Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tris-ureas as transmembrane anion transporters†
Martina
Olivari
a,
Riccardo
Montis
a,
Stuart N.
Berry
b,
Louise E.
Karagiannidis
b,
Simon J.
Coles
 b,
Peter N.
Horton
b,
Lucy K.
Mapp
b,
Philip A.
Gale
b,
Peter N.
Horton
b,
Lucy K.
Mapp
b,
Philip A.
Gale
 *b and
Claudia
Caltagirone
*a
*b and
Claudia
Caltagirone
*a
aDipartimento di Scienze Chimiche e Geologiche, Università degli Studi di Cagliari, S.S. 554 Bivio per Sestu, 09042 Monserrato (CA), Italy. E-mail: ccaltagirone@unica.it
bChemistry, University of Southampton, Southampton, SO17 1BJ, UK. E-mail: philip.gale@soton.ac.uk
First published on 1st July 2016
Abstract
Nine tris-urea receptors (L1–L9) have been synthesised and shown to coordinate to a range of anionic guests both by 1H NMR titration techniques and single crystal X-ray structural analysis. The compounds have been shown to be capable of mediating the exchange of chloride and nitrate and also chloride and bicarbonate across POPC or POPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cholesterol 7
cholesterol 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 vesicle bilayer membranes at low transporter loadings. An interesting dependency of anion transport on the nature of the cation is evidence to suggest that a M+/Cl− cotransport process may also contribute to the release of chloride from the vesicles.
3 vesicle bilayer membranes at low transporter loadings. An interesting dependency of anion transport on the nature of the cation is evidence to suggest that a M+/Cl− cotransport process may also contribute to the release of chloride from the vesicles.
Introduction
Transmembrane transport of anions across lipid bilayers is an important biological process that is normally regulated by complex membrane spanning proteins. A range of diseases, known as “channelopathies”, including cystic fibrosis, are caused by malfunctioning ion channels.1 There is currently interest in the design of synthetic membrane transporters for anions that can act as potential future therapeutic substitutes for these malfunctioning proteins and have other biological applications.2–4Gale and co-workers have recently described the anion binding properties of a series of ortho-phenylenediamine-based bis-ureas.5,6 These compounds are highly effective anion transporters that function by an anion antiport, and in some cases a HCl symport carrier mechanism. Addition of electron-withdrawing groups to either the central core or the peripheral phenyl groups improved the anion transport ability: the transporter activity increased with the electron withdrawing strength of the substituent with the trend H < F ≈ Cl < CF3 < CN < NO2. Indeed, the p-nitro functionalised compound was shown to possess a very high transport activity, facilitating chloride efflux at concentrations as low as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 transporter to lipid molar ratio.
000 transporter to lipid molar ratio.
Gale et al. have also reported the transmembrane anion transport of phosphoric triamide and thiophosphoric triamide-based receptors,7 and tris-urea tripodal receptors.8–10
Tris-urea receptors can be divided in two main families: tripodal receptors based on flexible linkers such as TREN (tris(2-aminoethyl)amine) that are able to preferentially bind oxo-anions5,11–19 and to work as organogelators,20 or rigid spacers such as cyanuric acid,21 benzene,22 or trindane.23
Recently Wu and co-workers designed and synthesised a new family of tris-ureas24,25 and tris-thioureas26 developed mimicking the scaffold of terpyridine as efficient receptors for phosphate and sulfate. Starting from the interesting results obtained by Gale with the ortho-phenylenediamine-based bis-ureas transporters we decided to expand the family of tris-ureas reported by Wu and therefore we synthesised nine receptors (L1–L9 in Scheme 1). We investigated the anion binding properties both in solution and in the solid state of the nine receptors and their ability to transport anions across lipid bilayers.
