Open Access Article
Open Access ArticleStimuli-responsive metal-directed self-assembly of a ring-in-ring complex†
Cristina
Alvariño
a,
Carlos
Platas-Iglesias
a,
Víctor
Blanco
b,
Marcos D.
García
*a,
Carlos
Peinador
*a and
José M.
Quintela
a
aDepartamento de Química Fundamental and Centro de Investigacións Científicas Avanzadas (CICA), Facultad de Ciencias, Universidade da Coruña, E-15071 A Coruña, Spain. E-mail: carlos.peinador@udc.es; marcos.garcia1@udc.es
bDepartamento de Química Orgánica, Facultad de Ciencias, Universidad de Granada, Campus Fuentenueva S/N, 18071 Granada, Spain
First published on 6th June 2016
Abstract
Concentration, temperature and/or solvent polarity control the speciation on the metal-directed self-assembly of a ditopic pyridyl ligand L with cis-protected Pd(II) metal centers. This results into a controllable dynamic system, involving a [Pd2L2]6+ metallacycle and a [Pd4L4]12+ ring-in-ring complex.
The ultimate goal of supramolecular chemistry1 is to achieve control over the structure and dynamics of self-assembled systems.2 Nature is the undisputed master of the discipline, creating complex functional architectures by exquisite regulation of the spatial and temporal self-organization of a handful of simple building blocks.3
Among different strategies developed by chemists to mimic Nature's self-sorting precision,4 coordination-driven self-assembly5 has emerged as a powerful tool enabling the construction of innumerable complex structures including interlocked molecules.6 Although catenated structures are almost routinely obtained, inclusion of a macrocycle within another to form ring-in-ring complexes is a synthetic challenge and few examples are known.7 Moreover, ring-in-ring assemblies are key intermediates for the preparation of non trivial molecular knots such as the Borromean rings.8
Despite the tremendous efforts dedicated to the creation of supramolecular complexity, by altering the structural (spatial) aspects of metal-coordination self-assembly, there is still a great lack of work focused on the dynamic (temporal) features of the strategy.9 The use of an external stimulus (pH, light, polarity, etc.), able to alter the speciation in supramolecular coordination self-assemblies,10 would certainly open the door for the development of supramolecular adaptive systems.2
Following our ongoing interest on the development of new metallacycles as molecular receptors11 and building blocks for interlocked architectures,12 we decided to enlarge the length of our previously reported dinuclear rectangular metallacyclophanes by self-assembling the 1,4-biphenylene-bridged 4,4′-bipyridinium-based ligand L with cis-protected Pd(II)/Pt(II) metal centers M (Scheme 1).
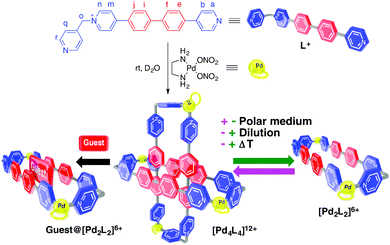 | ||
| Scheme 1 Representation of the different structures discussed in the text. The meaning of + and − is an increase and decrease of the corresponding stimulus. | ||
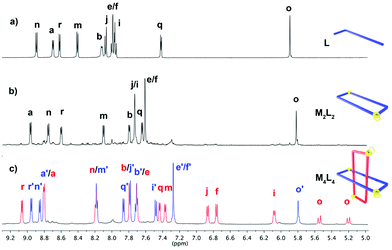
According to the typical methodology employed in our investigations,13 we firstly proceeded to test the self-assembly of the [Pd2L2]6+ metallacycle in organic media. The 1H and 13C NMR spectra of a 10 mM equimolar solution of L(PF6) and (en)Pd(OTf)2, in CD3NO2 at room temperature, showed signals and chemical shifts which are in good agreement with the self-assembly of the expected supramolecule (Fig. 1). Hereafter all concentrations will be referred to those of the starting components. Supported by 2D NMR experiments, we observed the usual downfield shift of protons Ha, Hb, Hq and Hr on the receptor as a result of the coordination of the non-quaternized nitrogen atoms of the ligand, and the upfield shift of the remaining protons of L because of the formation of the π-deficient cavity within [Pd2L2]6+.
