Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A pseudo-icosahedral cage {Gd12} based on aminomethylphosphonate†
Ze-Min
Zhang
ab,
Karzan H.
Zangana
bc,
Andreas K.
Kostopoulos
b,
Ming-Liang
Tong
*a and
Richard E. P.
Winpenny
*b
aKey Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry & Chemical Engineering, Sun Yat-Sen University, Guangzhou, 510275, P. R. China. E-mail: tongml@mail.sysu.edu.cn; Fax: +86 20 8411-2245
bSchool of Chemistry, The University of Manchester, Oxford Road, Manchester, M13 9PL, UK. E-mail: richard.winpenny@manchester.ac.uk
cDepartent of Chemistry, College of Education, Salahaddin University-Erbil, Kurdistan Region, Iraq
First published on 10th May 2016
Abstract
Reaction of (aminomethyl)phosphonic acid (ampH2) with a mixture of gadolinium and cobalt pivalates under solvothermal conditions, led to a pseudo-icosahedral cage {Gd12}, which shows a large magnetocaloric effect (MCE).
Metal phosphonate clusters have been extensively studied for their fascinating structures and interesting magnetic properties.1 3d–4f heterometallic cages2 and more recently lanthanide (4f) homometallic3 phosphonate clusters have been studied because of the range of magnetic anisotropies of the lanthanide ions and the weak coupling between the metal sites can produce unusual and potentially useful physics.4 Some cages such as {Dy2},3b {Dy4}3d and {Cu24Dy8},2a are single molecule magnets (SMM) because of the very anisotropic DyIII ions.5 When the isotropic GdIII ion is present, phosphonate clusters2c–h display impressive magnetocaloric effects (MCE), which have been proposed for application in magnetic refrigeration.6 For example, the observed magnetic entropy changes of a series of Co–Ln phosphonate grids and cages2d range from 20.0 to 28.6 J kg−1 K−1 for a magnetic field change ΔH = 70 kG and 3 K, while the highest magnetic entropy change is 33.7 J kg−1 K−1 found for {MnII4GdIII6}2e for ΔH = 70 kG and 3 K.
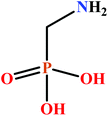
Previous studies have shown that the phosphonate moiety is an efficient functional group for the construction of molecular cages.7 Another frequently used ligand to prepare the polynuclear 3d–4f and 4f clusters is α-amino acids.8 Given this extensive body of previous work we thought that (aminomethyl)phosphonic acid (ampH2, Scheme 1), which is the smallest α-amino phosphonic acid, should be a useful ligand to synthesize 3d–4f and 4f cages.
Here we report our first explorations using ampH2 which have resulted in a dodecanuclear pseudo-icosahedral gadolinium cage. Firstly, ampH2 was dissolved in mixed solvent of MeOH/H2O and deprotonated by NEt3 to give a clear solution. Then [GdIII2(O2CtBu)6(HO2CtBu)6] and [CoII2(μ-OH2)(O2CtBu)4]·(HO2CtBu)4 were mixed and stirred in MeCN to generate a suspension. Reaction of the resulting solution and suspension under solvothermal conditions, followed by cooling to room temperature, gave light pink crystals of (HNEt3)4[CoII(H2O)6(H3NCH2PO3)12GdIII12(O2CtBu)24(OH)6]·8MeCN·18H2O (1·8MeCN·18H2O).
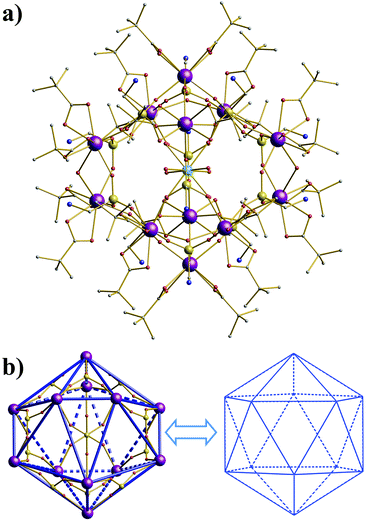
The X-ray crystal structure analysis shows that compound 1 crystallizes in the tetragonal space group P42/mmc and the molecular skeleton takes a shape of a pseudo-icosahedral cage, with a CoII hexahydrate cation trapped in the center of the molecule (Fig. 1). The molecule has 222 crystallographic symmetry with three twofold axes intersecting at the center of the molecule at the CoII ion site. As a result, only three of the GdIII ions and three of the ampH− ligands are crystallographically independent. The molecule also exhibits non-crystallographic triangular and pentagonal symmetry (Fig. S1†). Each of ten threefold pseudo-axes traverses the center of two {Gd3} triangles while the six fivefold pseudo-axes pass through Gd, Co and Gd′ sites. A previously reported cluster [Cp12Sm12(μ3-Cl)24] (Cp = η5-C5H5) possesses similar symmetrical features.9 Due to the regularity of the structure the phosphorus atoms are also arranged in an icosahedron (Fig. S2†).