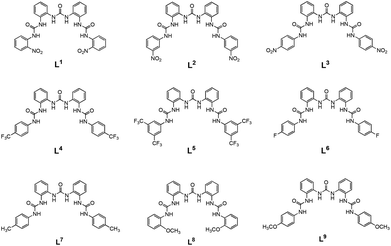 | ||
| Scheme 1 Representation of receptors L1–L9. Receptor L3 has already been published.25 | ||
Results and discussion
The synthesis of receptor L3 has previously been reported by Wu.25L1–L9 were synthesized via different reaction steps. Firstly 1,3-bis(2-aminophenyl)urea was prepared by reaction of ortho-phenylenediamine with ortho-nitro-phenylisocyanate in a mixed solvent THF/toluene at 0 °C and subsequent reduction of the 1-(2-nitrophenyl)-3-(2-aminophenyl)urea obtained by hydrazine and Pd/C 10%. 1,3-Bis(2-aminophenyl)urea was the reacted with the appropriate isocyanate (4-(trifluoromethyl)phenyl isocyanate; 3,5-bis(trifluoromethyl)phenyl isocyanate; 4-fluorophenyl isocyanate; p-tolyl isocyanate; 2-methoxyphenyl isocyanate; 4-methoxyphenyl isocyanate) in refluxing dichloromethane (DCM) under a N2 atmosphere to obtain L1–L9 in 60–90% yield (see ESI† for synthetic details).Anion-binding studies were performed by means of 1H-NMR titrations in DMSO-d6. Stability constants from the 1H-NMR titration curves obtained (see ESI Fig. S1–S13†) were calculated by fitting the data to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model using EQNMR27 as shown in Table 1.
1 binding model using EQNMR27 as shown in Table 1.
| Receptors | Anions | ||
|---|---|---|---|
| Cl− | HCO3− | NO3− | |
| a The NHs signals disappeared after the addition of one equivalent of anion. | |||
| L1 | <10 | Deprotonationa | No interaction |
| L2 | <10 | Deprotonationa | No interaction |
| L3 | <10 | Deprotonationa | No interaction |
| L4 | 262 | Deprotonationa | No interaction |
| L5 | <10 | 226 | No interaction |
| L6 | 205 | 203 | No interaction |
| L7 | 226 | 221 | No interaction |
| L8 | 28 | 802 | No interaction |
| L9 | 225 | 240 | No interaction |
Under the conditions of these experiments, the receptors did not interact with nitrate (i.e. no shift of the NH proton resonances occurred upon addition of tetrabutylammonium nitrate). Interestingly, receptors L1–L3 which contain nitro electron-withdrawing groups showed little interaction with chloride, while addition of bicarbonate caused the disappearance of the signals attributed to the urea NH groups, evidence in support of a deprotonation or exchange process. Receptor L4, bearing one CF3 substituent on the peripheral phenyl ring showed some affinity for chloride. On the other hand, receptors L6, L7, and L9 were able to bind both chloride and bicarbonate with comparable stability constants, while L5 and L8 bound bicarbonate preferentially.
A series of crystallization experiments of receptors L1–L9 in presence of an excess of anions such as acetate, chloride, bicarbonate and nitrate were carried out with the aim of investigating the anion-binding properties of the receptors in the solid state. In order to be consistent with solution studies, all the crystallization experiments were conducted in DMSO. However, (compatibly with the low solubility of receptors L1–L9 in common solvents) with the aim of investigating the possible influence of less polar solvents in the anion-binding process, other solvents (AcOEt, MeOH, EtOH, THF, MeNO2, MeCN) and mixture of solvents (MeOH/MeNO2 and THF/DMF) were employed. Details of the crystallization experiments are reported in ESI (Table S1†).
As shown in Table S1 (see ESI†) only a limited number of crystallizations were successful in producing single crystals. In particular, for a total of sixty-six crystallization experiments, only seven gave samples suitable for X-ray investigation. Within these, three gave the crystal structure of the simple tetrabutylammonium salt and the remaining four resulted in crystal structures [L4(Cl−)2](TBA+)2a, [L4(Cl−)2](TBA+)2b (isostructures obtained from MeOH/MeNO2 and MeCN respectively; for the structural description the code [L4(Cl−)2](TBA+)2 is used to identify both), [L5(Cl−)](TBA+) and [L5(AcO−)](TBA+) (both obtained from DMSO).
