Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stabilization of molecular lanthanide polysulfides by bulky scorpionate ligands†‡
Marcel
Kühling
a,
Robert
McDonald
b,
Phil
Liebing
a,
Liane
Hilfert
a,
Michael J.
Ferguson
b,
Josef
Takats
*b and
Frank T.
Edelmann
*a
aChemisches Institut der Otto-von-Guericke-Universität, 39106 Magdeburg, Germany. E-mail: frank.edelmann@ovgu.de
bDepartment of Chemistry, University of Alberta, Edmonton, Alberta, AB, Canada T6G 2G2. E-mail: joe.takats@ualberta.ca
First published on 28th April 2016
Abstract
Well-defined lanthanide polysulfide complexes containing S42− and S52− ligands, the samarium(III) pentasulfide complex Sm(TpiPr2)(κ1-3,5-iPr2Hpz)(S5) and the tetrasulfide-bridged binuclear ytterbium(III) complex (μ-S4)[Yb(TpiPr2)(κ1-3,5-iPr2Hpz)(κ2-3,5-iPr2pz)]2 (TpiPr2 = hydro-tris(3,5-diisopropylpyrazolyl)borate), have been synthesized and structurally characterized by single-crystal X-ray diffraction.
Introduction
Polysulfides of the rare-earth metals (Ln) have been known for over 100 years.1,2 The most common types of these materials are LnS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3,4 and LnS2−x,5,6 but well-defined, more complicated species such as Ln8S14.9 (Ln = Dy, Ho) have also been described.7 The synthetic routes to such lanthanide polysulfides normally require high-temperature and/or high-pressure techniques.8,9 In sharp contrast, molecular lanthanide complexes containing polysulfide ligands remain little explored, although a number of interesting inorganic10–17 and organometallic18–20 sulfido clusters have been reported in recent years. Most of these contain the S22− ligand, while (Cp*2Sm)2(S3)(THF) (Cp* = pentamethylcyclo-pentadienyl) appears to be the only lanthanide complex with an S32− ligand.10 We describe here the formation and structural characterization of the first lanthanide polysulfide complexes containing the S42− and S52− ligands, namely Sm(TpiPr2)(κ1-3,5-iPr2Hpz)(S5) (3) and (μ-S4)[Yb(TpiPr2)(κ1-3,5-iPr2Hpz)(κ2-3,5-iPr2pz)]2 (5) (TpiPr2 = hydro-tris(3,5-diisopropylpyrazolyl)borate; 3,5-iPr2pz = 3,5-diiso-propylpyrazolate; 3,5-iPr2Hpz = 3,5-diisopropylpyrazole), which are stabilized by bulky scorpionate ligands.
3,4 and LnS2−x,5,6 but well-defined, more complicated species such as Ln8S14.9 (Ln = Dy, Ho) have also been described.7 The synthetic routes to such lanthanide polysulfides normally require high-temperature and/or high-pressure techniques.8,9 In sharp contrast, molecular lanthanide complexes containing polysulfide ligands remain little explored, although a number of interesting inorganic10–17 and organometallic18–20 sulfido clusters have been reported in recent years. Most of these contain the S22− ligand, while (Cp*2Sm)2(S3)(THF) (Cp* = pentamethylcyclo-pentadienyl) appears to be the only lanthanide complex with an S32− ligand.10 We describe here the formation and structural characterization of the first lanthanide polysulfide complexes containing the S42− and S52− ligands, namely Sm(TpiPr2)(κ1-3,5-iPr2Hpz)(S5) (3) and (μ-S4)[Yb(TpiPr2)(κ1-3,5-iPr2Hpz)(κ2-3,5-iPr2pz)]2 (5) (TpiPr2 = hydro-tris(3,5-diisopropylpyrazolyl)borate; 3,5-iPr2pz = 3,5-diiso-propylpyrazolate; 3,5-iPr2Hpz = 3,5-diisopropylpyrazole), which are stabilized by bulky scorpionate ligands.