 | ||
| Fig. 1 Partial 1H NMR spectra (CD3NO2, 500 MHz) of L(PF6) (10 mM) (top) and Pd2L2(PF6)2(OTf)4(bottom). | ||
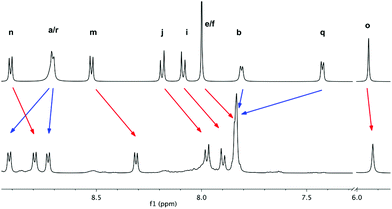
In sharp contrast, the results obtained for the attempted self-assembly of the [Pd2L2]6+ receptor in aqueous media were far more intriguing. Here, the 1H and 13C NMR spectra recorded for a 10 mM equimolar solution of L(NO3) and (en)Pd(NO3)2, in D2O at room temperature, showed two different sets of signals (twenty aromatic doublets) for the ligand in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (Fig. 2c). DOSY (diffusion-ordered NMR spectroscopy) experiments for this mixture showed the same diffusion coefficients for the two sets of signals for L and the palladium complex, implying that all the components of the sample are diffusing as a whole. Furthermore, the dilution experiments, carried out on the original sample within the 10–0.125 mM concentration range, revealed the initial two sets of signals for this species increasingly converging into a new one (Fig. S60†), and with the NMR data compatible with [Pd2L2]6+ being formed as the main product of the self-assembly on the 1–0.125 mM window (Fig. 2b). Additionally, variable-temperature 1H-NMR experiments carried out on the 10 mM sample also showed the conversion of the initially formed species into the expected dinuclear metallacycle upon heating (Fig. S63†). Finally, the addition of CD3OD to the 10 mM sample also leads to the formation of [Pd2L2]6+ (Fig. S64†).
1 ratio (Fig. 2c). DOSY (diffusion-ordered NMR spectroscopy) experiments for this mixture showed the same diffusion coefficients for the two sets of signals for L and the palladium complex, implying that all the components of the sample are diffusing as a whole. Furthermore, the dilution experiments, carried out on the original sample within the 10–0.125 mM concentration range, revealed the initial two sets of signals for this species increasingly converging into a new one (Fig. S60†), and with the NMR data compatible with [Pd2L2]6+ being formed as the main product of the self-assembly on the 1–0.125 mM window (Fig. 2b). Additionally, variable-temperature 1H-NMR experiments carried out on the 10 mM sample also showed the conversion of the initially formed species into the expected dinuclear metallacycle upon heating (Fig. S63†). Finally, the addition of CD3OD to the 10 mM sample also leads to the formation of [Pd2L2]6+ (Fig. S64†).
 | ||
| Fig. 2 Partial 1H NMR (D2O, 500 MHz) spectra of (a) L(NO3) (2.5 mM); equimolar solutions of L(NO3) and (en)Pd(NO3)2: (b) 0.25 mM, (c) 10 mM. | ||
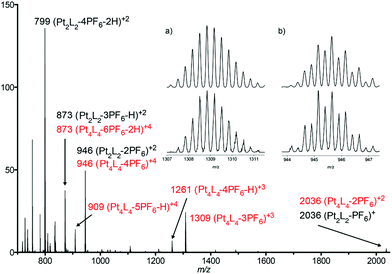
In order to gain insight into the nature of the species occurring by self-assembly of the components in aqueous solution, we decided to use our previously reported microwave-assisted protocol for the synthesis of Pt(II) analogues of the supramolecules formed.14a After heating an aqueous equimolar solution of L(NO3) and (en)Pt(NO3)2, the main species formed showed the same splitting of signals into two sets (twenty aromatic doublets) as the palladium analogue. Furthermore, after precipitation with KPF6, the HR-ESI spectra confirmed the self-assembly of an aggregate as being [Pt4L4](PF6)12 composed of two metallacycles, with a good correlation between the theoretical and experimental isotopic patterns of the peaks resulting from the loss of three and four counterions (Fig. 3).