In general, these results seem to be consistent with the low anion-binding affinity observed in solution studies (Table 1). This is particularly evident for receptors L1–L3, where the unsuccessful crystallization experiments agree with the negligible interactions observed in solution (Table 1). In the case of receptor L4, though the compound unexpectedly forms a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 [L4(Cl−)2](TBA+)2 complex, the affinity toward Cl− is confirmed. For the remaining receptors L5-L9, only in the case of receptor L5 it was possible to isolate single crystals of anion complexes with chloride and acetate (not investigated in solution).
2 [L4(Cl−)2](TBA+)2 complex, the affinity toward Cl− is confirmed. For the remaining receptors L5-L9, only in the case of receptor L5 it was possible to isolate single crystals of anion complexes with chloride and acetate (not investigated in solution).
[L4(Cl−)2](TBA+)2 crystallised in the triclinic crystal system (space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) with an asymmetric unit consisting of one receptor L4, two chloride anions and two tetrabutylammonium counterions, resulting in a 1
) with an asymmetric unit consisting of one receptor L4, two chloride anions and two tetrabutylammonium counterions, resulting in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
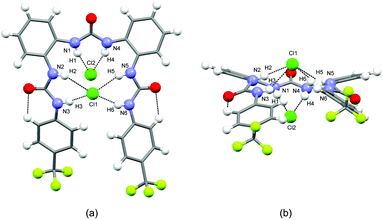
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex. L4 adopts a closed conformation, with the urea NHs all oriented toward the centre of a pseudo-cavity. The three urea groups are slightly tilted to interact via N–H⋯Cl hydrogen bonds (N–H⋯Cl distances are in the range 2.36(2)–2.51(2) Å, average 2.43 Å) with the two Cl− anions which respectively lie below (Cl1) and above (Cl2) the pseudo-cavity (Fig. 1a and b). In particular it is worth noticing that the shortest distances are observed for Cl2 (N1–H1⋯Cl2 2.36(2) Å, N1–H1⋯Cl2 2.34(2) Å).
2 complex. L4 adopts a closed conformation, with the urea NHs all oriented toward the centre of a pseudo-cavity. The three urea groups are slightly tilted to interact via N–H⋯Cl hydrogen bonds (N–H⋯Cl distances are in the range 2.36(2)–2.51(2) Å, average 2.43 Å) with the two Cl− anions which respectively lie below (Cl1) and above (Cl2) the pseudo-cavity (Fig. 1a and b). In particular it is worth noticing that the shortest distances are observed for Cl2 (N1–H1⋯Cl2 2.36(2) Å, N1–H1⋯Cl2 2.34(2) Å).
[L5(Cl−)](TBA+) also adopts a triclinic crystal system (space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ). The asymmetric unit consists of one independent receptor L5, one independent chloride and one independent tetrabutylammonium resulting in a 1
). The asymmetric unit consists of one independent receptor L5, one independent chloride and one independent tetrabutylammonium resulting in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
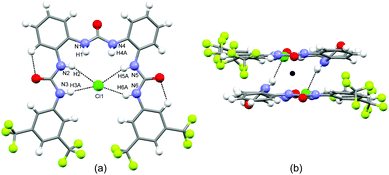
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex. Similarly to L4 the receptor L5 shows a closed conformation with the two peripheral urea NHs (Fig. 2a) pointing at the centre of the pseudo-cavity and interacting with the chloride via four N–H⋯Cl hydrogen bonds (N–H⋯Cl) distances are in the range 2.32(2)–2.75(3) Å (average distance 2.53 Å). The central urea NHs are tilted to interact with an adjacent receptor-chloride unit via a second set of N–H⋯Cl hydrogen bonds (N–H⋯Cl distances are 2.44(3) Å and 2.74(3) Å respectively) forming a centrosymmetric dimer (Fig. 2b).