It is well established from the chemistry of the bent metallocenes Cp*2Ln (Ln = Sm, Eu, Yb) that these strongly reducing lanthanide(II) precursors are easily oxidized by elemental sulfur or diorganodisulfides (RSSR), resulting in the formation of trivalent metallocenes containing S2− or RS− ligands.10,21,22 Thus, it was of interest to investigate the reactivity of the recently reported “bent sandwich-like” divalent lanthanide scorpionates, Ln(TpiPr2)2 (Ln = Sm, Eu, Tm, Yb)23,24 with S8 with a view to establish whether the presence of bulky scorpionate ligands might lead to the formation of polysulfide-ligated species. Here we report the results of the reactions of Ln(TpiPr2)2 (Ln = Sm (1), Yb (2)) with sulfur.
Synthesis and characterization
The reaction of Sm(TpiPr2)2 (1) with sulfur was first carried out in toluene. Addition of one equiv. of S8 to a toluene solution of 1 at r.t., resulted in a rapid color change from dark green to orange, with some unreacted sulfur remaining at the bottom of the flask. Recovery of the sulfur showed that only half an equivalent of sulfur (S8) reacted with 1. Solvent removal produced a somewhat sticky yellow solid, the usual form of crude products due to the greasy, lipophilic nature of the TpiPr2 ligand. Curiously, crystallization attempts from various solvent mixtures (Et2O/THF; Et2O/hexane) first produced small amounts of yellow crystals, which proved to be elemental sulfur, orthorhombic and monoclinic forms. From further crystallization attempts only a few suitable crystals for X-ray analysis could be isolated, which were shown to be Sm(TpiPr2)(κ1-3,5-iPr2Hpz)(S5) (3), Scheme 1. The observation that the first crystalline materials were elemental sulfur indicated either that the amount of sulfur in solution was more than needed for the reaction or that some intermediate Sm-polysulfide species underwent sulfur extrusion. To answer the question as to the stoichiometric amount of sulfur needed to oxidize the precursor Sm(TpiPr2)2 compound, a second reaction was carried out which showed that bleaching of the dark green THF solution of 1 at RT occurred after the addition of only 1 equiv. of sulfur (S) per Sm(II). Crystallization of the crude, yellow, sticky solid produced a small amount of white crystals which proved to be Sm(TpiPr2)2(κ2-3,5-iPr2pz) (4, Scheme 1). Unfortunately, numerous other crystallization attempts from various mixed solvent system did not yield crystals suitable for X-ray analysis, hence the nature of the sulfur containing species from this second reaction remain unknown.The analogous reaction of Yb(TpiPr2)2 (2) with sulfur was found to be more straightforward. Stirring of equimolar amounts (Yb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S) of 2 and sulfur in toluene for 24 h caused a color change from bright red to amber.
S) of 2 and sulfur in toluene for 24 h caused a color change from bright red to amber.
Recrystallization of the reaction product from n-pentane afforded orange, cube-like single crystals of 5·2C5H12 suitable for X-ray diffraction.
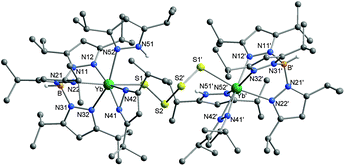
The constitution and molecular structure of compounds 3, 4 and 5 were revealed by single crystal X-ray diffraction studies. The structures of 3 and 5 are shown in Fig. 1 and 2, respectively. The structure of compound 4 can be found in the ESI.‡ The structure of 3 consists of a normal κ3-TpiPr2 ligand, a coordinated, neutral κ1-3,5-iPr2Hpz ligand, and the electronic demand of the Sm(III) ion is satisfied by the dianionic S52− polysulfide ligand. The most remarkable feature of the compound is the conformation of the SmS5 six-membered ring. As opposed to the classical chair conformation found in transition-metal complexes containing the MS5 moiety, such as Cp2MS5 (M = Ti, Zr, Hf), [FeS5(μ-S)]22− and [Pt(S5)3]2−,25–30 and that in cyclo-S6,31 the SmS5 six-membered ring adopts the twist-boat conformation. It is noteworthy that a similar ring conformation was also observed in the related actinide f-element compound, (C5Me5)2ThS5,32 and may signal that the twist-boat conformation is a common feature of An/LnS5 containing compounds. The similarities between the two structures extend to the M–S distances as well. Interestingly not only the terminal S(alpha) atoms are bonded to Sm (Sm–S1/S5 2.755(1)/2.792(1) Å) but also the two S(beta) atoms (Sm–S2/S4 2.952(1)/3.090(1) Å); the corresponding distances in the C2 symmetric (C5Me5)2ThS5 compound are Th–S1/S1′ and Th–S2/S2′, 2.768(4) and 3.036(4), respectively. In the transition-metal MS5 compounds with the chair conformation only the terminal sulfurs of the chelating S52− ligand are coordinated to the metal.
