CSI [M + H]+, m/z 374.1976). The 31P NMR spectrum of 3 shows a broad resonance at δ 24.5 (Δδ = −10.8 ppm) and the doublet in 11B NMR is found in the typical region for tetracoordinated boron atoms (δ 5.1, 1JBP = 106 Hz), confirming its connectivity to phosphorus. The 1H NMR spectrum of 3 showed a doublet at δ 3.46 (2JHP = 5.1 Hz) attributed to the proton of the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–PPh2 arm.
CH–PPh2 arm.
 | ||
| Scheme 3 Preparation of the phosphorus-stabilized amidoborane 3 and its reaction with GaCl3 to form the zwitterion anti-3·GaCl3. | ||
Addition of 1 eq. of GaCl3 to 3 instantaneously yielded a yellow precipitate, which was characterized by a broad signal at δ 34.0 in the 31P NMR spectrum (Δδ = 9.5 ppm) and a slightly upfield shifted 11B NMR signal (δ 5.6); (Δδ = 0.5 ppm). The presence of a doublet at δ 3.67 in the 1H NMR spectrum with a relatively large 2JHP coupling constant of 13.4 Hz (ΔJ = 8.3 Hz compared to 3) indicates rearomatization of the N-heterocycle and supports addition of GaCl3 to the methylene carbon spacer. Single crystal X-ray structure determination confirmed the diastereoselective formation of the formally zwitterionic anti-3·GaCl3 (anti refers to the relative positions of the chloride at boron and the GaCl3 fragment with respect to the G13-pyridine plane, Fig. 3). The new C1–Ga1 bond is in the range of previously reported carbogallates.12 The boronium fragment is strongly pyramidalized (sum of angles 333.5°). DFT calculations (Gaussian 09, B3PW91, 6-31G**) show that the syn-3·GaCl3 diastereoisomer is 3.7 kcal mol−1 higher in energy, which is within the upper limit of the precision of the methods. Heating a solution of anti-3·GaCl3 for 10 h at 100 °C led to partial isomerization to syn-3·GaCl3, as evidenced by multinuclear NMR spectroscopy.
Although there is some precedent for nucleophilic attack of dearomatized pincer transition metal complexes on a Group 13 Lewis acid,13 this reactivity is unprecedented for main group elements or metalloids. Moreover, given the propensity of GaCl3 to act as halide scavenger toward main group halides, including boron for the generation of boron cations,14 the observed preference to act as electrophile in a carbon–gallium bond formation step is deemed highly remarkable. This bond is indeed reactive, as evidenced by the reaction of anti-3·GaCl3 with HCl, which led to protonation at the methylene carbon with the formation of tetrachlorogallate salt 4 (Scheme 4), which was fully characterized, including by single crystal X-ray structure determination (Fig. 4).
The electronegativity of Ga lies in-between that of B and Al. We decided to explore the reaction of gallane 2 with boron trichloride as Lewis acid in order to probe whether the resulting reactivity of these Group 13 elements bound to a dearomatized P,N ligand follows the same trend. Upon addition of BCl3 to 2, the bright orange reaction mixture turned colorless, which is diagnostic of re-aromatization (Scheme 5).
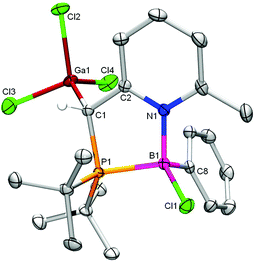
The broad 31P NMR signal appeared at lower field (δ 31.1 ppm, Δδ −10.8 ppm) with no evidence for P–B coupling, indicative that the phosphine is still connected to gallium. In the 1H NMR spectrum, the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–PPh2 proton is deshielded (δ 3.93 ppm) and broadened due to scalar proximity of a 11B nucleus (confirmed by 1H{11B} NMR analysis). Single crystal X-ray structure determination of 5·BCl2 confirmed the incorporation of both Ga and B (Fig. 5). However, concomitant with exchange of Ga for B for the coordination to the heterocycle nitrogen atom, the latter has undergone an unprecedented change from a P,N to a C,N binding pocket.
CH–PPh2 proton is deshielded (δ 3.93 ppm) and broadened due to scalar proximity of a 11B nucleus (confirmed by 1H{11B} NMR analysis). Single crystal X-ray structure determination of 5·BCl2 confirmed the incorporation of both Ga and B (Fig. 5). However, concomitant with exchange of Ga for B for the coordination to the heterocycle nitrogen atom, the latter has undergone an unprecedented change from a P,N to a C,N binding pocket.
The boron atom is incorporated in a rare four-membered ring that is fused to a rearomatized pyridine ring (judging from the almost identical carbon–carbon bond lengths in the ring). The carbon atom binds to boron while the pendant phosphine binds the trichlorogallane. The C1–B1 bond length of 1.682(3) Å likely reflects the ring-strain in this structure. To the best of our knowledge, this is the first example wherein the methine spacer of a dearomatized lutidinylphosphine engages in direct coordination to a metal(loid). The only two structurally characterized fused heterobicyclic structures with boron and pyridine resulted either from (i) C–H activation of a lutidine–borenium adduct in the presence of an external Lewis base15 or (ii) ring-closure of a bis(pentafluorophenyl)boryl derived FLP.16 In both cases, extremely strong Lewis acids are involved.
Given its unique nature, we decided to get further insight into the bonding situation within 5·BCl2 by DFT calculations. Natural bond order (NBO) analysis demonstrated that the C1–B1 and the N1–B1 bonds are very polarized toward C and N (70% and 80%, respectively). In line with this observation, the Natural Population Analysis (NPA) showed a positively charged boron atom (+0.4). Interestingly, the C1 carbon atom bears a significant negative charge (−0.9). As a result, 5·BCl2 is best described as the combination of a boronium and an anionic C,N ligand. The carbanion might be partially stabilized by negative hyperconjugation into the σ*(P–C) orbitals as in reported α-monolithiated17 and α-dilithiated18 phosphine-boranes, although second order NBO analysis does not provide clear evidence for this. The analogous 5·B(Cl)(Ph) was obtained as a mixture of diastereomers by reacting 2 with BCl2Ph.
Compounds 3·GaCl3 and 5·B(Cl)(Ph) are linkage isomers (Scheme 6). The average DFT calculated energy differences of the linkage isomers 3·GaCl3 and 5·B(Cl)(Ph) (syn and anti) are within the upper limit of the precision of the methods (3.6 kcal mol−1 in favor of 5·B(Cl)(Ph)). Given this small energy difference, we speculated that 5·B(Cl)(Ph) could conceivably still be of relevance in the formal transmetallation of 2 with BCl2Ph to provide 3·GaCl3 under forcing conditions. To verify this hypothesis, a solution of 5·B(Cl)(Ph) in CD2Cl2 was heated in a pressure tube to 100 °C for 6 hours, which cleanly generated 3·GaCl3 as a mixture of diastereoisomers. It is plausible that this structural rearrangement, involving both C–B and P–Ga bond cleavage and C–Ga as well as P–B bond formation, proceeds via reconstitution of a mixture of 3 (with the dearomatized N-heterocycle) and GaCl3, which were found to react instantaneously at room temperature.
 | ||
| Scheme 6 Thermal linkage isomerization of 5·B(Cl)(Ph) into 3·GaCl3, proposedly with intermediacy of 3 and free GaCl3. | ||
With the well-defined coordination chemistry for the heterodinuclear species in hand, we wondered if the reaction of 1 with boron trichloride would also lead to selective transmetallation and generation of product BCl2(L) (as observed with GaCl3) or an analogous reaction as observed for 3 to 5 but with a phosphino-alane pendant arm. The addition of one equivalent of BCl3 to a solution of alane 1 at room temperature led to only 50% conversion of starting material, but the reaction smoothly went to completion when two equivalents of BCl3 were used (Scheme 7).
