Aerobic epoxidation catalysed by transition metal substituted polyfluorooxometalates
Abstract
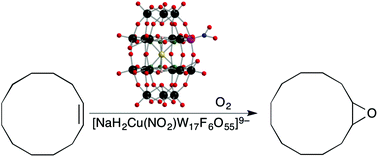
First row transition metal substituted polyfluorooxmetalates with quasi Wells–Dawson structures and a nitro terminal ligand, [NaH2M(NO2)W17F6O55]q−, were used as catalysts for the aerobic epoxidation of cyclic alkenes. The Cu(NO2) analog combined the best traits of conversion and selectivity. Some C–C bond cleavage was also observed and cis isomers reacted preferentially without stereochemical inversion indicating an oxygen atom to double bond concerted reaction.
- This article is part of the themed collection: Small Molecule Activation

 Please wait while we load your content...
Please wait while we load your content...