Compound 5 is a dinuclear ytterbium(III) polysulfide complex in which two [Yb(TpiPr2)(κ1-3,5-iPr2Hpz)(κ2-3,5-iPr2pz)] moieties are bridged by a μ-S42− ligand. Each formally 7-coordinated ytterbium ion is bonded to an original κ3-TpiPr2 ligand as well as a neutral κ1-3,5-iPr2Hpz ligand and an anionic κ2-3,5-iPr2pz ligand which were formed as a result of ligand fragmentation.33 Certainly this accounts for the fairly low isolated yield of compound 5 (32%). In accordance with the overall C2-symmetry of the molecule, the 11B NMR spectrum of 5 shows only a single resonance (δ = −4.4 ppm). The conformation of the bridging μ-S42− ligand closely resembles that of the tetrasulfide dianion in crystalline Na2S4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34,35 with a dihedral angle of 69.5(6)° (97.81° in Na2S4). The Yb–S bond length is 2.703(2) Å.
34,35 with a dihedral angle of 69.5(6)° (97.81° in Na2S4). The Yb–S bond length is 2.703(2) Å.
Conclusions
In summarizing the results reported here, although formation of compounds 3, 4 and 5 was accompanied by TpiPr2 ligand fragmentation, their isolation underscores the following points: 1. The fact that Sm(TpiPr2)2(κ2-3,5-iPr2pz) (4) contains a κ2-pyrazolide wedged between two (TpiPr2)2 ligand, holds out the hope that under suitable conditions, highly reducing Ln(TpiPr2)2 compounds might be able to bind and activate interesting small molecules like CO, CO2, etc. 2. The very rapid reaction of compound 1 with sulfur and the rather non-selective formation of compound 3 underlines the stronger reducing power of the Sm(II) precursor compared with Yb(II). Thus, the reactivity of Yb(TpiPr2)2 is significantly reduced and allows for the more straightforward preparation of the tetrasulfide derivative 5. 3. The successful isolation and structural characterization of the first lanthanide(III) polysulfide complexes, Sm(TpiPr2)(κ1-3,5-iPr2Hpz)(S5) (3) and (μ-S4)[Yb(TpiPr2)(κ1-3,5-iPr2Hpz)(κ2-3,5-iPr2pz)]2 (5) encourages the quest for more rational synthesis of similar lanthanide polysulfides and the study of their bonding characteristics. Such studies are underway in our laboratories.This work was financially supported by the Otto-von-Guericke-Universität Magdeburg and the University of Alberta. JT thanks Jackie Kiplinger (Los Alamos National Laboratory) for useful discussions and we thank the reviewers for helpful comments.
Experimental section
Synthesis of complex 3
(i) Sulfur flakes (56.7 mg, 0.22 mmol S8) were added to a stirred toluene solution of 1 (239 mg, 0.22 mmol) at r.t. After only about a minute the color changed from dark green to orange, with substantial amount of unreacted sulfur (ca. 28 mg) at the bottom of the round-bottom flask. Decantation from the unreacted sulfur, followed by solvent removal gave a slightly sticky, yellow solid. Extraction with n-pentane left behind a small amount of pale yellow solid, shown to be sulfur (orthorhombic) by X-ray diffraction on crystals obtained from Et2O/THF solvent mixture. Crystallization attempts from n-pentane only produced powdery solids. Crystallization from Et2O/n-hexane solvent mixtures, two attempts, produced small amounts of yellow crystals which were again shown to be sulfur (orthorhombic and monoclinic forms). Ultimately from THF/hexamethyldisiloxane (HMDSO), a small number of pale yellow crystals were harvested, which proved to be compound 3.(ii) Small amounts of sulfur flakes were added to a stirred THF solution of 1 (523 mg, 0.484 mmol) at r.t. After the addition of only 16 mg (0.5 mmol of S) the original dark green color changed to orange. Solvent removal gave a slightly sticky, yellow solid. Crystallizations from Et2O/n-hexane solvent mixtures, twice produced crystals of compound 3. Numerous other crystallization attempts failed to produce crystals suitable for X-ray analysis and failed to give sulfur-containing material.