The new species gives rise to a quadruplet in 31P NMR at δ 22.8, which is characteristic of the direct coordination of the phosphine to a boron atom. In line with the required stoichiometry, the 11B NMR shows two signals, a doublet at δ 4.7 with a 1JBP = 139.4 Hz and a broad singlet at δ 6.6. Single crystal X-ray structure determination of 6 confirmed the incorporation of two boron atoms in the molecule (Fig. 6). Complex 6 is structurally related to borane–gallane 5.BCl2, with the same strained four-membered ring including the boron atom, but featuring a trichloroborane-coordinated phosphine arm. It is likely that the interaction of AlClEt2 with the pendant phosphine is relatively weak (perhaps partly due to steric hindrance), resulting in facile displacement by an additional equivalent of BCl3.
In summary, we demonstrate the first examples of Group 13 complexes (boron, gallium and aluminum) stabilized by a dearomatized P,N ligand. Depending on the nature of the metal(loid) involved, very different reactivity toward Group 13 halogenides is observed. The rich coordination chemistry of this reactive ligand scaffold with G13 elements may open up e.g. new avenues for non-transition metal based small molecule activation and cooperative catalysis. Research in this direction is currently ongoing in our laboratories.
Experimental section
General comments
All reactions and manipulations were carried out under an atmosphere of dry dinitrogen using standard Schlenk techniques or in a glove-box. All solvents were purged with dinitrogen and dried using an MBRAUN Solvent Purification System (SPS). 1H, 13C, 31P, 11B and 27Al NMR spectra were recorded on Bruker AV 300, Bruker DRX 300 or Bruker AV 400 spectrometers. Chemical shifts were expressed in positive sign, in parts per million, calibrated to residual 1H (5.32 ppm) and 13C (53.84 ppm) solvent signals, external BF3·Et2O, 85% H3PO4 and Al(NO3)3 1 M in D2O (0 ppm) respectively. Mass spectra were recorded on an AccuTOF LC, JMS-T100LP or an AccuTOF GC v 4 g, JMS-T100GCV Mass spectrometer. Di-(tert-butylphosphino)methyl-6-methyl-pyridine LH was prepared according to a literature procedure.19
1H NMR (300 MHz, CD2Cl2, δ): 0.18 (m, 4H, CH2Et), 1.05 (t, 6H, 3JHH = 8.0 Hz, CH3Et), 1.25 (d, 18H, 3JHP = 13.9 Hz, tBu), 2.06 (s, 3H, CH3Me), 3.46 (s br., 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(
C(![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) )(PtBu2)), 5.28 (d, 1H, 3JHH = 6.5 Hz, Csp2–H), 6.07 (d, 1H, 3JHH = 9.1 Hz, Csp2–H), 6.42 (ddd, 1H, 3JHH = 9.1 Hz, 3JHH = 6.5 Hz, 5JHP = 1.5 Hz, Csp2–H). 31P {1H} NMR (121 MHz, CD2Cl2, δ): −0.9 (br.). 27Al {1H} NMR (78 MHz, CD2Cl2, δ): 162 (br.) 13C {1H} NMR (75 MHz, CD2Cl2, δ): 2.5 (br., 2C, CH2Et), 9.5 (s, 2C, CH3Et), 23.1 (s, 1C, CH3), 29.3 (d, 6C, 2JCP = 4.8 Hz, CH3tBu), 34.3 (d, 2C, 1JCP = 23.5 Hz, P–CtBu), 57.4 (d, 1C, 1JCP = 50.5 Hz, P–Csp2), 102.7 (s, 1C, Csp2–H), 118.1 (d, 1C, JCP = 12.4 Hz, Csp2–H), 133.9 (d, 1C, JCP = 2.5 Hz, Csp2–H), 150.9 (d, 1C, JCP = 4.4 Hz, Cquat.), 167.2 (d, 1C, JCP = 14.9 Hz, Cquat.). The highly air-sensitive nature of this oil (slow decomposition even in an N2-filled glovebox) interfered during our repetitive attempts to obtain high-resolution mass analysis and elemental analysis.
)(PtBu2)), 5.28 (d, 1H, 3JHH = 6.5 Hz, Csp2–H), 6.07 (d, 1H, 3JHH = 9.1 Hz, Csp2–H), 6.42 (ddd, 1H, 3JHH = 9.1 Hz, 3JHH = 6.5 Hz, 5JHP = 1.5 Hz, Csp2–H). 31P {1H} NMR (121 MHz, CD2Cl2, δ): −0.9 (br.). 27Al {1H} NMR (78 MHz, CD2Cl2, δ): 162 (br.) 13C {1H} NMR (75 MHz, CD2Cl2, δ): 2.5 (br., 2C, CH2Et), 9.5 (s, 2C, CH3Et), 23.1 (s, 1C, CH3), 29.3 (d, 6C, 2JCP = 4.8 Hz, CH3tBu), 34.3 (d, 2C, 1JCP = 23.5 Hz, P–CtBu), 57.4 (d, 1C, 1JCP = 50.5 Hz, P–Csp2), 102.7 (s, 1C, Csp2–H), 118.1 (d, 1C, JCP = 12.4 Hz, Csp2–H), 133.9 (d, 1C, JCP = 2.5 Hz, Csp2–H), 150.9 (d, 1C, JCP = 4.4 Hz, Cquat.), 167.2 (d, 1C, JCP = 14.9 Hz, Cquat.). The highly air-sensitive nature of this oil (slow decomposition even in an N2-filled glovebox) interfered during our repetitive attempts to obtain high-resolution mass analysis and elemental analysis.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(
C(![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) )(PtBu2)), 5.53 (d, 1H, JHH = 6.6 Hz, Csp2–H), 6.24 (d, 1H, JHH = 9.0 Hz, Csp2–H), 6.62 (ddd, 1H, JHH = 9.0 Hz, JHH = 6.6 Hz, JHP = 1.3 Hz, Csp2–H). 31P {1H} NMR (121 MHz, CD2Cl2, δ): −3.1 (br.). 13C {1H} NMR (75 MHz, CD2Cl2, δ): 22.7 (s, 1C, CH3), 28.9 (d, 6C, 2JCP = 2.8 Hz, CH3tBu), 36.8 (d, 2C, 1JCP = 30.4 Hz, P–CtBu), 50.7 (d, 1C, 1JCP = 67.6 Hz, P–Csp2), 105.5 (d, 1C, JCP = 1.2 Hz, Csp2–H), 119.2 (d, 1C, JCP = 12.0 Hz, Csp2–H), 135.7 (d, 1C, JCP = 2.3 Hz, Csp2–H), 151.2 (d, 1C, JCP = 4.3 Hz, Cquat.), 166.7 (d, 1C, JCP = 6.7 Hz, Cquat.). HRMS (FD): exact mass (monoisotopic) calcd for [C15H2569GaNP35Cl2 + H]+, 390.04357; found 390.04338. Despite several attempts, no satisfactory combustion analysis data was obtained for this compound, likely as a result of its air-sensitive nature.
