 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nanoparticles of lanthanide oxysulfate/oxysulfide for improved oxygen storage/release†
Wuyuan
Zhang
,
Isabel. W. C. E.
Arends
and
Kristina
Djanashvili
*
Department of Biotechnology, Delft University of Technology, Julianalaan 136, 2628 BL, Delft, the Netherlands. E-mail: k.djanashvili@tudelft.nl
First published on 4th August 2016
Abstract
Lanthanide oxysulfates have the ability to store and release large volumes of oxygen under oxidizing/reducing conditions, rendering them interesting as automotive catalysts. Herein we demonstrate a remarkable improvement of both processes by utilization of nanoparticles compared to the bulk materials. A further improvement of the catalytic activity was achieved by cost-effective doping with 1.9 wt% of Ni.
Materials with a capacity for oxygen storage and release are important oxygen carriers (OCs) and are therefore of great interest for application in automotive catalytic combustion. Under oxidative conditions, metal oxides are formed, which then in turn can be reduced by fuel components, such as CO, hydrocarbons, and NOx. In this process, OCs undergo a reversible and quantifiable redox reaction with oxygen in the gas phase or at the gas–solid interphase, and are therefore useful for regulation of the oxygen concentration under oxygen lean conditions.1–3 Commonly, transition metal oxides on various supports, e.g. dendrimers, TiO2, Al2O3, zeolites, SiO2, etc. have been studied for this purpose.4 CeO2 is an attractive material in this respect because of the reversible and fast redox reactions between Ce4+ and Ce3+ at relatively mild temperatures (<400 °C). Much effort has been put into control of the surface properties of this material to minimize deactivation due to sintering at operation temperatures. Strategies have been developed to increase surface area and/or to create more distorted structures, such as dispersion of ceria into porous carriers,5 creation of defects,6,7 chemical doping,8–10 and reduction of the particle size.11–14 The latter objective is among the most important factors that are influencing the oxygen storage capacity of OCs.
However, the maximum capacity of oxygen storage per mole of CeO2 is limited to 0.25 mole of O2 and its stability is not sufficient under operating conditions.15 Another disadvantage of metal oxides is their susceptibility to sulfur poisoning when using common carbon fuels.16,17 CaSO4 has been extensively studied due to its sulfur tolerance along with the capacity to store up to 2 mol of O2 per mol of sulfate.18,19 However, high reduction rates can be only achieved above 1000 °C, accompanied with some undesired release of SO2.20
In 2004, Machida et al. reported a promising alternative by using lanthanide oxysulfates (Ln2O2SO4) with much larger capacities of oxygen storage (2 mole of O2 per mole of S).21 The mechanism of the oxygen storage, in this case, does not involve the metal ions, but is based on the reversible redox of sulfur from +6 (SO42−) to −2 in sulfide (S2−),1 as shown in the following reactions:
| Ln2O2SO4 + 4H2 → Ln2O2S + 4H2O | (1) |
| Ln2O2S + 2O2 → Ln2O2SO4 | (2) |
Another advantage of Ln2O2SO4 is the very large stability and catalytic activity up to very high temperatures (>1000 °C), without the loss of sulfur. Moreover, a wide range of lanthanides can be applied for this purpose.1,22 On the other hand, the practical application is limited due to the still high temperatures (>700 °C) required. Impregnation of Ln2O2SO4 materials with noble metals (Pt or Pd) resulted in significant reduction of the operative temperatures by 100–200 °C for both oxygen release and storage processes due to the activation of hydrogen and oxygen spillover.21 The reaction rates could also be enhanced by increasing the surface of the Ln2O2SO4 materials by using layered Ln-dodecyl sulfate mesophases as precursors during its preparation.23 Doping by Ce offered another effective way to improve the activity of Ln2O2SO4 (Ln ≠ Ce). It causes structural distortion of tetrahedral SO4 units, promoting the rates of oxygen release and storage,24 while the co-presence of Ce3+/Ce4+ ions on the surface of Ln2O2SO4 further accelerated the redox of sulfur. The detailed X-ray structural study revealed that the oxygen release and storage behavior is accompanied by noticeable differences in S–O distances and O–S–O angles of the SO4 units, as well as differences in the crystal structure of Ln2O22+ units.22,25 In a very recent report, Lisi et al. demonstrated that Cu-doping can enhance the oxygen mobility in the La2O2SO4 structure, leading to decreased reaction temperatures for both reduction and oxidation.20