1 complex. Similarly to L4 the receptor L5 shows a closed conformation with the two peripheral urea NHs (Fig. 2a) pointing at the centre of the pseudo-cavity and interacting with the chloride via four N–H⋯Cl hydrogen bonds (N–H⋯Cl) distances are in the range 2.32(2)–2.75(3) Å (average distance 2.53 Å). The central urea NHs are tilted to interact with an adjacent receptor-chloride unit via a second set of N–H⋯Cl hydrogen bonds (N–H⋯Cl distances are 2.44(3) Å and 2.74(3) Å respectively) forming a centrosymmetric dimer (Fig. 2b).
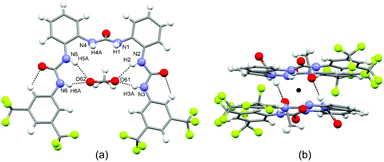
Similarly to the previous two structures, [L5(AcO−)](TBA+) crystallises in the triclinic crystal system (space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ). The asymmetric unit consists of one independent receptor L5, one independent acetate and one independent tetrabutylammonium. The receptor molecule adopts a closed conformation (Fig. 3a) to form a pseudo-cavity similar to those observed for [L4(Cl−)2](TBA+)2 and [L5(Cl−)](TBA+). The peripheral NHs are oriented toward the centre of the pseudo-cavity interacting with the AcO−via N–H⋯O hydrogen bonds (N–H⋯O distances are in the range 1.89(2)–2.34 (2) Å, average distance 2.13 Å). This is oriented perpendicularly to the plane of the pseudo-cavity and interacts via two N–H⋯O hydrogen bonds (N–H⋯O distances are 1.97(2) Å and 1.99(2) Å respectively) with the central urea NHs of an adjacent receptor molecule (Fig. 3b) to form a centrosymmetric dimer similar to that observed for [L5(Cl−)](TBA+).
). The asymmetric unit consists of one independent receptor L5, one independent acetate and one independent tetrabutylammonium. The receptor molecule adopts a closed conformation (Fig. 3a) to form a pseudo-cavity similar to those observed for [L4(Cl−)2](TBA+)2 and [L5(Cl−)](TBA+). The peripheral NHs are oriented toward the centre of the pseudo-cavity interacting with the AcO−via N–H⋯O hydrogen bonds (N–H⋯O distances are in the range 1.89(2)–2.34 (2) Å, average distance 2.13 Å). This is oriented perpendicularly to the plane of the pseudo-cavity and interacts via two N–H⋯O hydrogen bonds (N–H⋯O distances are 1.97(2) Å and 1.99(2) Å respectively) with the central urea NHs of an adjacent receptor molecule (Fig. 3b) to form a centrosymmetric dimer similar to that observed for [L5(Cl−)](TBA+).
The anion transport properties of receptors L1–L9 were studied using vesicle-based methods.28 A sample of unilamellar POPC vesicles was prepared containing 489 mM NaCl buffered to pH 7.2 with 5 mM sodium phosphate salts. The vesicles were suspended at a lipid concentration of 1 mM in 489 mM NaNO3 buffered to pH 7.2 with 5 mM sodium phosphate salts.
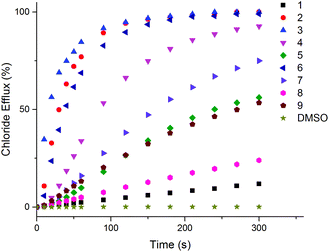
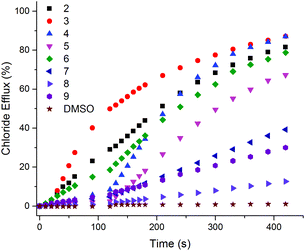
A small amount of DMSO solution of the receptor (0.02–2 mol% with respect to lipid) was added to the vesicles suspension, and the resulting chloride efflux was monitored using a chloride ion selective electrode (ISE) for 300 s. At the end of the experiment, the vesicles were lysed by the addition of detergent, and the final electrode reading was used to calibrate 100% chloride release. We found that all the compounds except L1 (i.e.L2–L9) (at 2 mol% with respect to lipid) were capable of mediating chloride transport.