Synthesis of complex 5
Powdered sulfur (14 mg, 0.44 mmol) was added to a solution of compound 2 (484 mg, 0.44 mmol) in toluene (20 ml). After 24 h stirring at r.t. the color had changed from bright red to amber. Removal of the solvent and recrystallization from n-pentane (15 ml, −5 °C) afforded 152 mg (32%) of 5 as orange, cube-like crystals. Mp. 130 °C. 1H NMR (toluene-d8, 400 MHz): δ 10.53 (s br, 6H, pz), 5.40 (s br, 2H, C–HiPr), 3.04 (s, 2H, pz), 2.0–2.2 (s br, 2H, B–H), 2.38 (s, 12H, CH3iPr), 1.23 (m, 12H, CH2 of n-pentane), 0.87 (t, 12H, CH3 of n-pentane), −0.20 (s br, 6H, C–H iPr), −0.65 (s, 2H, pz), −0.99 (s, 2H, C–H iPr), −2.33 (s br, 12H, CH3iPr), −6.22 (s br, 48H, CH3iPr), −12.00 (s br, 48H, CH3iPr).13C NMR (toluene-d8, 100 MHz): δ = 173.8 (q-C pz), 150.9 (q-C pz), 102.3 (CH pz) 87.3 (CH pz), 34.4 (CH2 of n-pentane), 27.3 (CH iPr), 23.5(CH3iPr), 22.7 (CH2 of n-pentane), 21.5 (CH3iPr), 19.5 (C–H iPr), 15.6 (CH3iPr), 14.1 (CH3 of n-pentane). 11B NMR (toluene-d8, 128 MHz): δ = −4.4 ppm. Elemental analysis calcd for C100H178B2N20S4Yb2 (5·2C5H12): C 55.69, H 8.32, N 12.99; found C 56.04, H 8.64, N 12.50.References
- W. Biltz, Z. Elektrochem., 1911, 17, 668 CAS.
- J. Flahaut, M. Guitard and M. Patrie, Bull. Soc. Chim. Fr., 1959, 1917 CAS.
- Y. Yanagisawa and S. Kume, Mater. Res. Bull., 1986, 21, 379 CrossRef CAS.
- L. C. Otero-Diaz, M. J. Torralvo and R. M. Rojas, Solid State Ionics, 1993, 63–65, 318 CrossRef CAS.
- R. Tamazyan, S. van Smaalen, I. G. Vasilyeva and H. Arnold, Acta Crystallogr., Sect. B: Struct. Sci., 2003, 59, 709 Search PubMed.
- T. Doert, C. Graf, P. Laux and T. Schleid, Z. Anorg. Allg. Chem., 2007, 633, 2719 CrossRef CAS.
- T. Doert, C. Graf, I. G. Vailyeva and W. Schnelle, Inorg. Chem., 2012, 51, 282 CrossRef CAS PubMed.
- A. W. Webb and H. T. Hall, Inorg. Chem., 1970, 9, 1084 CrossRef CAS.
- C. J. Müller, U. Schwarz and T. Doert, Z. Anorg. Allg. Chem., 2012, 638, 2477 CrossRef , and references cited therein.
- W. J. Evans, G. W. Rabe, J. W. Ziller and R. J. Doedens, Inorg. Chem., 1994, 33, 2719 CrossRef CAS.
- J. H. Melman, T. J. Emge and J. G. Brennan, Chem. Commun., 1997, 2269 RSC.
- D. Freedman, T. J. Emge and J. G. Brennan, Inorg. Chem., 1999, 38, 4400 CrossRef CAS PubMed.
- J. H. Melman, T. J. Emge and J. G. Brennan, Inorg. Chem., 1999, 38, 2117 CrossRef CAS PubMed.
- M. Fitzgerald, T. J. Emge and J. G. Brennan, Inorg. Chem., 2002, 41, 3528 CrossRef CAS PubMed.