At this point it is worth noting that, in terms of topology, a [M4L4]12+ species can be assembled in two manners: a [2]catenane formed by two interlocking metallacycles and a ring-in-ring complex assembled by self-complexation. Even though it would be in good agreement with the NMR and mass spectrometry data and the two proposed models, the formation of the [2]catenane structure could be ruled out on the basis of the Pt(II) species displaying a concentration-dependent behavior and the kinetic inertness of the N(Py)–Pt bond at room temperature.14
Thus, the dilution experiment of a solution of [Pt4L4]12+ (8 mM in D2O) monitored by 1H NMR showed the gradual conversion of [Pt4L4]12+ to [Pt2L2]6+. Moreover, the 1H NMR spectrum recorded upon the addition of 1,5-bis[2-(hydroxyethoxy)ethoxy]naphthalene, a likely good guest for [Pt2L2]6+, to a solution of the [Pt4L4]12+ species in D2O, showed signals in concordance with those obtained for the inclusion complex between [Pd2L2]6+ and the same substrate. In this context, 1H–1H and 1H–13C correlation experiments allowed us to assign the relative shifts of each of the two different sets of resonances found for L within the ring-in-ring complex [Pd4L4]12+ (Fig. 2c). It is remarkable that the shielding effect experimented by the biphenylene (BiPh) protons of one set of signals (red signals, Fig. 2c) was compared with those of the free ligand L (especially Hi: δ = 6.1 ppm, Δδ = 1.9 ppm; Hf: δ = 6.8 ppm, Δδ = 1.3 ppm, and Hj: δ = 6.9 ppm, Δδ = 1.2 ppm); as would be expected for the insertion of the BiPh moieties of one [Pd2L2]6+ metallacycle inside the cavity of the other [Pd2L2]6+. Moreover, the signal attributable to the methylene protons of the species splits into two doublets because of the loss of symmetry of [Pd4L4]12+ compared to [Pd2L2]6+. In addition, the [Pd2L2]6+ and [Pd4L4]12+ structures were characterized by means of DFT calculations (Fig. 4). According to the calculations the ring-in-ring species is slightly more stable (1.99 kJ mol−1) than the [2]catenane in the aqueous phase. Finally, the comparable length and size of [Pd2L2]6+ and Ex2Box,15 which form ring-in-ring structures also support the non-catenated topology.
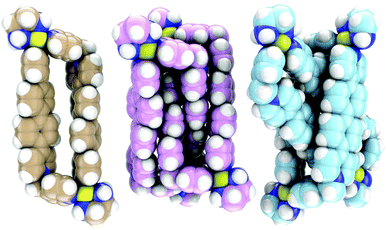 | ||
| Fig. 4 CPK representations of optimized structures of metallacycle [Pd2L2]6+ (left), [2]catenane (center), and ring-in-ring (right). | ||
Considering the results obtained for the self-assembly of the components in aqueous media, the system behaves as constitutionally dynamic, as a change in an external parameter (concentration, temperature and solvent polarity), which reversibly produces constitutional changes in the supramolecular structure. In this context, we tested the conversion of [Pd2L2]6+ into [Pd4L4]12+ by increasing the polarity of the reaction media. Therefore, the 1H NMR spectra recorded upon addition of NaNO3 to a 0.25 mM solution of [Pd2L2]6+ showed the partial conversion of the metallacycle into the [Pd4L4]12+ ring-in-ring complex.
BiPh units play an indispensable role in the self-assembly of the ring-in-ring complex, as we could verify when ligand L′, with a different configuration of the phenylene and pyridinium rings, was subjected to the same experimental conditions. Thus, ligand L′(NO3) leads to the metallacycle [Pd2L′2]6+ in D2O (Fig. 5, see also Fig. S12–S16†). The crystal structure of [Pd2L′2]6+ revealed the expected rectangular metallacycle with a similar size and shape to [Pd2L2]6+.16 However, L′ did not form any other structure than [Pd2L′2]6+ under the conditions tested for the self-assembly of L. Probably, the electrostatic repulsion between positively charged pyridinium units, much closer in the hypothetical [Pd4L′4]12+ structure, prevents its formation (Fig. 5).
 | ||
| Fig. 5 Metallacyles [Pd2L2]6+ (left) and [Pd2L′2]6+ (right) and schematic representation for the corresponding ring-in-ring structures. | ||
The fact that the speciation is conditioned by the solvent polarity seems to indicate that hydrophobic interactions are crucial in this dynamic process. Consequently, the rearrangement towards larger species can be considered as an effort of the system to hide from the solvent the more apolar region (BiPh units). In order to quantify this hypothesis, we determined the solvent-accessible surface area (SASA) and the buried solvent-accessible surface area (BSASA) (Table 1) for the BiPh units in each calculated structure. Although the SASA increases as expected with the number of BiPh, the SASA per BiPh unit decreases clearly with a maximum of 44% of reduction/BiPh in the ring-in-ring structure. Likewise, BSASA follows the same tendency, becoming greater, as the polarity increases. Moreover, it is worth noting that the SASA reduction per BiPh on the ring-in-ring complex (44%) is larger than that on the [2]catenane (39%).