The Ln2O2SO4 materials for oxygen storage and release reported so far have been prepared by several methods, such as calcination of Ln2(SO4)3·nH2O,1,26 utilization of precursors of layered Ln-dodecyl sulfate mesophases,23,27 or Ln-precipitation.28,29 All these procedures lead to bulk materials with an irregular morphology. The correlation between the size and shape of the catalyst and the catalytic performance has been mentioned in the literature,30–32 but the effects on oxygen storage/release performance have yet not been demonstrated. Herein we report on a remarkable enhancement of the oxygen storage/release capacity by (i) using nanosized Pr2O2SO4 rather than bulk and (ii) by doping the Pr2O2SO4 with Ni(II). Pr2O2SO4 was selected, because among the lanthanides it can act as oxidation catalyst with high rates at relatively low temperatures (<600 °C).1
Recently, we have developed a facile method for the preparation of nanosized Ln2O2SO4 (Ln = Gd and Ho) based on thermal decomposition of nanodroplets (NDs) formed by Ln-acetylacetonates (Ln(acac)3) under emulsifying conditions.33 The choice of the surfactant for the formation of NDs was found to determine the elemental composition of the nanoparticles (NPs) obtained after the calcination of the dried NDs. In the present study sodium dodecyl sulfate was selected as the surfactant. The thermogravimetric analysis (TGA) profile of the fluffy powders resulting from freeze drying of the obtained NDs, showed two major weight losses: dehydration and combustion of organic moieties below 300 °C (Fig. S1†). The formation of Pr2O2SO4 takes place between 300–800 °C by the alternative stacking between SO42− and Pr2O22+.23,24 Above 800 °C a stabilized curve was observed, indicating the full formation of inorganic NPs after this temperature. Therefore, to obtain the solid NPs, the calcination was carried out at 800 °C for 1 h to give Pr2O2SO4 NPs in 82% yield with respect to Pr(acac)3.
Fig. 1 demonstrates the X-Ray Diffraction (XRD) patterns of the crystalline Pr2O2SO4 as well as the oxysulfide Pr2O2S, which was obtained after reduction of the oxysulfate by H2 (10%) in Ar. The XRD pattern reveals an orthorhombic structure of Pr2O2SO4 with calculated lattice constants a = 4.240 Å, b = 4.138 Å, and c = 13.422 Å, which are in a good agreement with the reported values (PDF#41-0679). Additionally, the XRD pattern of Pr2O2S shows lattice dimensions of a = 3.574 Å, b = 3.974 Å and c = 6.798 Å, corresponding to a hexagonal cell (p3ml-164, PDF#65-3453). TEM images show that fairly spherical particles NPs were obtained with a diameter of 28 ± 5.1 (Fig. S2†).
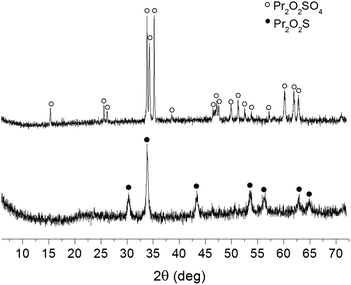 | ||
| Fig. 1 Powder XRD patterns of nanoparticulate Pr2O2SO4 obtained by miniemulsion method, and Pr2O2S, resulted from the subsequent reduction. | ||
Following the successful preparation of Pr2O2SO4 NPs, their redox behavior was investigated (Fig. 2a and b). The dynamic reduction was evaluated by the temperature programmed reduction (TPR), which was carried out in a conventional flow system by heating the sample at 10 °C min−1 in a stream of 10% H2 in Ar. As shown in Fig. 2a, the reduction started at about 700 °C and gave a peak in H2-uptake at 790 °C, whereas the reaction was completed at around 800 °C. The asymmetric peak in the narrow temperature range indicated a very fast reduction. The oxysulfate was reduced into oxysulfide (Pr2O2S), as proven by its XRD pattern as shown in Fig. 1. The obtained oxysulfide was then subjected to temperature programmed re-oxidation (TPRO) in a stream of 20% O2 in He (Fig. 2b). The oxygen consumption started at about 480 °C and exhibited a maximal peak at 580 °C. Above this temperature, the re-oxidation rate became slower and was not even completed until 900 °C. Based on the integration of TPR and TPRO profiles, the amount of consumed H2 and O2 was 3.97 and 1.34 mol−1 for Pr2O2SO4 and Pr2O2S, respectively. The ratio of oxygen consumption per mol of Pr2O2S is somewhat below 2, which confirms that the re-oxidation was not finished under the conditions applied.