)(PtBu2)), 5.53 (d, 1H, JHH = 6.6 Hz, Csp2–H), 6.24 (d, 1H, JHH = 9.0 Hz, Csp2–H), 6.62 (ddd, 1H, JHH = 9.0 Hz, JHH = 6.6 Hz, JHP = 1.3 Hz, Csp2–H). 31P {1H} NMR (121 MHz, CD2Cl2, δ): −3.1 (br.). 13C {1H} NMR (75 MHz, CD2Cl2, δ): 22.7 (s, 1C, CH3), 28.9 (d, 6C, 2JCP = 2.8 Hz, CH3tBu), 36.8 (d, 2C, 1JCP = 30.4 Hz, P–CtBu), 50.7 (d, 1C, 1JCP = 67.6 Hz, P–Csp2), 105.5 (d, 1C, JCP = 1.2 Hz, Csp2–H), 119.2 (d, 1C, JCP = 12.0 Hz, Csp2–H), 135.7 (d, 1C, JCP = 2.3 Hz, Csp2–H), 151.2 (d, 1C, JCP = 4.3 Hz, Cquat.), 166.7 (d, 1C, JCP = 6.7 Hz, Cquat.). HRMS (FD): exact mass (monoisotopic) calcd for [C15H2569GaNP35Cl2 + H]+, 390.04357; found 390.04338. Despite several attempts, no satisfactory combustion analysis data was obtained for this compound, likely as a result of its air-sensitive nature.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(
C(![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) )(PtBu2)), 5.40 (d, 1H, 3JHH = 6.5 Hz, Csp2–H), 6.25 (d, 1H, 3JHH = 8.9 Hz, Csp2–H), 6.59 (ddd, 1H, 3JHH = 8.9 Hz, 3JHH = 6.5 Hz, 5JHP = 1.4 Hz, Csp2–H), 7.10–7.24 (m, 3H, Csp2–H), 7.33 (t, 1H, 3JHH = 7.4 Hz, Csp2–H), 7.91 (d, 1H, 3JHH = 7.4 Hz, Csp2–H). 11B {1H} NMR (96 MHz, CD2Cl2, δ): 5.1 (d, 1JBP = 106.0 Hz). 31P {1H} NMR (121 MHz, CD2Cl2, δ): 24.5 (s br.). 13C {1H} NMR (75 MHz, CD2Cl2, δ): 22.6 (s, 1C, CH3), 28.7 (s, 3C, CH3tBu), 29.4 (s, 3C, CH3tBu), 34.7 (d, 1C, 1JCP = 24.4 Hz, P–CtBu), 38.8 (d, 1C, 1JCP = 23.1 Hz, P–CtBu), 53.0 (d, 1C, 1JCP = 65.2 Hz, P–Csp2), 106.0 (d, 1C, JCP = 1.7 Hz, Csp2–H), 116.2 (d, 1C, JCP = 14.3 Hz, Csp2–H), 127.2 (d, 1C, JCP = 1.2 Hz, Csp2–H), 127.3 (d, 1C, JCP = 2.3 Hz, Csp2–H), 127.8 (d, 1C, JCP = 1.6 Hz, Csp2–H), 133.4 (d, 1C, JCP = 2.9 Hz, Csp2–H), 135.0 (d, 1C, JCP = 2.1 Hz, Csp2–H), 136.0 (d, 1C, JCP = 2.3 Hz, Csp2–H), 151.3 (d, 1C, JCP = 9.1 Hz, Cquat.), 167.6 (d, 1C, JCP = 13.8 Hz, Cquat.), the aromatic carbon connected to boron is not observed. HRMS (CSI, −30 °C): exact mass (monoisotopic) calcd for [C21H30BClNP + H]+, 374.1976; found 374.1980. This compound proved too hydrolysis-sensitive for satisfactory combustion analysis data.
)(PtBu2)), 5.40 (d, 1H, 3JHH = 6.5 Hz, Csp2–H), 6.25 (d, 1H, 3JHH = 8.9 Hz, Csp2–H), 6.59 (ddd, 1H, 3JHH = 8.9 Hz, 3JHH = 6.5 Hz, 5JHP = 1.4 Hz, Csp2–H), 7.10–7.24 (m, 3H, Csp2–H), 7.33 (t, 1H, 3JHH = 7.4 Hz, Csp2–H), 7.91 (d, 1H, 3JHH = 7.4 Hz, Csp2–H). 11B {1H} NMR (96 MHz, CD2Cl2, δ): 5.1 (d, 1JBP = 106.0 Hz). 31P {1H} NMR (121 MHz, CD2Cl2, δ): 24.5 (s br.). 13C {1H} NMR (75 MHz, CD2Cl2, δ): 22.6 (s, 1C, CH3), 28.7 (s, 3C, CH3tBu), 29.4 (s, 3C, CH3tBu), 34.7 (d, 1C, 1JCP = 24.4 Hz, P–CtBu), 38.8 (d, 1C, 1JCP = 23.1 Hz, P–CtBu), 53.0 (d, 1C, 1JCP = 65.2 Hz, P–Csp2), 106.0 (d, 1C, JCP = 1.7 Hz, Csp2–H), 116.2 (d, 1C, JCP = 14.3 Hz, Csp2–H), 127.2 (d, 1C, JCP = 1.2 Hz, Csp2–H), 127.3 (d, 1C, JCP = 2.3 Hz, Csp2–H), 127.8 (d, 1C, JCP = 1.6 Hz, Csp2–H), 133.4 (d, 1C, JCP = 2.9 Hz, Csp2–H), 135.0 (d, 1C, JCP = 2.1 Hz, Csp2–H), 136.0 (d, 1C, JCP = 2.3 Hz, Csp2–H), 151.3 (d, 1C, JCP = 9.1 Hz, Cquat.), 167.6 (d, 1C, JCP = 13.8 Hz, Cquat.), the aromatic carbon connected to boron is not observed. HRMS (CSI, −30 °C): exact mass (monoisotopic) calcd for [C21H30BClNP + H]+, 374.1976; found 374.1980. This compound proved too hydrolysis-sensitive for satisfactory combustion analysis data.
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) )–Ga), 7.04 (m, 1H, Harom.), 7.18 (m, 1H, Harom.), 7.29 (m, 1H, Harom.), 7.34 (m, 1H, Harom.), 7.43 (m, 1H, Harom.), 7.96–8.05 (m, 2H, Harom.), 8.51 (d, 1H, 3JHH = 8.2 Hz, Harom.). 31P {1H} NMR (121 MHz, CD2Cl2, δ): 34.0 (br.). 11B {1H} NMR (96 MHz, CD2Cl2, δ): 5.6. 13C {1H} NMR (75 MHz, CD2Cl2, δ): 23.4 (s, 1C, CH3), 28.8 (s, 3C, CH3tBu), 30.0(s, 3C, CH3tBu), 32.6 (br., P–
)–Ga), 7.04 (m, 1H, Harom.), 7.18 (m, 1H, Harom.), 7.29 (m, 1H, Harom.), 7.34 (m, 1H, Harom.), 7.43 (m, 1H, Harom.), 7.96–8.05 (m, 2H, Harom.), 8.51 (d, 1H, 3JHH = 8.2 Hz, Harom.). 31P {1H} NMR (121 MHz, CD2Cl2, δ): 34.0 (br.). 11B {1H} NMR (96 MHz, CD2Cl2, δ): 5.6. 13C {1H} NMR (75 MHz, CD2Cl2, δ): 23.4 (s, 1C, CH3), 28.8 (s, 3C, CH3tBu), 30.0(s, 3C, CH3tBu), 32.6 (br., P–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) (H)–Ga), 36.6 (d, 1C, 1JCP = 24.4 Hz, P–CtBu), 39.6 (d, 1C, 1JCP = 23.1 Hz, P–CtBu), 123.5 (d, 1C, JCP = 9.2 Hz, CHarom.), 127.1 (d, 1C, JCP = 1.9 Hz, CHarom.), 128.4 (s, 2C, CHarom.), 129.2 (d, 1C, JCP = 2.3 Hz, CHarom.), 134.7 (d, 1C, JCP = 2.2 Hz, CHarom.), 135.1 (d, 1C, JCP = 2.9 Hz, CHarom.), 143.0 (d, 1C, JCP = 1.7 Hz, CHarom.), 157.9 (d, 1C, JCP = 7.4 Hz, Cquat.), 164.5 (d, 1C, JCP = 3.7 Hz, Cquat.), the aromatic carbon connected to boron is not observed. Anal. Calcd For C21H30BCl4GaNP; C, 45.88; H, 5.50; N, 2.55. Found: C, 45.81; H, 5.43; N, 2.49. M. p.: decomposition above 200 °C.