| BiPh | [Pd2L2] | [2]Catenane | Ring-in-ring | |
|---|---|---|---|---|
| a Only the BiPh units of the calculated structures were taken into account. A 1.4 Å radii probe was used to calculate SASA and BSASA. b Average of the two different BiPh units. | ||||
| BiPh units | 1 | 2 | 4 | 4 |
| SASA | 334.6 | 594.4 | 815.3 | 743.9 |
| SASA/BiPh | 334.6 | 297.2 | 203.8 | 186.0 |
| BSASA/BiPh | — | 35.2 | 128.3b | 137.8b |
| SASA reduction (%) | — | 11 | 39 | 44 |
In summary, the self-assembly of the dinuclear metallacycle [Pd2L2]6+ has been achieved in organic and aqueous media. In aqueous media, hydrophobic forces promote the threading of two [Pd2L2]6+ metallacyles into a ring-in-ring structure, as could be demonstrated by dilution and complexation experiments using the platinum-based metallacyclic analogue. Formation and dissociation of the ring-in-ring complex can be controlled by temperature, concentration or medium polarity. An increase of the polarity of the reaction media promotes the assembly of the ring-in-ring complex, possibly due to less exposure to the solvent of the Bipy units within the supramolecule and, consequently, due to more favourable solvation energy.
This research was supported by the Ministerio de Economía y Competitividad (MINECO FEDER, CTQ-201341097-P) and Xunta de Galicia (EM2014/056). V. B. thanks MINECO for a “Juan de la Cierva” postdoctoral contract. The authors are also indebted to Centro de Supercomputación de Galicia (CESGA) for providing the computer facilities.
Notes and references
- (a) J.-M. Lehn, Supramolecular Chemistry: Concepts and Perspectives, VCH, Weinheim, Germany, 1995 Search PubMed; (b) Comprehensive Supramolecular Chemistry, ed. J. L. Atwood, J. E. D. Davies, D. D. MacNicol, F. Vögtle and J.-M. Lehn, Pergamon, Oxford, 1996 Search PubMed.
- (a) J.-M. Lehn, Science, 2002, 295, 2400 CrossRef CAS PubMed; (b) J.-M. Lehn, Top. Curr. Chem., 2012, 322, 1 CrossRef CAS PubMed; (c) J.-M. Lehn, Angew. Chem., Int. Ed., 2013, 52, 2836 CrossRef CAS PubMed; (d) J.-M. Lehn, Angew. Chem., Int. Ed., 2015, 54, 3276 CrossRef CAS PubMed.
- (a) M. Eigen, Naturwissenschaften, 1971, 58, 465 CrossRef CAS PubMed; (b) M. Groll, L. Dizel, J. Lowe, D. Stock, M. Bochter, H. D. Bartunik and R. Huber, Nature, 1997, 386, 463 CrossRef CAS PubMed; (c) P. Ball, The Self-Made Tapestry: Pattern Formation in Nature, Oxford Univ. Press, Oxford, 1999 Search PubMed; (d) S. De, K. Mahata and M. Schmittel, Chem. Soc. Rev., 2010, 39, 1555 RSC.
- (a) J. S. Lindsey, New J. Chem., 1991, 15, 153 CAS; (b) C. T. Matthew and J. F. Stoddart, Acc. Chem. Res., 1997, 30, 393 CrossRef; (c) P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 483 CrossRef; (d) G. M. Whitesides and B. A. Grzybowski, Science, 2002, 295, 2418 CrossRef CAS PubMed; (e) L. Brammer, Chem. Soc. Rev., 2004, 33, 476 RSC; (f) G. R. Desiraju, Angew. Chem., Int. Ed., 2007, 46, 8342 CrossRef CAS PubMed; (g) K. J. M. Bishop, C. E. Wilmer, S. Soh and B. A. Grzybowski, Small, 2009, 5, 1600 CrossRef CAS PubMed.
- (a) P. J. Stang and B. Olenyuk, Acc. Chem. Res., 1997, 30, 502 CrossRef CAS; (b) M. Fujita, Chem. Soc. Rev., 1998, 27, 417 RSC; (c) D. L. Caulder and K. N. Raymond, Acc. Chem. Res., 1999, 32, 975 CrossRef CAS; (d) B. H. Northrop, Y.-R. Zheng, K.-W. Chi and P. J. Stang, Acc. Chem. Res., 2009, 42, 1554 CrossRef CAS PubMed; (e) P. Wei, X. Yan and F. Huang, Chem. Soc. Rev., 2015, 44, 815 RSC.