These results can be compared with those for the bulk material as reported in literature.1 The nanosized Pr2O2SO4 displays a fast reduction in TPR between 700 and 800 °C, and a low temperature for the maximum uptake of oxygen in TPRO (580 °C). In contrast, the catalytic performance of the previously reported bulk Pr2O2SO4 was clearly less effective: the reduction took place above 900 °C and the maximum oxygen uptake was observed only at 700 °C.1
Aiming at further enhancement of the redox reactions, we next doped the Pr2O2SO4 with Ni(II) as a cost-effective alternative for Pt or Pd for activation of both hydrogen and oxygen.2,34 The Pr2O2SO4 NPs described above were impregnated with an aqueous solution of NiCl2 and then calcined at 450 °C for 90 min to give Pr2O2SO4 doped with 1.9 wt% of Ni. The extent of Ni-doping was calculated from the Energy Dispersive Spectrum (EDS) of the prepared materials (Fig. S3†). The identical XRD-patterns (Fig. S4†) confirm the unchanged crystallinity of Pr2O2SO4 NPs after doping with Ni, as it was already expected from the literature data.35 Additionally, the calculations of the lattice space selected from the HRTEM images (Fig. S5†) resulted in 1.57, 0.72, 0.55, 0.45 and 0.36 nm, corresponding to the interplanar space of (001), (002), (100), (100), (310) and (202) crystallographic plane, which is a fair agreement with the interplanar space of the standard (PDF#41-0679).
The TPR/TPRO profile of these Ni-doped NPs (Fig. 2c and d) appeared to release the oxygen in the temperature range 570–730 °C under consumption of 3.89 mol−1 of H2. The oxygen uptake started at about 400 °C, reached a maximum at 580 °C, and was completed at about 700 °C with 1.93 mol−1 of the total O2 uptake. The ratio H2/O2 uptake is 2, which is in perfect agreement with fully reversible redactions.
The rate of oxygen release and storage is another important property that characterizes the performance of the Ln2O2SO4 as a storage material. To compare this property of the present nanosized Pr2O2SO4 with those of the bulk material, we performed the redox reaction at both 700 and 600 °C, as shown in Fig. 3. Because this material has demonstrated perfect dynamic oxygen release and storage cycles, we only calculated the reaction rates based on the first cycle. For the 1.9 wt% Ni-doped Pr2O2SO4 NPs, both reduction and re-oxidation reactions were completed within 10 min at 700 °C. The reaction rates calculated from the redox profiles are 0.51 mmol g−1 min−1 for the reduction, and 0.66 mmol g−1 min−1 for the re-oxidation. The rate of oxygen storage is more than 2 times higher than that of the best bulk Pr2O2SO4 materials doped with 1 wt% Pd reported in the literature (see Table 1). At 600 °C, the storage rate of Ni-doped Pr2O2SO4 is still faster than that of bulk material measured at 700 °C. The observed faster oxygen storage compared to release is in agreement with bulk materials, and is characteristic for the Pr-based systems. This is due to the coexistence of Pr3+ and Pr4+ ions on the particle surface as demonstrated by Machida and coworkers.22,25
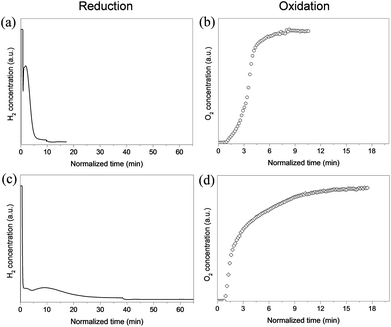 | ||
| Fig. 3 Redox reactions of 1.9 wt% Ni-doped Pr2O2SO4 at 700 °C (a, b) and 600 °C (c, d) under feed stream of 10% H2/Ar and 5% O2/He. | ||
These results show that the Ni-doped nanosized Pr2O2SO4 system has an improved performance in terms of faster reaction rate at lower temperatures compared to bulk materials. This is likely to be due to the reduced size of the particles: the higher surface-to-volume ratio of smaller NPs leads to rapid gas diffusion and solid–gas reactions that facilitate oxygen storage and release.23,26 Additionally, smaller size leads to an increased number of Pr3+ and Pr4+ species on the surface of NPs. As discussed above, the obtained Pr2O2SO4 NPs exhibit orthorhombic structure with shortened a (4.240 Å) but extended c (13.422 Å) of lattice parameters, compared to those of the bulk material with a monoclinic structure (a = 14.047 Å, and c = 8.281 Å).22 Stacking of SO42− and Pr2O22+ layers along the a-axis changes the crystal structure of Pr2O2SO4 NPs by distortion of the SO4 tetrahedral units in which each oxygen atom is coordinated to a Pr atom.30 This is, therefore, probably advantageous for the faster release of oxygen observed in the present study.