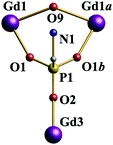
The ampH− ligand links three GdIII ions using the 3.111 binding mode (Fig. 2), with the nitrogen atom uncoordinated. Six μ-OH groups bridge six pairs of GdIII ions, respectively. Two tBuCOO− ligands chelate to each GdIII ion. Therefore, all of the GdIII ions are eight-coordinate with distorted triangular dodecahedral geometries, formed by four oxygens of two tBuCOO− ligands, three oxygens of three ampH− ligands, and one oxygen of an OH group. The Gd–O bond lengths are in the range of 2.258–2.565 Å, comparable to the values found for previous gadolinium-containing phosphonate clusters.2 The shortest Gd⋯Gd contacts are 4.553–4.625 Å and are those bridged by μ-OH groups with Gd–O–Gd′ angle of 128.75–132.61° (Fig. 2). These separations are longer than those found for similar μ-OH-bridged GdIII clusters ca. 3.8 Å,10 which may result in weaker magnetic interaction between GdIII ions. The longer Gd⋯Gd separations are bridged by ampH− ligands (e.g. Gd1⋯Gd3 in Fig. 2) and are in the range of 6.874–6.898 Å. The CoII ion is coordinated by six water molecules which are disordered over eight positions, with Co–O bond lengths of 2.083 Å. The Co⋯Gd distances are ca. 6.0 Å. The isolated CoII hexahydrate cation filling the cavity in the cage is crucial to stabilize the whole molecule, as water molecules are interacting with the cage by hydrogen bonding. No identifiable products or crystals are obtained if the reaction is carried out in the absence of the cobalt pivalate complex.
The closest Gd⋯Gd separation between neighbouring molecules is ca. 10.6 Å, which preclude any significant intermolecular interactions. Space-filling representations demonstrate that the overall molecule of compound 1 is in a spherical shape with a approximate diameter of 2.0 nm (Fig. S3†). The packing of molecules generates two types of one-dimensional square channels in the c axis direction with diagonal separations of 1.3 and 1.1 nm (Fig. S4†), respectively.
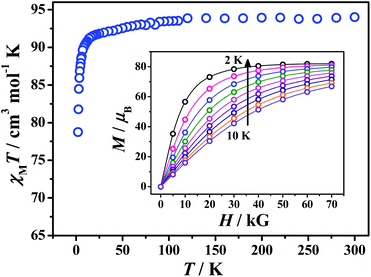
Variable-temperature (2–300 K) magnetic susceptibility data were collected for polycrystalline samples of compound 1 in an applied direct-current (dc) magnetic field of 5 kG (Fig. 3). The room-temperature χMT value for 1 is 94.0 cm3 K mol−1, which is in agreement with the theoretically expected value of 96.4 cm3 K mol−1 for spin-only twelve GdIII (S = 7/2, g = 2) and one CoII (S = 3/2, g = 2).4a Upon lowering the temperature, χMT values decrease gradually to 90.4 cm3 K mol−1 at 10 K and then fall abruptly to a minimum of 78.7 cm3 K mol−1 at 2 K. The overall magnetic behaviour is due in the main to the cobalt(II) ion, where the orbitally degenerate ground state will show significant temperature dependent behaviour. There may be antiferromagnetic interactions between the Gd⋯Gd ions bridged by hydroxides, but this cannot be modelled sensibly due to the presence of the six-coordinate CoII ion.