(H)–Ga), 36.6 (d, 1C, 1JCP = 24.4 Hz, P–CtBu), 39.6 (d, 1C, 1JCP = 23.1 Hz, P–CtBu), 123.5 (d, 1C, JCP = 9.2 Hz, CHarom.), 127.1 (d, 1C, JCP = 1.9 Hz, CHarom.), 128.4 (s, 2C, CHarom.), 129.2 (d, 1C, JCP = 2.3 Hz, CHarom.), 134.7 (d, 1C, JCP = 2.2 Hz, CHarom.), 135.1 (d, 1C, JCP = 2.9 Hz, CHarom.), 143.0 (d, 1C, JCP = 1.7 Hz, CHarom.), 157.9 (d, 1C, JCP = 7.4 Hz, Cquat.), 164.5 (d, 1C, JCP = 3.7 Hz, Cquat.), the aromatic carbon connected to boron is not observed. Anal. Calcd For C21H30BCl4GaNP; C, 45.88; H, 5.50; N, 2.55. Found: C, 45.81; H, 5.43; N, 2.49. M. p.: decomposition above 200 °C.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPPh2 signal in the 1H NMR spectrum, D1 = 10 s). The mixture of diastereoisomers was fully characterized by NMR spectroscopy: anti-3·GaCl3 = A; syn-3·GaCl3 = B. 1H NMR (400 MHz, CD2Cl2, δ): 1.29 (d, 9H, 3JHP = 15.2 Hz, tBu(B)), 1.31 (d, 9H, 3JHP = 15.5 Hz, tBu(A)), 1.50 (d, 9H, 3JHP = 13.3 Hz, tBu(A)), 1.53 (d, 9H, 3JHP = 12.7 Hz, tBu(B)), 2.42 (s, 3H, CH3(A)), 2.53 (s, 3H, CH3(B)), 3.27 (d, 1H, 2JHP = 13.6 Hz, P–C(
CHPPh2 signal in the 1H NMR spectrum, D1 = 10 s). The mixture of diastereoisomers was fully characterized by NMR spectroscopy: anti-3·GaCl3 = A; syn-3·GaCl3 = B. 1H NMR (400 MHz, CD2Cl2, δ): 1.29 (d, 9H, 3JHP = 15.2 Hz, tBu(B)), 1.31 (d, 9H, 3JHP = 15.5 Hz, tBu(A)), 1.50 (d, 9H, 3JHP = 13.3 Hz, tBu(A)), 1.53 (d, 9H, 3JHP = 12.7 Hz, tBu(B)), 2.42 (s, 3H, CH3(A)), 2.53 (s, 3H, CH3(B)), 3.27 (d, 1H, 2JHP = 13.6 Hz, P–C(![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) )–Ga (B)), 3.68 (d, 1H, 2JHP = 13.5 Hz, P–C(
)–Ga (B)), 3.68 (d, 1H, 2JHP = 13.5 Hz, P–C(![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) )–Ga (A)), 6.44 (d, 1H, 3JHH = 7.7 Hz, Harom.(B)), 7.04 (m, 1H, Harom.(A)), 7.14–7.21 (m, 1Harom.(A) + 1Harom.(B)), 7.26–7.37 (m, 2Harom.(A) + 1Harom.(B)), 7.95–8.04 (m, 1Harom.(A) + 1Harom.(B)), 8.07 (d br., 1H, 3JHH = 7.4 Hz, Harom.(B)), 8.13 (t, 1H, 3JHH = 8.0 Hz, Harom.(B)), 8.51 (d, 1H, 3JHH = 8.5 Hz, Harom.(A)), 8.60 (d, 1H, 3JHH = 8.1 Hz, Harom.(B)). 31P {1H} NMR (162 MHz, CD2Cl2, δ): 34.1 (br., A), 43.4 (br., B). 11B {1H} NMR (128 MHz, CD2Cl2, δ): 5.3 (br.). 13C {1H} NMR (101 MHz, CD2Cl2, δ): 23.4 (s, 1C, CH3(A)), 24.4 (s, 1C, CH3(B)), 28.1 (br., 1C, P–
)–Ga (A)), 6.44 (d, 1H, 3JHH = 7.7 Hz, Harom.(B)), 7.04 (m, 1H, Harom.(A)), 7.14–7.21 (m, 1Harom.(A) + 1Harom.(B)), 7.26–7.37 (m, 2Harom.(A) + 1Harom.(B)), 7.95–8.04 (m, 1Harom.(A) + 1Harom.(B)), 8.07 (d br., 1H, 3JHH = 7.4 Hz, Harom.(B)), 8.13 (t, 1H, 3JHH = 8.0 Hz, Harom.(B)), 8.51 (d, 1H, 3JHH = 8.5 Hz, Harom.(A)), 8.60 (d, 1H, 3JHH = 8.1 Hz, Harom.(B)). 31P {1H} NMR (162 MHz, CD2Cl2, δ): 34.1 (br., A), 43.4 (br., B). 11B {1H} NMR (128 MHz, CD2Cl2, δ): 5.3 (br.). 13C {1H} NMR (101 MHz, CD2Cl2, δ): 23.4 (s, 1C, CH3(A)), 24.4 (s, 1C, CH3(B)), 28.1 (br., 1C, P–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) (H)–Ga(B)), 28.8 (s, 1C, CH3tBu(A)), 29.3 (s, 2C, CH3tBu(B)), 30.0 (s, 1C, CH3tBu(A)), 32.6 (br., 1C, P–
(H)–Ga(B)), 28.8 (s, 1C, CH3tBu(A)), 29.3 (s, 2C, CH3tBu(B)), 30.0 (s, 1C, CH3tBu(A)), 32.6 (br., 1C, P–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) (H)–Ga(A)), 35.2 (d, 1C, 1JCP = 23.7 Hz, P–CtBu(B)), 36.6 (d, 1C, 1JCP = 24.5 Hz, P–CtBu(A)), 38.3 (d, 1C, 1JCP = 22.2 Hz, P–CtBu(B)), 39.5 (d, 1C, 1JCP = 23.0 Hz, P–CtBu(A)), 123.5 (d, 1C, JCP = 9.1 Hz, CHarom.(A)), 126.2 (d, 1C, JCP = 7.8 Hz, CHarom.(B)), 127.1 (d, 1C, JCP = 1.9 Hz, CHarom.(A)), 128.4 (br., 2C, CHarom.(A)), 128.7 (br, 1C, CHarom.(B)), 128.9 (d, 1C, JCP = 2.4 Hz, CHarom.(B)), 129.0 (br, 1C, CHarom.(B)), 129.2 (d, 1C, JCP = 2.4 Hz, CHarom.(A)), 129.4 (d, 1C, JCP = 2.2 Hz, CHarom.(B)), 131.6 (s br., 1C, CHarom.(B)), 134.7 (d, 1C, JCP = 2.2 Hz, CHarom.(A)), 135.1 (d, 1C, JCP = 2.9 Hz, CHarom.(A)), 136.1 (s br., 1C, CHarom.(B)), 143.0 (d, 1C, JCP = 1.6 Hz, CHarom.(A)), 143.9 (s br., 1C, CHarom.(B)), 157.9 (d, 1C, JCP = 7.8 Hz, Cquat.(A)), 159.0 (d, 1C, JCP = 7.2 Hz, Cquat.(B)), 161.3 (d, 1C, JCP = 2.5 Hz, Cquat.(B)), 164.4 (d, 1C, JCP = 3.7 Hz, Cquat.(A)), the aromatic ipso-carbon connected to boron is not observed for A or B.
(H)–Ga(A)), 35.2 (d, 1C, 1JCP = 23.7 Hz, P–CtBu(B)), 36.6 (d, 1C, 1JCP = 24.5 Hz, P–CtBu(A)), 38.3 (d, 1C, 1JCP = 22.2 Hz, P–CtBu(B)), 39.5 (d, 1C, 1JCP = 23.0 Hz, P–CtBu(A)), 123.5 (d, 1C, JCP = 9.1 Hz, CHarom.(A)), 126.2 (d, 1C, JCP = 7.8 Hz, CHarom.(B)), 127.1 (d, 1C, JCP = 1.9 Hz, CHarom.(A)), 128.4 (br., 2C, CHarom.(A)), 128.7 (br, 1C, CHarom.(B)), 128.9 (d, 1C, JCP = 2.4 Hz, CHarom.(B)), 129.0 (br, 1C, CHarom.(B)), 129.2 (d, 1C, JCP = 2.4 Hz, CHarom.(A)), 129.4 (d, 1C, JCP = 2.2 Hz, CHarom.(B)), 131.6 (s br., 1C, CHarom.(B)), 134.7 (d, 1C, JCP = 2.2 Hz, CHarom.(A)), 135.1 (d, 1C, JCP = 2.9 Hz, CHarom.(A)), 136.1 (s br., 1C, CHarom.(B)), 143.0 (d, 1C, JCP = 1.6 Hz, CHarom.(A)), 143.9 (s br., 1C, CHarom.(B)), 157.9 (d, 1C, JCP = 7.8 Hz, Cquat.(A)), 159.0 (d, 1C, JCP = 7.2 Hz, Cquat.(B)), 161.3 (d, 1C, JCP = 2.5 Hz, Cquat.(B)), 164.4 (d, 1C, JCP = 3.7 Hz, Cquat.(A)), the aromatic ipso-carbon connected to boron is not observed for A or B.