In summary, the nanoparticulate Pr2O2SO4 showed a remarkable enhancement of oxygen storage/release reaction rates allowing operation with good performance at lower temperatures than comparable bulk Pr-oxysulfates. Further improvement was achieved by 1.9 wt% doping with Ni, due to increased oxygen mobility known to occur at the surface of the catalysts with available d-orbitals. The results of TPR/TPRO for the Ni-doped Pr2O2SO4 show catalytic activity already at 600 °C, and 700 °C, and the rates for oxygen storage and release are respectively 2 and 4.6 times higher than these of the best bulk material reported up to now (1 wt% Pd-doped Pr2O2SO4). The promoting effect of the presented system could be attributed to a collective effect of (i) higher surface-to-volume ratio of NPs, (ii) co-presence of Pr3+ and Pr4+ at the NP surface, (iii) distorted crystal structure leading to more reactive SO4 units, and (iv) Ni-doping as a cost-effective alternative to much more expensive Pt and Pd. These effects result in an overall enhanced ability in storing and releasing oxygen.
Acknowledgements
This research was supported by China Scholarship Council (W. Z.) and the Netherlands Organization for Scientific Research (K. D., Veni grant-722.012.009). The authors thank Bart van der Linden (Catalysis Engineering, TU Delft) for the TPR/TPRO measurements.Notes and references
- M. Machida, K. Kawamura, K. Ito and K. Ikeue, Chem. Mater., 2005, 17, 1487–1492 CrossRef CAS.
- J. Wang, H. Chen, Z. Hu, M. Yao and Y. Li, Catal. Rev.: Sci. Eng., 2015, 57, 79–144 CAS.
- T. Motohashi, T. Ueda, Y. Masubuchi, M. Takiguchi, T. Setoyama, K. Oshima and S. Kikkawa, Chem. Mater., 2010, 22, 3192–3196 CrossRef CAS.
- W. Yu, M. D. Porosoff and J. G. Chen, Chem. Rev., 2012, 112, 5780–5817 CrossRef CAS PubMed , and references therein.
- T. Osaki, K. Yamada, K. Watari and K. Tajiri, React. Kinet., Mech. Catal., 2014, 114, 561–570 CrossRef.
- N. J. Lawrence, J. R. Brewer, L. Wang, T.-S. Wu, J. Wells-Kingsbury, M. M. Ihrig, G. Wang, Y.-L. Soo, W.-N. Mei and C. L. Cheung, Nano Lett., 2011, 11, 2666–2671 CrossRef CAS PubMed.
- B. Huang, R. Gillen and J. Robertson, J. Phys. Chem. C, 2014, 118, 24248–24256 CAS.
- A. Simson, K. Roark and R. Farrauto, Appl. Catal., B, 2014, 158–159, 106–111 CrossRef CAS.
- N. Qiu, J. Zhang and Z. Wu, Phys. Chem. Chem. Phys., 2014, 16, 22659–22664 RSC.
- Q. Dong, S. Yin, C. Guo, X. Wu, T. Kimura, T. Le, T. Sakanakura and T. Sato, IOP Conf. Ser.: Mater. Sci. Eng., 2013, 47, 012065 CrossRef.