- Z. Zhang, L. Zhang, Y. Li, L. Hong, Z. Chen and X. Zhou, Inorg. Chem., 2010, 49, 5715 CrossRef CAS PubMed.
- K. Norton, S. Banerjee, S. Das, L. Hübner, T. J. Emge and J. G. Brennan, Dalton Trans., 2010, 39, 6794 RSC.
- J. F. Corbey, M. Fang, J. W. Ziller and W. J. Evans, Inorg. Chem., 2015, 54, 801 CrossRef CAS PubMed.
- Y. Li, C. Pi, J. Zhang, X. Zhou, Z. Chen and L. Weng, Organometallics, 2005, 24, 1982 CrossRef CAS.
- N. Arleth, S. Bestgen, M. T. Gamer and P. W. Roesky, J. Am. Chem. Soc., 2014, 136, 14023 CrossRef CAS PubMed.
- J. Li, J. Hao and C. Cui, Dalton Trans., 2015, 44, 767 RSC.
- D. J. Berg, C. J. Burns, R. A. Andersen and A. Zalkin, Organometallics, 1989, 8, 1865 CrossRef CAS.
- A. Recknagel, M. Noltemeyer, D. Stalke, U. Pieper, H.-G. Schmidt and F. T. Edelmann, J. Organomet. Chem., 1991, 411, 347 CrossRef CAS.
- A. Momin, L. Carter, Y. Yang, R. McDonald, S. Essafi, F. Nief, I. Del Rosal, A. Sella, L. Maron and J. Takats, Inorg. Chem., 2014, 53, 12066 CrossRef CAS PubMed.
- M. Kühling, C. Wickleder, M. J. Ferguson, C. G. Hrib, R. McDonald, M. Suta, L. Hilfert, J. Takats and F. T. Edelmann, New J. Chem., 2015, 39, 7617 RSC.
- H. Köpf, B. Block and M. Schmidt, Chem. Ber., 1968, 101, 272 CrossRef.
- E. F. Epstein, I. Bernal and H. Köpf, J. Organomet. Chem., 1971, 26, 229 CrossRef CAS.
- E. G. Muller, J. L. Petersen and L. F. Dahl, J. Organomet. Chem., 1976, 111, 91 CrossRef CAS.
- M. Draganjac and T. B. Rauchfuss, Angew. Chem., Int. Ed. Engl., 1985, 24, 742 CrossRef , and references therein.
- A. Müller and E. Diemann, Adv. Inorg. Chem., 1987, 31, 89 CrossRef.
- N. Takeda, N. Tokito and R. Okazaki, Top. Curr. Chem., 2003, 231, 153 CrossRef CAS.
- M. Schmidt, B. Block, H. D. Block, H. Köpf and E. Wilhelm, Angew. Chem., Int. Ed. Engl., 1968, 7, 632 CrossRef CAS.
- D. A. Wrobleski, D. T. Cromer, J. V. Ortiz, T. B. Rauchfuss, R. R. Ryan and A. P. Sattelberger, J. Am. Chem. Soc., 1986, 108, 174 CrossRef CAS.
- Fragmentation of substituted Tp ligands is well documented ( A. Domingos, M. R. J. Elsegood, A. C. Hillier, G. Lin, S. Y. Liu, I. Lopes, N. Marques, G. H. Maunder, R. McDonald, A. Sella, J. W. Steed and J. Takats, Inorg. Chem., 2002, 41, 6761 CrossRef CAS PubMed , and references therein), but it seems to be even more prevalent with the TpiPr2 ligand as seen in reactivity studies of Ln(TpiPr2)2 (Ln = Sm, Tm) (J. Takats, unpublished work.).
- R. Tegman, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1973, 29, 1463 CrossRef CAS.
- S. J. Chadwell, D. Rickard and G. W. Luther III, Electroanalysis, 2001, 13, 21 CrossRef CAS.
Footnotes |
| † Dedicated to Professor R. Dieter Fischer on the occasion of his 80th birthday. |
| ‡ Electronic supplementary information (ESI) available: Full crystallographic data for 3, 4, and 5. CCDC 1472924, 1472923 and 1472811. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt01439a |
| This journal is © The Royal Society of Chemistry 2016 |