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) )–B), 7.46 (d, 1H, 3JHH = 8.0 Hz, Hm-py), 8.12 (pseudo-t, 1H, 3JHH = 8.0 Hz, Hp-py), 8.32 (d, 1H, 3JHH = 8.1 Hz, Hm-py). 31P {1H} NMR (121 MHz, CD2Cl2, δ): 31.1 (br.). 11B {1H} NMR (96 MHz, CD2Cl2, δ): 6.4. 13C {1H} NMR (75 MHz, CD2Cl2, δ): 18.1 (s, 1C, CH3), 29.3 (d, 3C, 2JCP = 1.5 Hz, CH3tBu), 30.3 (s, 3C, 2JCP = 2.5 Hz, CH3tBu), 32.9–36.1 (br., 1C, P–
)–B), 7.46 (d, 1H, 3JHH = 8.0 Hz, Hm-py), 8.12 (pseudo-t, 1H, 3JHH = 8.0 Hz, Hp-py), 8.32 (d, 1H, 3JHH = 8.1 Hz, Hm-py). 31P {1H} NMR (121 MHz, CD2Cl2, δ): 31.1 (br.). 11B {1H} NMR (96 MHz, CD2Cl2, δ): 6.4. 13C {1H} NMR (75 MHz, CD2Cl2, δ): 18.1 (s, 1C, CH3), 29.3 (d, 3C, 2JCP = 1.5 Hz, CH3tBu), 30.3 (s, 3C, 2JCP = 2.5 Hz, CH3tBu), 32.9–36.1 (br., 1C, P–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) (H)–B), 37.5 (d, 1C, 1JCP = 20.8 Hz, P–CtBu), 38.5 (d, 1C, 1JCP = 15.2 Hz, P–CtBu), 123.8 (s, 1C, CHpy), 126.1 (s, 1C, CHpy), 144.7 (s, 1C, JCP = 1.2 Hz, CHpy), 153.6 (s, 1C, Cquat.), 159.0 (s br., 1C, Cquat.). HRMS (CSI, −35 °C): exact mass (monoisotopic) calcd for [C15H25BCl2NP − (GaCl3) + H]+, 332.1276; found 332.1301. Anal. Calcd For C15H25BCl5GaNP; C, 35.46; H, 4.96; N, 2.76. Found: C, 35.56; H, 4.86; N, 2.67. M. p.: decomposition above 177 °C.
(H)–B), 37.5 (d, 1C, 1JCP = 20.8 Hz, P–CtBu), 38.5 (d, 1C, 1JCP = 15.2 Hz, P–CtBu), 123.8 (s, 1C, CHpy), 126.1 (s, 1C, CHpy), 144.7 (s, 1C, JCP = 1.2 Hz, CHpy), 153.6 (s, 1C, Cquat.), 159.0 (s br., 1C, Cquat.). HRMS (CSI, −35 °C): exact mass (monoisotopic) calcd for [C15H25BCl2NP − (GaCl3) + H]+, 332.1276; found 332.1301. Anal. Calcd For C15H25BCl5GaNP; C, 35.46; H, 4.96; N, 2.76. Found: C, 35.56; H, 4.86; N, 2.67. M. p.: decomposition above 177 °C.
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) )–PPh2 proton and the protons of the phenyl ring due to their spatial proximity, no correlation is observed with the anti isomer). For NMR: A = syn-5·B(Cl)(Ph); B = anti-5·B(Cl)(Ph). 1H NMR (400 MHz, CD2Cl2, δ): 0.95 (d, 9H, 3JHP = 16.4 Hz, tBu(B)), 1.44 (d, 9H, 3JHP = 15.4 Hz, tBu(A)), 1.61 (d, 9H, 3JHP = 17.9 Hz, tBu(A)), 1.61 (d, 9H, 3JHP = 15.0 Hz, tBu(B)), 2.30 (s, 3H, CH3(A)), 2.48 (s, 3H, CH3(B)), 3.74 (d, 1H, 2JHP = 15.2 Hz, P–C(
)–PPh2 proton and the protons of the phenyl ring due to their spatial proximity, no correlation is observed with the anti isomer). For NMR: A = syn-5·B(Cl)(Ph); B = anti-5·B(Cl)(Ph). 1H NMR (400 MHz, CD2Cl2, δ): 0.95 (d, 9H, 3JHP = 16.4 Hz, tBu(B)), 1.44 (d, 9H, 3JHP = 15.4 Hz, tBu(A)), 1.61 (d, 9H, 3JHP = 17.9 Hz, tBu(A)), 1.61 (d, 9H, 3JHP = 15.0 Hz, tBu(B)), 2.30 (s, 3H, CH3(A)), 2.48 (s, 3H, CH3(B)), 3.74 (d, 1H, 2JHP = 15.2 Hz, P–C(![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) )–B (B)), 3.77 (d, 1H, 2JHP = 12.5 Hz, P–C(
)–B (B)), 3.77 (d, 1H, 2JHP = 12.5 Hz, P–C(![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) )–B (A)), 7.23–7.42 (m, 5 HPh (B) and 5 HPh (A)and 1 Hm-py (A)), 7.49 (d, 1H, 3JHH = 8.0 Hz, Hm-py (B)), 8.10 (pseudo-t, 1H, 3JHH = 8.0 Hz, Hp-py (A)), 8.16 (pseudo-t, 1H, 3JHH = 8.0 Hz, Hp-py (B)), 8.34(d, 1H, 3JHH = 8.1 Hz, Hm-py (B)), 8.38(d, 1H, 3JHH = 8.1 Hz, Hm-py (B)). 31P {1H} NMR (121 MHz, CD2Cl2, δ): 32.6 (br.). 11B {1H} NMR (96 MHz, CD2Cl2, δ): 7.4. 13C {1H} NMR (75 MHz, CD2Cl2, δ): 18.0 (s, 1C, CH3(A)), 18.4 (s, 1C, CH3(B)), 29.0 (s, 3C, CH3tBu(B)), 29.2 (s, 3C, CH3tBu(A)), 29.5 (s, 3C, CH3tBu(B)), 30.3 (d, 3C, 2JCP = 2.1 Hz, CH3tBu(A)), 32.8 (br., C–B(A) + C–B(B)), 37.1 (d, 1C, 1JCP = 22.7 Hz, P–CtBu(B)), 37.3 (d, 1C, 1JCP = 21.8 Hz, P–CtBu(A)), 37.6 (d, 1C, 1JCP = 16.8 Hz, P–CtBu(B)), 38.5 (d, 1C, 1JCP = 15.9 Hz, P–CtBu(A)), 123.7 (s, 1C, CHarom.(B)), 123.9 (s, 1C, CHarom.(A)), 125.4 (s, 1C, CHarom.(B)), 125.6 (s, 1C, CHarom.(A)), 127.9–128.6 (m, 3CHarom.(A) + 4CHarom.(B)), 128.7 (s, 1C, CHarom.(B)), 131.7 (s, 2C, CHarom.(A)), 143.6 (s, 1C, CHarom.(A)), 143.9 (s, 1C, CHarom.(B)), 153.6 (s, 1C, Cquat.(A)), 154.1 (s, 1C, Cquat.(B)), 160.7 (s, 1C, Cquat.(A)), 161.1 (br., 1C, Cquat.(B)), the aromatic ipso-carbon connected to boron is not observed for A or B. HRMS (CSI, −35 °C): exact mass (monoisotopic) calcd for [C21H29B1Cl1N1P1 − (GaCl3) + H]+, 374.1980; found 374.2062. Anal. Calcd For C21H30BCl4GaNP; C, 45.88; H, 5.50; N, 2.55. Found: C, 45.61; H, 5.46; N, 2.50.