Magnetization measurements on compound 1 were performed at the 2–10 K temperature range under 0–70 kG field (Fig. 3 inset). The M vs. H data display a steady increase in magnetization to reach 81.9μB at 70 kG at 2 K without achieving saturation. This value is a little lower than the value that would be expected for twelve isolated GdIII ions (ca. 83.6μB), which suggests some weak anti-ferromagnetic exchange are present. Given the large magnetization values, we investigated the magnetocaloric properties for compound 1. The magnetic entropy change can be estimated from the magnetization change as a function of applied field and temperature (Fig. 4) by using the Maxwell equation ΔSm(T) = ∫[∂M(T,H)/∂T]dH. The resulting maximum magnetic entropy change is 26.7 J kg−1 K−1 for ΔH = 70 kG at 2 K. This value is comparable to reported 3d–4f phosphonate clusters.6
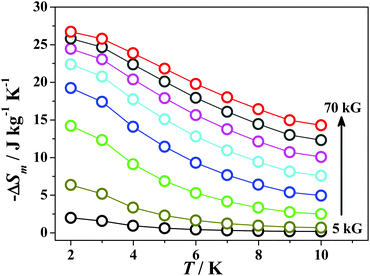 | ||
| Fig. 4 −ΔSm(T) calculated from magnetization data of 1 at various fields (5–70 kG) and temperature (2–10 K). | ||
In summary, we have shown that (aminomethyl)phosphonic acid reacts with gadolinium and cobalt pivalates to give a pseudo-icosahedral cage {Gd12}. The molecular skeleton, including twelve Gd ions and twelve P atoms, possesses a fascinating icosahedron-in-icosahedron topology. Magnetic studies revealed that the compound exhibits a large MCE at the ultralow temperature. The present work suggests that ampH2 is an efficient ligand to construct 4f cages. Further studies on 4f and 3d–4f phosphonate cages using ampH2 are in progress.
The icosahedron is Platonic solid, and the presence of five-membered metal rings should lead to significant frustration effects.11 Unfortunately, the presence of the divalent Co site prevents informative magnetic studies of this polyhedron in this case. This is particularly unfortunate as there have been very interesting recent reports of spin frustration effects in gadolinium cage complexes.12 We are attempting to synthesise the analogue of compound 1 with a diamagnetic divalent ion at the centre.
This work was supported by the NSFC (grant no. 21371183 and 91422302), the NSF of Guangdong (S2013020013002) and Chinese Government Scholarship. We also thank EPSRC (UK) for funding an X ray diffractometer (grant number EP/K039547/1).
Notes and references
- Metal Phosphonate Chemistry: From Synthesis to Applications, ed. A. Clearfield and K. Demadis, Royal Society of Chemistry, Cambridge, 2011, and references therein Search PubMed.
- Representative examples: (a) V. Baskar, K. Gopal, M. Helliwell, F. Tuna, W. Wernsdorfer and R. E. P. Winpenny, Dalton Trans., 2010, 39, 4747 RSC; (b) M. Wang, D.-Q. Yuan, C.-B. Ma, M.-J. Yuan, M.-Q. Hu, N. Li, H. Chen, C.-N. Chen and Q.-T. Liu, Dalton Trans., 2010, 39, 7276 RSC; (c) Y.-Z. Zheng, M. Evangelisti and R. E. P. Winpenny, Angew. Chem., Int. Ed., 2011, 50, 3692 CrossRef CAS PubMed; (d) Y.-Z. Zheng, M. Evangelisti, F. Tuna and R. E. P. Winpenny, J. Am. Chem. Soc., 2012, 134, 1057 CrossRef CAS PubMed; (e) Y.-Z. Zheng, E. M. Pineda, M. Helliwell, M. Evangelisti and R. E. P. Winpenny, Chem. – Eur. J., 2012, 18, 4161 CrossRef CAS PubMed; (f) E. Moreno-Pineda, F. Tuna, R. G. Pritchard, A. C. Regan, R. E. P. Winpenny and E. J. L. McInnes, Chem. Commun., 2013, 49, 3522 RSC; (g) K. H. Zangana, E. Moreno-Pineda, I. J. Vitorica-Yrezabal, E. J. L. McInnes and R. E. P. Winpenny, Dalton Trans., 2014, 43, 13242 RSC; (h) E. Moreno-Pineda, F. Tuna, Y.-Z. Zheng, S. J. Teat, R. E. P. Winpenny, J. Schnack and E. J. L. McInnes, Inorg. Chem., 2014, 53, 3032 CrossRef PubMed.