Under these experimental conditions the most active compounds are to be L2, L3, L4 and L6 (Fig. 4 and S14–S29 in ESI†) In order to determine the mechanism of chloride release by receptors L2–L9, the transport assays were repeated suspending POPC vesicles loaded with NaCl (451 mM with 20 mM phosphate buffer at pH 7.2) in a solution of Na2SO4 (150 mM with 20 mM phosphate buffer at pH 7.2). DMSO suspensions of compounds L2–L9 were then added to the suspension. Usually during this experiment the antiport mechanism (2Cl−/SO42− exchange) would not be expected to be observed due to the high hydrophilicity of the SO42− anion29 which would inhibit the chloride efflux from the liposomes.9 However, as shown in Fig. 5, during the first two minutes of the experiment significant chloride release was observed for compounds L3, L2 and L6. There is also some chloride efflux mediated by the other compounds except for L4 and L5 under these conditions. After 120 s, a pulse of NaHCO3 solution was added, and we observed an increase of chloride efflux (Fig. 5 and S31–S46 in ESI†), evidence in support of a chloride/bicarbonate exchange process.
A lucigenin assay for chloride/sulfate exchange was run with compounds L2–L9 which showed that these compounds do not mediate sulfate transport (see ESI, Fig. S56† in the case of L6, as a representative compound).10
Although it has been recently reported that ureas and thioureas can facilitate proton or hydroxide transport,30 a HPTS assay to test for H+/Cl− co-transport resulted in inconclusive results for the class of molecules presented herein (see ESI, Fig. S57† in the case of L6 as a representative compound).
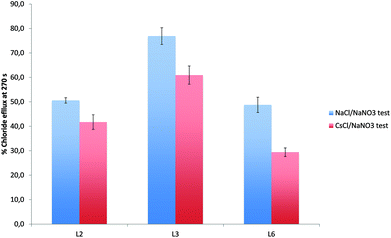
To further examine the origin of the chloride transport during the first two minutes of this assay, we examined the possibility of a mechanism involving sodium/chloride co-transport. The Cl−/NO3− transport assays were repeated using vesicles containing caesium chloride (489 mM NaCl buffered to pH 7.2 with 5 mM sodium phosphate salts) instead of sodium chloride, suspended in an isotonic sodium nitrate solution. In the event of NaCl co-transport we would expect the rate of chloride release to be different in the presence of CsCl. As shown in Fig. 6 in the case of L2, L3, and L6 (and Fig. S47–S55 in the ESI†) the release of chloride is dependent on the nature of metal cation and it is reduced when the internal solution was replaced by CsCl.
The results described above suggest, from the first assay, that the receptors can facilitate Cl−/NO3− exchange; the second assay, supports the hypothesis of Cl−/HCO3− antiport mechanism although in the first two minutes in the presence of sulfate for all the compounds except L4 and L5 a small amount of chloride efflux was observed. The caesium chloride assay demonstrates that for L2, L3, L6–L9 the counter cation is involved in transport possibly via a M+/Cl− co-transport process.