)–B (A)), 7.23–7.42 (m, 5 HPh (B) and 5 HPh (A)and 1 Hm-py (A)), 7.49 (d, 1H, 3JHH = 8.0 Hz, Hm-py (B)), 8.10 (pseudo-t, 1H, 3JHH = 8.0 Hz, Hp-py (A)), 8.16 (pseudo-t, 1H, 3JHH = 8.0 Hz, Hp-py (B)), 8.34(d, 1H, 3JHH = 8.1 Hz, Hm-py (B)), 8.38(d, 1H, 3JHH = 8.1 Hz, Hm-py (B)). 31P {1H} NMR (121 MHz, CD2Cl2, δ): 32.6 (br.). 11B {1H} NMR (96 MHz, CD2Cl2, δ): 7.4. 13C {1H} NMR (75 MHz, CD2Cl2, δ): 18.0 (s, 1C, CH3(A)), 18.4 (s, 1C, CH3(B)), 29.0 (s, 3C, CH3tBu(B)), 29.2 (s, 3C, CH3tBu(A)), 29.5 (s, 3C, CH3tBu(B)), 30.3 (d, 3C, 2JCP = 2.1 Hz, CH3tBu(A)), 32.8 (br., C–B(A) + C–B(B)), 37.1 (d, 1C, 1JCP = 22.7 Hz, P–CtBu(B)), 37.3 (d, 1C, 1JCP = 21.8 Hz, P–CtBu(A)), 37.6 (d, 1C, 1JCP = 16.8 Hz, P–CtBu(B)), 38.5 (d, 1C, 1JCP = 15.9 Hz, P–CtBu(A)), 123.7 (s, 1C, CHarom.(B)), 123.9 (s, 1C, CHarom.(A)), 125.4 (s, 1C, CHarom.(B)), 125.6 (s, 1C, CHarom.(A)), 127.9–128.6 (m, 3CHarom.(A) + 4CHarom.(B)), 128.7 (s, 1C, CHarom.(B)), 131.7 (s, 2C, CHarom.(A)), 143.6 (s, 1C, CHarom.(A)), 143.9 (s, 1C, CHarom.(B)), 153.6 (s, 1C, Cquat.(A)), 154.1 (s, 1C, Cquat.(B)), 160.7 (s, 1C, Cquat.(A)), 161.1 (br., 1C, Cquat.(B)), the aromatic ipso-carbon connected to boron is not observed for A or B. HRMS (CSI, −35 °C): exact mass (monoisotopic) calcd for [C21H29B1Cl1N1P1 − (GaCl3) + H]+, 374.1980; found 374.2062. Anal. Calcd For C21H30BCl4GaNP; C, 45.88; H, 5.50; N, 2.55. Found: C, 45.61; H, 5.46; N, 2.50.
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) )–B), 7.42 (d, 1H, 3JHH = 8.0 Hz, Hm-py), 8.06 (pseudo-t, 1H, 3JHH = 8.1 Hz, Hp-py), 8.48 (d, 1H, 3JHH = 8.3 Hz, Hm-py). 31P {1H} NMR (121 MHz, CD2Cl2, δ): 22.8 (q, 1JPB = 139.4 Hz). 11B {1H} NMR (96 MHz, CD2Cl2, δ): 4.7 (d, 1JPB = 139.4 Hz, P–
)–B), 7.42 (d, 1H, 3JHH = 8.0 Hz, Hm-py), 8.06 (pseudo-t, 1H, 3JHH = 8.1 Hz, Hp-py), 8.48 (d, 1H, 3JHH = 8.3 Hz, Hm-py). 31P {1H} NMR (121 MHz, CD2Cl2, δ): 22.8 (q, 1JPB = 139.4 Hz). 11B {1H} NMR (96 MHz, CD2Cl2, δ): 4.7 (d, 1JPB = 139.4 Hz, P–![[B with combining low line]](https://www.rsc.org/images/entities/char_0042_0332.gif) Cl3), 6.6 (br.,
Cl3), 6.6 (br., ![[B with combining low line]](https://www.rsc.org/images/entities/char_0042_0332.gif) Cl2). 13C {1H} NMR (75 MHz, CD2Cl2, δ): 18.0 (s, 1C, CH3), 29.4 (s, 3C, CH3tBu), 30.0 (s, 3C, CH3tBu), 35.2 (br., 1C, B–C), 37.4 (d, 1C, 1JCP = 27.0 Hz, P–CtBu), 38.0 (d, 1C, 1JCP = 21.1 Hz, P–CtBu), 124.9 (s, 1C, CHm-py), 125.7 (s, 1C, CHm-py), 144.3 (s, 1C, CHp-py), 153.0 (s, 1C, Co-py), 159.7 (s br., 1C, Co-py). Anal. Calcd For C15H25B2Cl5NP; C, 40.10; H, 5.61; N, 3.12. Found: C, 40.13; H, 5.63; N, 3.13.
Cl2). 13C {1H} NMR (75 MHz, CD2Cl2, δ): 18.0 (s, 1C, CH3), 29.4 (s, 3C, CH3tBu), 30.0 (s, 3C, CH3tBu), 35.2 (br., 1C, B–C), 37.4 (d, 1C, 1JCP = 27.0 Hz, P–CtBu), 38.0 (d, 1C, 1JCP = 21.1 Hz, P–CtBu), 124.9 (s, 1C, CHm-py), 125.7 (s, 1C, CHm-py), 144.3 (s, 1C, CHp-py), 153.0 (s, 1C, Co-py), 159.7 (s br., 1C, Co-py). Anal. Calcd For C15H25B2Cl5NP; C, 40.10; H, 5.61; N, 3.12. Found: C, 40.13; H, 5.63; N, 3.13.
X-ray crystallography
X-ray intensities were measured on a Bruker D8 Quest Eco diffractometer equipped with a Triumph monochromator (λ = 0.71073 Å) and a CMOS Photon 50 detector at a temperature of 150(2) K. Intensity data were integrated with the Bruker APEX2 software.20 Absorption correction and scaling was performed with SADABS.21 The structures were solved with the program SHELXL.20 Least-squares refinement was performed with SHELXL-201322 against F2 of all reflections. Non-hydrogen atoms were refined with anisotropic displacement parameters. The H atoms were placed at calculated positions using the instructions AFIX 13, AFIX 43 or AFIX 137 with isotropic displacement parameters having values 1.2 or 1.5 times Ueq of the attached C atoms. CCDC 1455266–1455269 contain the supplementary crystallographic data for this paper.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 709 reflections were measured up to a resolution of (sin
709 reflections were measured up to a resolution of (sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/λ)max = 0.65 Å−1. 7005 reflections were unique (Rint = 0.0634), of which 5482 were observed [I > 2σ(I)]. 188 Parameters were refined with 0 restraints. R1/wR2 [I > 2σ(I)]: 0.0329/0.0687. R1/wR2 [all refl.]: 0.0517/0.0752. S = 1.008. Residual electron density between −0.760 and 0.667 e Å−3. CCDC 1455266.
θ/λ)max = 0.65 Å−1. 7005 reflections were unique (Rint = 0.0634), of which 5482 were observed [I > 2σ(I)]. 188 Parameters were refined with 0 restraints. R1/wR2 [I > 2σ(I)]: 0.0329/0.0687. R1/wR2 [all refl.]: 0.0517/0.0752. S = 1.008. Residual electron density between −0.760 and 0.667 e Å−3. CCDC 1455266.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 499 reflections were measured up to a resolution of (sin
499 reflections were measured up to a resolution of (sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/λ)max = 0.84 Å−1. 4370 reflections were unique (Rint = 0.0384), of which 3954 were observed [I > 2σ(I)]. 269 Parameters were refined with 252 restraints. R1/wR2 [I > 2σ(I)]: 0.0240/0.0563. R1/wR2 [all refl.]: 0.0288/0.0591. S = 1.091. Residual electron density between −0.382 and 0.431 e Å−3. CCDC 1455267.