- (a) S. Comby, R. Scopelliti, D. Imbert, L. Charbonnière, R. Ziessel and J.-C. G. Bünzli, Inorg. Chem., 2006, 45, 3158 CrossRef CAS PubMed; (b) M. Ren, S.-S. Bao, N. Hoshino, T. Akutagawa, B. Wang, Y.-C. Ding, S. Wei and L.-M. Zheng, Chem. – Eur. J., 2013, 19, 9619 CrossRef CAS PubMed; (c) K. H. Zangana, E. Moreno-Pineda, J. Schnack and R. E. P. Winpenny, Dalton Trans., 2013, 42, 14045 RSC; (d) K. H. Zangana, E. Moreno-Pineda and R. E. P. Winpenny, Dalton Trans., 2014, 43, 17101 RSC.
- (a) O. Kahn, Molecular Magnetism, VCH, New York, 1993 Search PubMed; (b) C. Benelli and D. Gatteschi, Chem. Rev., 2002, 102, 2369 CrossRef CAS PubMed; (c) Y.-G. Huang, F.-L. Jiang and M.-C. Hong, Coord. Chem. Rev., 2009, 253, 2814 CrossRef CAS.
- (a) R. Sessoli and A. K. Powell, Coord. Chem. Rev., 2009, 253, 2328 CrossRef CAS; (b) P. Zhang, Y.-N. Guo and J. Tang, Coord. Chem. Rev., 2013, 257, 1728 CrossRef CAS.
- (a) V. Corradini, A. Ghirri, A. Candini, R. Biagi, U. del Pennino, G. Dotti, E. Otero, F. Choueikani, R. J. Blagg, E. J. L. McInnes and M. Affronte, Adv. Mater., 2013, 25, 2816 CrossRef CAS PubMed; (b) Y.-Z. Zheng, G.-J. Zhou, Z. Zheng and R. E. P. Winpenny, Chem. Soc. Rev., 2014, 43, 1462 RSC; (c) J.-L. Liu, Y.-C. Chen, F.-S. Guo and M.-L. Tong, Coord. Chem. Rev., 2014, 281, 26 CrossRef CAS.
- D. J. Tranchemontagne, Z. Ni, M. O. Keeffe and O. M. Yaghi, Angew. Chem., Int. Ed., 2008, 47, 5136 CrossRef CAS PubMed.
- (a) R. Wang, Z. Zheng, T. Jin and R. J. Staples, Angew. Chem., Int. Ed., 1999, 38, 1813 CrossRef CAS; (b) J.-J. Zhang, T.-L. Sheng, S.-Q. Xia, G. Leibeling, F. Meyer, S.-M. Hu, R.-B. Fu, S.-C. Xiang and X.-T. Wu, Inorg. Chem., 2004, 43, 5472 CrossRef CAS PubMed; (c) X.-J. Kong, Y. Wu, L.-S. Long, L.-S. Zheng and Z. Zheng, J. Am. Chem. Soc., 2009, 131, 6918 CrossRef CAS PubMed.
- W. P. Kretschmer, J. H. Teuben and S. I. Troyanov, Angew. Chem., Int. Ed., 1998, 37, 88 CrossRef CAS.
- (a) J.-P. Zhao, B.-W. Hu, F.-C. Liu, X. Hu, Y.-F. Zeng and X.-H. Bu, CrystEngComm, 2007, 9, 902 RSC; (b) C. Walbaum, I. Pantenburg and G. Meyer, Z. Anorg. Allg. Chem., 2009, 635, 1083 CrossRef CAS; (c) Z.-M. Zhang, F.-S. Guo, P.-H. Guo, J.-L. Liu, Z.-P. Ni and M.-L. Tong, Sci. China: Chem., 2012, 55, 934 CrossRef CAS; (d) S. Biswas, S. Das, J. van Leusen, P. Kögerler and V. Chandrasekhar, Eur. J. Inorg. Chem., 2014, 25, 4159 CrossRef.
- E. I. Tolis, L. P. Engelhardt, P. V. Mason, G. Rajaraman, K. Kindo, M. Luban, A. Matsuo, H. Nojiri, J. Raftery, C. Schröder, G. A. Timco, F. Tuna, W. Wernsdorfer and R. E. P. Winpenny, Chem. – Eur. J., 2006, 12, 8961–8968 CrossRef CAS PubMed.
- J. W. Sharples, D. Collison, E. J. L. McInnes, J. Schnack, E. Palacios and M. Evangelisti, Nat. Commun., 2014, 5, 5321 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, X-ray crystallography and structural figures. CCDC 1450005. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt00876c |
| This journal is © The Royal Society of Chemistry 2016 |