- For selected reviews, see: (a) J. D. Crowley, S. M. Goldup, A.-L. Lee, D. A. Leigh and R. T. McBurney, Chem. Soc. Rev., 2009, 38, 1530 RSC; (b) E. Zangrando, M. Casanova and E. Alessio, Chem. Rev., 2008, 108, 4979 CrossRef CAS PubMed; (c) R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Chem. Rev., 2011, 111, 6810 CrossRef CAS PubMed; (d) C. Dietrich-Buchecker, B. X. Collason and J.-P. Sauvage, Top. Curr. Chem., 2005, 249, 261 CAS; (e) A. Mishra, S. C. Kang and K.-W. Chi, Eur. J. Inorg. Chem., 2013, 5222 CrossRef CAS; (f) S. Mukherjee and P. S. Mukherjee, Chem. Commun., 2014, 50, 2239 RSC; (g) T. R. Cook and P. J. Stang, Chem. Rev., 2015, 115, 7001 CrossRef CAS PubMed.
- (a) S.-Y. Kim, I.-S. Jung, E. Lee, J. Kim, S. Sakamoto, K. Yamaguchi and K. Kim, Angew. Chem., Int. Ed., 2001, 40, 2119 CrossRef CAS; (b) T. Iwanaga, R. Nakamoto, M. Yasutake, H. Takemura, K. Sako and T. Shinmyozu, Angew. Chem., Int. Ed., 2006, 45, 3643 CrossRef CAS PubMed; (c) S. J. Dalgarno, J. Fisher and C. L. Raston, Chem. – Eur. J., 2006, 12, 2772 CrossRef CAS PubMed; (d) R. S. Forgan, C. Wang, D. C. Friedman, J. M. Spruell, C. L. Stern, A. A. Sarjeant, D. Cao and J. F. Stoddart, Chem. – Eur. J., 2012, 18, 202 CrossRef CAS PubMed; (e) S.-H. Chiu, A. R. Pease, J. F. Stoddart, A. J. P. White and D. J. Williams, Angew. Chem., Int. Ed., 2002, 41, 270 CrossRef CAS; (f) V. Vajpayee, Y. H. Song, T. R. Cook, H. Kim, Y. Lee, P. J. Stang and K.-W. Chi, J. Am. Chem. Soc., 2011, 133, 19646 CrossRef CAS PubMed; (g) R. S. Forgan, D. C. Friedman, C. L. Stern, C. J. Bruns and J. F. Stoddart, Chem. Commun., 2010, 46, 5861 RSC.
- (a) J. L. Sun, M. Frasconi, Z. C. Liu, J. C. Barnes, Y. P. Wang, D. Y. Chen, C. L. Stern and J. F. Stoddart, Chem. Commun., 2015, 51, 1432 RSC; (b) J. K. Klosterman, J. Veliks, D. K. Frantz, Y. Yasui, M. Loepfe, E. Zysman-Colman, A. Linden and J. S. Siegel, Org. Chem. Front., 2016, 3, 661 RSC.
- For selected examples, see: (a) S.-S. Sun, J. A. Anspach and A. Lees, Inorg. Chem., 2002, 41, 1862 CrossRef CAS PubMed; (b) S.-S. Sun, C. L. Stern, S. T. Nguyen and J. T. Hupp, J. Am. Chem. Soc., 2004, 126, 6314 CrossRef CAS PubMed; (c) A. M. Brown, M. V. Ovchinnikov, C. L. Stern and C. A. Mirkin, J. Am. Chem. Soc., 2004, 126, 14316 CrossRef CAS PubMed; (d) L. Zhao, B. H. Northrop and P. J. Stang, J. Am. Chem. Soc., 2008, 130, 11886 CrossRef CAS PubMed; (e) V. Blanco, M. D. García, C. Platas-Iglesias, C. Peinador and J. M. Quintela, Chem. Commun., 2010, 46, 6672 RSC; (f) M. L. Saha, S. Pramanik and M. Schmittel, Chem. Commun., 2012, 48, 9459 RSC; (g) E. F. V. Dry, J. K. Clegg, B. Breiner, D. E. Whitaker, R. Stefak and J. R. Nitschke, Chem. Commun., 2011, 47, 6021 RSC; (h) S. Chen, L.-J. Chen, H.-B. Yang, H. Tian and W. Zhu, J. Am. Chem. Soc., 2012, 134, 13596 CrossRef CAS PubMed.