θ/λ)max = 0.84 Å−1. 4370 reflections were unique (Rint = 0.0384), of which 3954 were observed [I > 2σ(I)]. 269 Parameters were refined with 252 restraints. R1/wR2 [I > 2σ(I)]: 0.0240/0.0563. R1/wR2 [all refl.]: 0.0288/0.0591. S = 1.091. Residual electron density between −0.382 and 0.431 e Å−3. CCDC 1455267.
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no: 1), a = 9.7845(7), b = 11.8465(9), c = 12.0322(9) Å, α = 83.458(3), β = 83.640(3), γ = 77.501(3)°, V = 1347.41(17) Å3, Z = 2, Dx = 1.358 g cm−3, μ = 1.486 mm−1. 70
(no: 1), a = 9.7845(7), b = 11.8465(9), c = 12.0322(9) Å, α = 83.458(3), β = 83.640(3), γ = 77.501(3)°, V = 1347.41(17) Å3, Z = 2, Dx = 1.358 g cm−3, μ = 1.486 mm−1. 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 054 reflections were measured up to a resolution of (sin
054 reflections were measured up to a resolution of (sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/λ)max = 0.64 Å−1. 10
θ/λ)max = 0.64 Å−1. 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 873 reflections were unique (Rint = 0.0501), of which 8259 were observed [I > 2σ(I)]. 278 Parameters were refined with 0 restraints. R1/wR2 [I > 2σ(I)]: 0.377/0.746. R1/wR2 [all refl.]: 0.647/0.897. S = 1.076. Residual electron density between −1.04 and 0.86 e Å−3. CCDC 1455269.
873 reflections were unique (Rint = 0.0501), of which 8259 were observed [I > 2σ(I)]. 278 Parameters were refined with 0 restraints. R1/wR2 [I > 2σ(I)]: 0.377/0.746. R1/wR2 [all refl.]: 0.647/0.897. S = 1.076. Residual electron density between −1.04 and 0.86 e Å−3. CCDC 1455269.
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no: 1), a = 8.483(6), b = 8.751(5), c = 16.791(7) Å, α = 87.39(2), β = 80.71(2), γ = 63.25(3)°, V = 1098.0(16) Å3, Z = 2, Dx = 1.560 g cm−3, μ = 1.963 mm−1. 33
(no: 1), a = 8.483(6), b = 8.751(5), c = 16.791(7) Å, α = 87.39(2), β = 80.71(2), γ = 63.25(3)°, V = 1098.0(16) Å3, Z = 2, Dx = 1.560 g cm−3, μ = 1.963 mm−1. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 460 reflections were measured up to a resolution of (sin
460 reflections were measured up to a resolution of (sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/λ)max = 0.74 Å−1. 5489 reflections were unique (Rint = 0.0589), of which 4453 were observed [I > 2σ(I)]. 224 Parameters were refined with 252 restraints. R1/wR2 [I > 2σ(I)]: 0.321/0.628. R1/wR2 [all refl.]: 0.491/0.685. S = 1.008. Residual electron density between −0.469 and 0.503 e Å−3. CCDC 1455268.
θ/λ)max = 0.74 Å−1. 5489 reflections were unique (Rint = 0.0589), of which 4453 were observed [I > 2σ(I)]. 224 Parameters were refined with 252 restraints. R1/wR2 [I > 2σ(I)]: 0.321/0.628. R1/wR2 [all refl.]: 0.491/0.685. S = 1.008. Residual electron density between −0.469 and 0.503 e Å−3. CCDC 1455268.
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no: 1), a = 8.2835(19), b = 8.5491(18), c = 16.876(4) Å, α = 87.103(6), β = 81.076(7), γ = 61.850(5)°, V = 1040.6(4) Å3, Z = 2, Dx = 1.434 g cm−3, μ = 0.773 mm−1. 34
(no: 1), a = 8.2835(19), b = 8.5491(18), c = 16.876(4) Å, α = 87.103(6), β = 81.076(7), γ = 61.850(5)°, V = 1040.6(4) Å3, Z = 2, Dx = 1.434 g cm−3, μ = 0.773 mm−1. 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 045 reflections were measured up to a resolution of (sin
045 reflections were measured up to a resolution of (sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/λ)max = 0.75 Å−1. 5215 reflections were unique (Rint = 0.658), of which 4319 were observed [I > 2σ(I)]. 227 Parameters were refined with 0 restraints. R1/wR2 [I > 2σ(I)]: 0.357/0.722. R1/wR2 [all refl.]: 0.502/0.774. S = 1.072. Residual electron density between −0.32 and 0.58 e Å−3. CCDC 1481714.
θ/λ)max = 0.75 Å−1. 5215 reflections were unique (Rint = 0.658), of which 4319 were observed [I > 2σ(I)]. 227 Parameters were refined with 0 restraints. R1/wR2 [I > 2σ(I)]: 0.357/0.722. R1/wR2 [all refl.]: 0.502/0.774. S = 1.072. Residual electron density between −0.32 and 0.58 e Å−3. CCDC 1481714.
DFT calculations
Calculations were carried out with the Gaussian09 software23 at the DFT level, using the hybrid functional B3PW91.24 Aluminum, gallium, chlorine and phosphorus atoms were treated with small-core pseudopotentials from the Stuttgart group, with additional polarization orbitals.25 Nitrogen, carbon, boron and H atoms have been treated with the all-electron 6-31G** Pople basis set. The geometry optimizations were done without any symmetry constraints. Analytical calculations of the vibrational frequencies of the optimized geometries were performed to confirm that the structures obtained were minimums, and also obtained the thermal corrections over the energies. The Natural Bond Orbital analysis was done over the optimized structure, with the version of the NBO software included in Gaussian09.26Acknowledgements
This research is funded by the European Research Council through ERC Starting Grant EuReCat 279097 to J. I. v. d. V. We thank Prof.'s Bas de Bruin and Joost Reek for general support and interest in our work and Ed Zuidinga for MS analysis.References
- R. Kinjo, B. Donnadieu, M. A. Celik, G. Frenking and G. Bertrand, Science, 2011, 333, 610 CrossRef CAS PubMed; C. Fliedel, G. Schnee, T. Avilés and S. Dragorne, Coord. Chem. Rev., 2014, 275, 63 CrossRef.
- I. A. Cade and M. J. Ingleson, Chem. – Eur. J., 2014, 20, 12874 CrossRef CAS PubMed; M. Devillard, R. Brousses, K. Miqueu, G. Bouhadir and D. Bourissou, Angew. Chem., Int. Ed., 2015, 54, 5722 CrossRef PubMed; J. M. Farrell, R. T. Posaratnanathan and D. W. Stephan, Chem. Sci., 2015, 6, 2010 RSC; Z. Yang, M. Zhong, X. Ma, K. Nijesh, S. De, P. Parameswaran and H. W. Roesky, J. Am. Chem. Soc., 2016, 138, 2548 CrossRef PubMed.
- D. W. Stephan, J. Am. Chem. Soc., 2015, 137, 10018 CrossRef CAS PubMed; D. W. Stephan and G. Erker, Angew. Chem., Int. Ed., 2015, 54, 6400 CrossRef PubMed; D. W. Stephan, Acc. Chem. Res., 2015, 48, 306–316 CrossRef PubMed.
- C. Gunanathan and D. Milstein, Acc. Chem. Res., 2011, 44, 588 CrossRef CAS PubMed; K. Junge, K. Schröder and M. Beller, Chem. Commun., 2011, 47, 4849 RSC; J. I. van der Vlugt, Eur. J. Inorg. Chem., 2012, 363 CrossRef; D. Gelman and S. Musa, ACS Catal., 2012, 2, 2456 CrossRef; W.-H. Wang, J. T. Muckerman, E. Fujita and Y. Himeda, New J. Chem., 2013, 37, 1860 RSC; S. Kuwata and T. Ikariya, Chem. Commun., 2014, 50, 14290 RSC; R. H. Morris, Acc. Chem. Res., 2015, 48, 1494 CrossRef PubMed.