We tested the Cl−/NO3−antiport activity of L2–L9 in vesicles composed of POPC–cholesterol (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
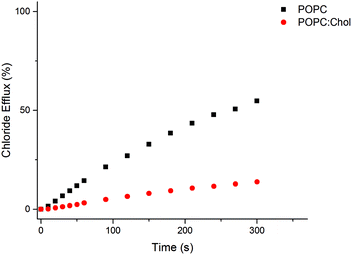
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). This mixture is a closer mimic of biological membranes than pure POPC lipid bilayers. The presence of cholesterol is known to increase the order in the bilayer and its viscosity. All the receptors tested showed a reduced rate of transport in the POPC–cholesterol system (see Fig. 7 for L2 and Fig. S58–S64 in ESI† for L3–L9), providing evidence that for this class of receptor diffusion through the interior of the bilayer may be the rate determining step.31
3). This mixture is a closer mimic of biological membranes than pure POPC lipid bilayers. The presence of cholesterol is known to increase the order in the bilayer and its viscosity. All the receptors tested showed a reduced rate of transport in the POPC–cholesterol system (see Fig. 7 for L2 and Fig. S58–S64 in ESI† for L3–L9), providing evidence that for this class of receptor diffusion through the interior of the bilayer may be the rate determining step.31
To quantify the transport activity of compounds L2–L9 Hill analyses32,33 for the chloride/nitrate and chloride/bicarbonate antiport assays were performed (see Fig. S14–S29, S31–S46 in ESI†). Hill analysis allows the calculation of the EC50,270 s which is a measure of transporter efficiency, defined as the required receptor concentration to mediate 50% of the total chloride efflux 270 s after the addition of the carrier (or after the bicarbonate ‘pulse’). This allows us to compare the transport activity of the compounds. These values are summarised in Table 2, together with the Hill coefficients, which can be correlated to the number of transporter molecules required to transport a single anion and can provide further evidence for a mobile carrier mechanism.
| Compound | EC50 at 270 s (Cl−/NO3−) | n | EC50 at 270 s (Cl−/HCO3−) | n |
|---|---|---|---|---|
| L2 | 0.095 | 1.207 | 0.50 | 0.76 |
| L3 | 0.047 | 1.67 | 0.21 | 0.77 |
| L4 | 0.039 | 1.07 | 0.14 | 0.98 |
| L5 | 0.066 | 0.88 | 0.25 | 0.97 |
| L6 | 0.10 | 1.39 | 0.76 | 0.80 |
| L7 | 0.6 | 0.96 | 4.27 | 0.88 |
| L8 | 4.98 | 1.29 | 21.93 | 0.86 |
| L9 | 1.96 | 0.88 | 8.43 | 0.84 |
From the EC50,270 s values reported in Table 2 the most active transporter among the series appears to be L4 (EC50,270 s 0.039 mol% and 0.14 mol% with respect to lipid for nitrate and bicarbonate antiport, respectively).
Further, Hill coefficients of ∼1 provides further evidence that these compounds are functioning via a mobile carrier mechanism and are not forming membrane spanning channels in which we would expect the Hill coefficient to be higher.
Conclusions
In conclusion we have synthesised nine tris-urea receptors bearing a range of substituents attached to the pendant arms. We have studied the anion binding properties of the receptors both in solution and in the solid state and we have tested their ability to mediate chloride transport through membranes. Solution studies demonstrate that these receptors bind anions with moderate stability constants (as reported in Table 1).Solid state studies confirm the results observed in solution and indeed, despite many attempts, only three crystal structures were obtained. In particular, the crystal structure of L4 in the presence of chloride suggests that the receptor has a good degree of pre-organization and binds chloride via the urea NH groups with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry. We also demonstrated that these systems are able to mediate transmembrane chloride transport as mobile carriers with different mechanisms, Cl−/NO3− and Cl−/HCO3− antiport, and metal-dependent cation co-transport. Hill-plot analysis demonstrates that the most active compound of the series is L4.34
2 stoichiometry. We also demonstrated that these systems are able to mediate transmembrane chloride transport as mobile carriers with different mechanisms, Cl−/NO3− and Cl−/HCO3− antiport, and metal-dependent cation co-transport. Hill-plot analysis demonstrates that the most active compound of the series is L4.34
Acknowledgements
CC would like to thank Fondazione Banco di Sardegna for financial support. PAG thanks the University of Southampton and the A*STAR ARAP programme for a studentship (SNB), the Royal Society and the Wolfson Foundation for a Research Merit Award and the EPSRC (EP/K039466/1) (Core Capability for Chemistry Research in Southampton). We also thank the EPSRC for access to the crystallographic facilities at the University of Southampton.35Notes and references
- F. M. Ashcroft, Ion Channels and Disease, Academic Press, San Diego, 2000 Search PubMed.