- (a) A. J. McConnell, C. S. Wood, P. P. Neelakandan and J. R. Nitschke, Chem. Rev., 2015, 115, 7729 CrossRef CAS PubMed; (b) P. Wei, T. R. Cook, X. Yan, F. Huang and P. J. Stang, J. Am. Chem. Soc., 2014, 136, 15497 CrossRef CAS PubMed.
- (a) M. Chas, D. Abella, V. Blanco, E. Pía, G. Blanco, A. Fernández, C. Platas-Iglesias, C. Peinador and J. M. Quintela, Chem. – Eur. J., 2007, 13, 8572 CrossRef CAS PubMed; (b) V. Blanco, A. Gutiérrez, C. Platas-Iglesias, C. Peinador and J. M. Quintela, J. Org. Chem., 2009, 74, 6577 CrossRef CAS PubMed; (c) C. Peinador, E. Pía, V. Blanco, M. D. García and J. M. Quintela, Org. Lett., 2010, 12, 1380 CrossRef CAS PubMed; (d) V. Blanco, M. D. García, A. Terenzi, E. Pía, A. Fernández-Mato, C. Peinador and J. M. Quintela, Chem. – Eur. J., 2010, 16, 12373 CrossRef CAS PubMed; (e) C. Alvariño, E. Pía, M. D. García, V. Blanco, A. Fernández, C. Peinador and J. M. Quintela, Chem. – Eur. J., 2013, 19, 15329 CrossRef PubMed; (f) T. Rama, E. M. López-Vidal, M. D. García, C. Peinador and J. M. Quintela, Chem. – Eur. J., 2015, 21, 9482 CrossRef CAS PubMed.
- (a) V. Blanco, M. Chas, D. Abella, C. Peinador and J. M. Quintela, J. Am. Chem. Soc., 2007, 129, 13978 CrossRef CAS PubMed; (b) C. Peinador, V. Blanco and J. M. Quintela, J. Am. Chem. Soc., 2009, 131, 920 CrossRef CAS PubMed; (c) V. Blanco, D. Abella, E. Pía, C. Platas-Iglesias, C. Peinador and J. M. Quintela, Inorg. Chem., 2009, 48, 4098 CrossRef CAS PubMed; (d) V. Blanco, M. D. García, C. Peinador and J. M. Quintela, Chem. Sci., 2011, 2, 2407 RSC; (e) C. Alvariño, A. Terenzi, V. Blanco, M. D. García, C. Peinador and J. M. Quintela, Dalton Trans., 2012, 41, 11992 RSC; (f) E. M. López-Vidal, M. D. García, C. Peinador and J. M. Quintela, Chem. – Eur. J., 2015, 21, 2259 CrossRef PubMed.
- (a) C. Peinador, V. Blanco, M. D. García and J. M. Quintela, in Molecular Self-Assembly: Advances and Applications, ed. A. D. Q. Li, Pan Stanford Publishing, Singapore, 2012, p. 351 Search PubMed; (b) M. D. García, C. Alvariño, E. M. López-Vidal, T. Rama, C. Peinador and J. M. Quintela, Inorg. Chim. Acta, 2014, 417, 27 CrossRef.
- (a) E. López-Vidal, V. Blanco, M. D. García, C. Peinador and J. M. Quintela, Org. Lett., 2012, 14, 580 CrossRef PubMed; (b) M. Fujita, F. Ibukuro, K. Yamaguchi and K. Ogura, J. Am. Chem. Soc., 1995, 117, 4175 CrossRef CAS; (c) F. Ibukuro, K. Kusukawa and M. Fujita, J. Am. Chem. Soc., 1998, 120, 8561 CrossRef CAS.
- M. Juricek, J. C. Barnes, E. J. Dale, W. G. Liu, N. L. Strutt, C. J. Bruns, N. A. Vermeulen, K. C. Ghooray, A. A. Sarjeant, C. L. Stern, Y. Y. Botros, W. A. Goddard and J. F. Stoddart, J. Am. Chem. Soc., 2013, 135, 12736 CrossRef CAS PubMed.
- The length of the metallacycle (distance Pt-CH2: 18.95 Å) was obtained from the crystal structure of [Pt2L′2](PF6)6 (see the ESI†) which was almost identical to the obtained from the calculated structure of [Pd2L2]6+ (distance Pd-CH2: 19.15 Å). CCDC 1470000 contains the supplementary crystallographic data for this paper.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details, full characterization data of the new compounds (1D and 2D NMR spectra), and details of modelling calculations. CCDC 1470000. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt01835a |
| This journal is © The Royal Society of Chemistry 2016 |