- Recent reviews: J. I. van der Vlugt and J. N. H. Reek, Angew. Chem., Int. Ed., 2009, 48, 8832 CrossRef CAS PubMed; C. Gunanathan and D. Milstein, Chem. Rev., 2014, 114, 12024 CrossRef PubMed; J. R. Khusnutdinova and D. Milstein, Angew. Chem., Int. Ed., 2015, 54, 12236 CrossRef PubMed . See also: H. Li, B. Zheng and K.-W. Huang, Coord. Chem. Rev., 2015, 293–294, 116 CrossRef . Recent examples from our group: J. I. van der Vlugt, E. A. Pidko, D. Vogt, M. Lutz, A. L. Spek and A. Meetsma, Inorg. Chem., 2008, 47, 4442 CrossRef PubMed; J. I. van der Vlugt, M. Lutz, E. A. Pidko, D. Vogt and A. L. Spek, Dalton Trans., 2009, 1016 RSC; S. Y. de Boer, Y. Gloaguen, J. N. H. Reek, M. Lutz and J. I. van der Vlugt, Dalton Trans., 2012, 41, 11276 RSC; S. Y. de Boer, Y. Gloaguen, M. Lutz and J. I. van der Vlugt, Inorg. Chim. Acta, 2012, 380, 336 CrossRef; L. S. Jongbloed, B. de Bruin, J. N. H. Reek, M. Lutz and J. I. van der Vlugt, Chem. – Eur. J., 2015, 21, 7297 CrossRef PubMed; L. S. Jongbloed, B. de Bruin, J. N. H. Reek, M. Lutz and J. I. van der Vlugt, Catal. Sci. Technol., 2016, 6, 1320 Search PubMed . See also: Y. Gloaguen, C. Rebreyend, M. Lutz, P. Kumar, M. I. Huber, J. I. van der Vlugt, S. Schneider and B. de Bruin, Angew. Chem., Int. Ed., 2014, 53, 6814 CrossRef PubMed; Z. Tang, S. Mandal, N. D. Paul, M. Lutz, P. Li, J. I. van der Vlugt and B. de Bruin, Org. Chem. Front., 2015, 2, 1561 RSC.
- Two known alternative binding modes are via phosphorus only or with P and N both binding to a different metal center. For an example, see: J. I. van der Vlugt, E. A. Pidko, R. C. Bauer, Y. Gloaguen, M. K. Rong and M. Lutz, Chem. – Eur. J., 2011, 17, 3850 CrossRef CAS PubMed.
- Transmetallation of P-only coordinated mononuclear Ag-species to e.g. Pd has been reported: J. I. van der Vlugt, M. A. Siegler, M. Janssen, D. Vogt and A. L. Spek, Organometallics, 2009, 28, 7025 CrossRef CAS.
- L. A. Körte, R. Warner, V. Y. Vishnevskiy, B. Neumann, H.-G. Stammler and N. W. Mitzel, Dalton Trans., 2015, 44, 9992 RSC; J. Zheng, Y.-J. Lin and H. Wang, Dalton Trans., 2016, 45, 6088 RSC.
- L.-C. Liang, M.-H. Huang and C.-H. Hung, Inorg. Chem., 2004, 43, 2166 CrossRef CAS PubMed; H. Naka, M. Uchiyama, Y. Matsumoto, A. E. H. Wheatley, M. McPartlin, J. V. Morey and Y. Kondo, J. Am. Chem. Soc., 2007, 129, 1921 CrossRef PubMed.
- J. I. van der Vlugt, E. A. Pidko, D. Vogt, M. Lutz and A. L. Spek, Inorg. Chem., 2009, 48, 7513 CrossRef CAS PubMed; M. Montag, J. Zhang and D. Milstein, J. Am. Chem. Soc., 2012, 134, 10325 CrossRef PubMed; C. A. Huff, J. W. Kampf and M. S. Sanford, Organometallics, 2012, 31, 4643 CrossRef; C. A. Huff, J. W. Kampf and M. S. Sanford, Chem. Commun., 2013, 49, 7147 RSC; S. Perdriau, D. S. Zijlstra, H. J. Heeres, J. G. de Vries and E. Otten, Angew. Chem., Int. Ed., 2015, 54, 4236 CrossRef PubMed . See also: Z. Tang, E. Otten, J. N. H. Reek, J. I. van der Vlugt and B. de Bruin, Chem. – Eur. J., 2015, 21, 12683 CrossRef PubMed.
- W. E. Piers, S. C. Bourke and K. D. Conroy, Angew. Chem., Int. Ed., 2005, 44, 5016 CrossRef CAS PubMed.
- J. L. Atwood and S. G. Bott, J. Chem. Soc., Dalton Trans., 1987, 747 RSC; C.-W. Tsang, C. A. Rohrick, T. S. Saini, B. O. Patrick and D. P. Gates, Organometallics, 2002, 21, 1008 CrossRef CAS; C.-W. Tsang, C. A. Rohrick, T. S. Saini, B. O. Patrick and D. P. Gates, Organometallics, 2004, 23, 5913 CrossRef; V. Bagutski, A. Del Grosso, J. A. Carillo, I. A. Cade, M. D. Helm, J. R. Lawson, P. J. Singleton, S. A. Solomon, T. Marcelli and M. J. Ingleson, J. Am. Chem. Soc., 2013, 135, 474 CrossRef PubMed.
- A. Alzamly, S. Gambarotta and I. Korobkov, Organometallics, 2013, 32, 7204 CrossRef CAS; A. Anaby, B. Butschke, Y. Ben-David, L. J. W. Shimon, G. Leitus, M. Feller and D. Milstein, Organometallics, 2014, 33, 3716 CrossRef; T. Cheisson and A. Auffrant, Dalton Trans., 2016, 45, 2069 RSC.
- P. Kölle and H. Nöth, Chem. Rev., 1985, 85, 399 CrossRef; D. P. Gates, R. Ziembinski, I. Manners, A. L. Rheingold and B. S. Haggerty, Angew. Chem., Int. Ed., 1994, 33, 2277 CrossRef.
- E. R. Clark, A. Del Grosso and M. J. Ingleson, Chem. – Eur. J., 2013, 19, 2462 CrossRef CAS PubMed.
- See ref. 8 and: J. Vergnaud, T. Ayed, K. Hussein, L. Vendier, M. Grellier, G. Bouhadir, J.-C. Barthelat, S. Sabo-Etienne and D. Bourissou, Dalton Trans., 2007, 2370 RSC.
- K. Izod, C. Wills, E. Anderson, R. W. Harrington and M. R. Probert, Organometallics, 2014, 33, 5283 CrossRef CAS.
- H. Heuclin, S. Y.-F. Ho, X. F. Le Goff, C.-W. So and N. Mézailles, J. Am. Chem. Soc., 2013, 135, 8774 CrossRef CAS PubMed.
- E. Kinoshita, K. Arashiba, S. Kuriyama, Y. Miyake, R. Shimazaki, N. Haruyuki and N. Yoshiaki, Organometallics, 2012, 31, 8437 CrossRef CAS . See also our 2012 contributions in ref. 5.
- Bruker, APEX2 software, Madison WI, USA, 2014 Search PubMed.
- G. M. Sheldrick, SADABS, Universität Göttingen, Germany, 2008 Search PubMed.
- G. M. Sheldrick, SHELXL2013, University of Göttingen, Germany, 2013 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS; J. P. Perdew and Y. Wang, Phys. Rev. B: Condens. Matter, 1992, 45, 13244 CrossRef; K. Burke, J. P. Perdew and W. Yang, Electronic Density Functional Theory: Recent Progress and New Directions, ed. J. F. Dobson, G. Vignale and M. P. Das, Plenum, New York, 1998 Search PubMed.
- A. Bergner, M. Dolg, W. Kuechle, H. Stoll and H. Preuss, Mol. Phys., 1993, 80, 1431 CrossRef CAS; T. Leininger, A. Berning, A. Nicklass, H. Stoll, H.-J. Werner and H.-J. Flad, Chem. Phys., 1997, 217, 19 CrossRef.
- E. D. Glendening, A. E. Reed, J. E. Carpenter and F. Weinhold, NBO Version 3.1 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1455266–1455269, 1481714. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt02087a |
| This journal is © The Royal Society of Chemistry 2016 |