- P. A. Gale, R. Pérez-Tomás and R. Quesada, Acc. Chem. Res., 2013, 46, 2801–2813 CrossRef CAS PubMed; N. Busschaert and P. A. Gale, Angew. Chem., Int. Ed., 2013, 52, 1374–1382 CrossRef PubMed.
- S. Matile, A. Vargas Jentzsch, J. Montenegro and A. Fin, Chem. Soc. Rev., 2011, 40, 2453–2474 RSC.
- (a) V. Soto-Cerrato, P. Manuel-Manresa, E. Hernando, S. Calabuig-Fariñas, A. Martínez-Romero, V. Fernández-Dueñas, K. Sahlholm, T. Knöpfel, M. García-Valverde, A. M. Rodilla, E. Jantus-Lewintre, R. Farràs, F. Ciruela, R. Pérez-Tomás and R. Quesada, J. Am. Chem. Soc., 2015, 137, 15892–15898 CrossRef CAS PubMed; (b) S. N. Berry, V. Soto-Cerrato, E. N. W. Howe, H. J. Clarke, I. Mistry, A. Tavassoli, Y.-T. Chang, R. Pérez-Tomás and P. A. Gale, Chem. Sci., 2016 10.1039/c6sc01643j; (c) W. Van Rossom, D. J. Asby, A. Tavassoli and P. A. Gale, Org. Biomol. Chem., 2016, 14, 2645–2650 RSC; (d) A. I. Share, K. Patel, C. Nativi, E. J. Cho, O. Francesconi, N. Busschaert, P. A. Gale, S. Roelens and J. L. Sessler, Chem. Commun., 2016, 52, 7560 RSC.
- S. J. Moore, C. J. E. Haynes, J. Gonzalez, J. L. Sutton, S. J. Brooks, M. E. Light, J. Herniman, G. J. Langley, V. Soto-Cerrato, R. Perez-Tomas, I. Marques, P. J. Costa, V. Felix and P. A. Gale, Chem. Sci., 2013, 4, 103–117 RSC.
- L. E. Karagiannidis, C. J. E. Haynes, K. J. Holder, I. L. Kirby, S. J. Moore, N. J. Wells and P. A. Gale, Chem. Commun., 2014, 50, 12050–12053 RSC.
- P. B. Cranwell, J. R. Hiscock, C. J. E. Haynes, M. E. Light, N. J. Wells and P. A. Gale, Chem. Commun., 2013, 49, 874–876 RSC.
- N. Busschaert, P. A. Gale, C. J. E. Haynes, M. E. Light, S. J. Moore, C. C. Tong, J. T. Davis and J. W. A. Harrell, Chem. Commun., 2010, 46, 6252–6254 RSC.
- N. Busschaert, L. E. Karagiannidis, M. Wenzel, C. J. E. Haynes, N. J. Wells, P. G. Young, D. Makuc, J. Plavec, K. A. Jolliffe and P. A. Gale, Chem. Sci., 2014, 5, 1118–1127 RSC.
- N. Busschaert, M. Wenzel, M. E. Light, P. Iglesias-Hernández, R. Pérez-Tomás and P. A. Gale, J. Am. Chem. Soc., 2011, 133, 14136–14148 CrossRef CAS PubMed.
- B. Akhuli, I. Ravikumar and P. Ghosh, Chem. Sci., 2012, 3, 1522–1530 RSC.
- P. Bose, R. Dutta and P. Ghosh, Org. Biomol. Chem., 2013, 11, 4581–4584 CAS.
- R. Custelcean, Chem. Commun., 2013, 49, 2173–2182 RSC.
- S. K. Dey, R. Chutia and G. Das, Inorg. Chem., 2012, 51, 1727–1738 CrossRef CAS PubMed.
- M. Emami Khansari, C. R. Johnson, I. Basaran, A. Nafis, J. Wang, J. Leszczynski and M. A. Hossain, RSC Adv., 2015, 5, 17606–17614 RSC.
- Y. Hao, C. Jia, S. Li, X. Huang, X. J. Yang, C. Janiak and B. Wu, Supramol. Chem., 2012, 24, 88–94 CrossRef CAS.