- J. Li, Z. Zhang, Z. Tian, X. Zhou, Z. Zheng, Y. Ma and Y. Qu, J. Mater. Chem. A, 2014, 2, 16459–16466 CAS.
- M. P. Yeste, J. C. Hernandez-Garrido, D. C. Arias, G. Blanco, J. M. Rodriguez-Izquierdo, J. M. Pintado, S. Bernal, J. A. Perez-Omil and J. J. Calvino, J. Mater. Chem. A, 2013, 1, 4836–4844 CAS.
- C. Sun and D. Xue, Phys. Chem. Chem. Phys., 2013, 15, 14414–14419 RSC.
- H. Imagawa, A. Suda, K. Yamamura and S. Sun, J. Phys. Chem. C, 2011, 115, 1740–1745 CAS.
- J. Kašpar and P. Fornasiero, J. Solid State Chem., 2003, 171, 19–29 CrossRef.
- N. S. Nasri, J. M. Jones, V. A. Dupont and A. Williams, Energy Fuels, 1998, 12, 1130–1134 CrossRef CAS.
- L. S. Carvalho, C. L. Pieck, M. do Carmo Rangel, N. S. Fígoli and J. M. Parera, Ind. Eng. Chem. Res., 2004, 43, 1222–1226 CrossRef CAS.
- H. Tian and Q. Guo, Ind. Eng. Chem. Res., 2009, 48, 5624–5632 CrossRef CAS.
- Q. Song, R. Xiao, Z. Deng, H. Zhang, L. Shen, J. Xiao and M. Zhang, Energy Convers. Manage., 2008, 49, 3178–3187 CrossRef CAS.
- L. Lisi, G. Mancino and S. Cimino, Int. J. Hydrogen Energy, 2015, 40, 2047–2054 CrossRef CAS.
- M. Machida, K. Kawamura and K. Ito, Chem. Commun., 2004, 662–663 RSC.
- M. Machida, T. Kawano, M. Eto, D. Zhang and K. Ikeue, Chem. Mater., 2007, 19, 954–960 CrossRef CAS.
- M. Machida, K. Kawamura, T. Kawano, D. Zhang and K. Ikeue, J. Mater. Chem., 2006, 16, 3084–3090 RSC.
- D. Zhang, F. Yoshioka, K. Ikeue and M. Machida, Chem. Mater., 2008, 20, 6697–6703 CrossRef CAS.
- K. Ikeue, T. Kawano, M. Eto, D. Zhang and M. Machida, J. Alloys Compd., 2008, 451, 338–340 CrossRef CAS.
- X. Ye, J. E. Collins, Y. Kang, J. Chen, D. T. N. Chen, A. G. Yodh and C. B. Murray, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 22430–22435 CrossRef CAS PubMed.
- D.-J. Zhang, M. Eto, K. Ikeue and M. Machida, J. Ceram. Soc. Jpn., 2007, 115, 597–601 CrossRef CAS.
- W. Shen and S. Naito, Adv. Mater. Res., 2014, 886, 196–199 CrossRef CAS.
- Y. Liu, D. Tu, H. Zhu and X. Chen, Chem. Soc. Rev., 2013, 42, 6924–6958 RSC.
- E. Aneggi, D. Wiater, C. de Leitenburg, J. Llorca and A. Trovarelli, ACS Catal., 2014, 4, 172–181 CrossRef CAS.
- C. Sun and D. Xue, Phys. Chem. Chem. Phys., 2013, 15, 14414–14419 RSC.
- H. Imagawa, A. Suda, K. Yamamura and S. Sun, J. Phys. Chem. C, 2011, 155, 1740–1745 Search PubMed.
- W. Zhang, J. Martinelli, F. Mayer, C. S. Bonnet, F. Szeremeta and K. Djanashvili, RSC Adv., 2015, 5, 69861–69869 RSC.
- R. Ran, X. Wu, D. Weng and J. Fan, J. Alloys Compd., 2013, 577, 288–294 CrossRef CAS.
- K. Ikeue, M. Eto, D.-J. Zhang, T. Kawano and M. Machida, J. Catal., 2007, 248, 46–52 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, TGA profiles, TEM images, and EDS spectra of Pr2O2SO4. See DOI: 10.1039/c6dt01667g |
| This journal is © The Royal Society of Chemistry 2016 |