- J. R. Hiscock, P. A. Gale and M. J. Hynes, Supramol. Chem., 2012, 24, 355–360 CrossRef CAS.
- M. Li, Y. Hao, B. Wu, C. Jia, X. Huang and X. J. Yang, Org. Biomol. Chem., 2011, 9, 5637–5640 CAS.
- M. Li, B. Wu, C. Jia, X. Huang, Q. Zhao, S. Shao and X. J. Yang, Chem. – Eur. J., 2011, 17, 2272–2280 CrossRef CAS PubMed.
- M. Yamanaka, J. Inclusion Phenom. Macrocyclic Chem., 2013, 77, 33–48 CrossRef CAS.
- R. Dutta and P. Ghosh, Eur. J. Inorg. Chem., 2013, 2673–2681 CrossRef CAS.
- V. K. Bhardwaj, S. Sharma, N. Singh, M. S. Hundal and G. Hundal, Supramol. Chem., 2011, 23, 790–800 CrossRef CAS.
- W. Kim, S. K. Sahoo, G. D. Kim and H. J. Choi, Tetrahedron, 2015, 71, 8111–8116 CrossRef CAS.
- C. Jia, B. Wu, S. Li, Z. Yang, Q. Zhao, J. Liang, Q. S. Li and X. J. Yang, Chem. Commun., 2010, 46, 5376–5378 RSC.
- R. Li, Y. Zhao, S. Li, P. Yang, X. Huang, X. J. Yang and B. Wu, Inorg. Chem., 2013, 52, 5851–5860 CrossRef CAS PubMed.
- Y. Zhang, R. Zhang, Y. Zhao, L. Ji, C. Jia and B. Wu, New J. Chem., 2013, 37, 2266–2270 RSC.
- M. J. Hynes, J. Chem. Soc., Dalton Trans., 1993, 311–312 RSC.
- B. D. Smith and T. N. Lambert, Chem. Commun., 2003, 2261–2268 RSC.
- Y. Marcus, J. Chem. Soc., Faraday Trans., 1991, 87, 2995–2999 RSC.
- X. Wu, L. W. Judd, E. N. W. Howe, A. M. Withecombe, V. Soto-Cerrato, H. Li, N. Busschaert, H. Valkenier, R. Perez-Tomas, D. N. Sheppard, Y.-B. Jiang, A. P. Davis and P. A. Gale, Chem, 2016 DOI:10.1016/j.chempr.2016.04.002.
- W. F. D. Bennett, J. L. MacCallum and D. P. Tieleman, J. Am. Chem. Soc., 2009, 131, 1972–1978 CrossRef CAS PubMed.
- A. V. Hill, Biochem. J., 1913, 7, 471 CrossRef CAS PubMed.
- S. Bhosale and S. Matile, Chirality, 2006, 18, 849–856 CrossRef CAS PubMed.
- N. Busschaert, S. J. Bradberry, M. Wenzel, C. J. E. Haynes, J. R. Hiscock, I. L. Kirby, L. E. Karagiannidis, S. J. Moore, N. J. Wells, J. Herniman, G. J. Langley, P. N. Horton, M. E. Light, I. Marques, P. J. Costa, V. Félix, J. G. Frey and P. A. Gale, Chem. Sci., 2013, 4, 3036–3045 RSC; N. J. Knight, E. Hernando, C. J. E. Haynes, N. Busschaert, H. J. Clarke, K. Takimoto, M. García-Valverde, J. G. Frey, R. Quesada and P. A. Gale, Chem. Sci., 2016, 7, 1600–1608 RSC.
- S. J. Coles and P. A. Gale, Chem. Sci., 2012, 3, 683–689 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Additional information as noted in the text including synthetic details for the preparation of L1–L9, fittings of 1H-NMR titrations, crystallographic tables, transport studies. CCDC 1481148–1481150 for [L5(Cl−)](TBA+), [L4(Cl−)2](TBA+)2, [L5(AcO−)](TBA+). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt02046a |
| This journal is © The Royal Society of Chemistry 2016 |