Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Complexes of Group 2 dications with soft thioether- and selenoether-containing macrocycles†
William
Levason
,
David
Pugh
,
Jamie M.
Purkis
and
Gillian
Reid
*
School of Chemistry, University of Southampton, Highfield, Southampton SO17 1BJ, UK. E-mail: g.reid@soton.ac.uk
First published on 1st April 2016
Abstract
A new route to cationic complexes of Mg, Ca, Sr and Ba with 18-membered ring O4S2, O4Se2 and O2S4 donor macrocycles from metal acetonitrile complexes with weakly coordinating [BArF]− anions is described. The precursors used were [M(MeCN)x][BArF]2 (M = Mg, x = 6; M = Ca, x = 8) and [M′(acacH)(MeCN)5][BArF]2 (M′ = Sr or Ba). Reaction of these with the heterocrowns, [18]aneO4S2 (1,4,10,13-tetraoxa-7,16-dithiacyclooctadecane), [18]aneO4Se2 (1,4,10,13-tetraoxa-7,16-diselenacyclooctadecane) or [18]aneO2S4 (1,10-dioxa-4,7,13,16-tetrathiacyclooctadecane) in anhydrous CH2Cl2 solution gave [M(heterocrown)(MeCN)2][BArF]2 for M = Mg, Ca or Sr, whilst the larger Ba forms [Ba(heterocrown)(acacH)(MeCN)][BArF]2. The complexes have been characterised by microanalysis, IR, 1H and 13C{1H} NMR spectroscopy. X-ray crystal structures are reported for [Ca([18]aneO2S4)(MeCN)2][BArF]2, [Ca([18]aneO4Se2)(MeCN)2][BArF]2, [Sr([18]aneO4S2)(MeCN)2][BArF]2, and [Sr([18]aneO4Se2)(MeCN)2][BArF]2 which contain 8-coordinate metal centres with trans-nitrile ligands and κ6-heterocrowns, and for the 9-coordinate [Ba([18]aneO4Se2)(acacH)(MeCN)][BArF]2. Adventitious hydrolysis of the magnesium complexes in solution results in six-coordinate complexes, [Mg(κ3-[18]aneO4Se2)(OH2)2(MeCN)][BArF]2 and [Mg(κ3-[18]aneO4S2)(OH2)2(MeCN)][BArF]2, whose structures were determined. X-ray crystal structures are also reported for [Mg(MeCN)6][BArF]2, [M(MeCN)8][BArF]2 (M = Ca, Sr) and [Ca(18-crown-6)(MeCN)2][BArF]2.
Introduction
The chemistries of the heavier alkaline earth metals (Ca, Sr and Ba) are dominated by the hard M2+ cations and their salts with oxo-anions or halides.1 Barium and strontium salts have niche applications, for example BaSO4 in the oil industry and medicine, but the key importance is of calcium salts, which range from engineering and construction (CaCO3, CaSO4, Ca(OH)2) to apatite in mammalian bones and CaCO3 in corals and shells. For many years their coordination chemistry with neutral ligands was very limited and the organometallic chemistry among the most restricted in the main group.1–3 However, the last 20 years have seen the development of the coordination chemistry with both charged and neutral oxygen and nitrogen ligands,4 and despite the synthetic challenges and high reactivities, of a significant organometallic chemistry.5 The coordination chemistry of magnesium is more familiar, not least because of the importance of chlorophyll and chlorophyll model compounds,4 while Grignard reagents have been known for over 100 years.6 Despite these recent developments, the synthesis of complexes of these elements retains significant challenges, not least the lability of the metal centres, which often results in unpredicted products or mixtures, even in apparently simple systems.7 We have recently been exploring the use of weakly coordinating fluorinated tetraarylborate anions, such as [B{3,5-(CF3)2C6H3}4]− (hereafter BArF) to obtain unusual complexes of the Group 1 metal ions, including the synthesis of homoleptic neutral diphosphine complexes of lithium and sodium, [M(L–L)3][BArF] (M = Li or Na; L–L = Me2PCH2CH2PMe2 or o-C6H4(PMe2)2),8 the homoleptic octathioether complex of sodium, [Na([24]aneS8)][BArF] ([24]aneS8 = 1,4,7,10,13,16,19,22-octathiacyclotetracosane),9 complexes of Li-Cs of N3-, N4- and N6-donor azamacrocycles,10 and with the heterocrowns, [18]aneO2S4 (1,10-dioxa-4,7,13,16-tetrathiacyclooctadecane), [18]aneO4S2 (1,4,10,13-tetraoxa-7,16-dithiacyclooctadecane) and [18]aneO4Se2 (1,4,10,13-tetraoxa-7,16-diselenacyclooctadecane).11 These contain rare coordination of the neutral sulfur or selenium donors to the alkali metal centre. Heterocrowns containing S- or Se-donor atoms have an extensive chemistry with d- and p-block metals,12 but examples with the s-block elements are few. In addition to the Group 1 examples described above, we reported examples of neutral eight-coordinate complexes of CaI2 and SrI2 with [18]aneO4S2, [18]aneO2S4 and [18]aneO4Se2.13 The complex [Ca(ClO4)2([18]aneO4S2)],14 the lamellar polymer [Ba{Cu(SCN)3([18]aneO4S2)}],15 and [Mg(R-benzo[18]aneO4S2)]16 (R-benzo[18]aneO4S2 = R-benzo-substituted [18]aneO4S2) are also known. The present paper describes the synthesis, structures and spectroscopic properties of complexes of [18]aneO4S2, [18]aneO2S4 and [18]aneO4Se2 with Mg, Ca, Sr and Ba dications, utilising the [BArF]− anions to afford soluble cationic precursor species.Experimental
All preparations were carried out under a dry dinitrogen atmosphere using standard Schlenk and glove box techniques. Anhydrous MgI2, CaI2, SrI2 and BaI2 were purchased from Sigma and used as received. Anhydrous [Sr(acac)2] was prepared by dehydrating commercial [Sr(acac)2(H2O)2] (Sigma): the hydrate was suspended in CH2Cl2 over 4 Å molecular sieves and stirred gently for 2 weeks, then the suspension was decanted away and concentrated to dryness. [Ba(acac)2] was synthesized by reacting BaI2 and K(acac) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio in MeCN followed by extraction of KI with anhydrous acetone. Na[BArF] and [H(OEt2)2][BArF] were synthesized using Brookhart's procedure.17 18-Crown-6 was purchased from Sigma and dried using SOCl2 and the macrocycles [18]aneO4S2, [18]aneO2S4 and [18]aneO4Se2 were synthesized using literature procedures.18 CH2Cl2 and MeCN were dried by distillation from CaH2 and n-hexane distilled from Na/K alloy. 1H and 13C{1H} NMR spectra were recorded in CD2Cl2 solution at 298 K using a Bruker AV II-400 spectrometer and are referenced to the residual CH2Cl2 resonance. IR spectra were recorded as mulls between CsI plates using a Perkin-Elmer Spectrum 100 spectrometer over the range 4000–200 cm−1. Microanalyses were undertaken at London Metropolitan University.
2 molar ratio in MeCN followed by extraction of KI with anhydrous acetone. Na[BArF] and [H(OEt2)2][BArF] were synthesized using Brookhart's procedure.17 18-Crown-6 was purchased from Sigma and dried using SOCl2 and the macrocycles [18]aneO4S2, [18]aneO2S4 and [18]aneO4Se2 were synthesized using literature procedures.18 CH2Cl2 and MeCN were dried by distillation from CaH2 and n-hexane distilled from Na/K alloy. 1H and 13C{1H} NMR spectra were recorded in CD2Cl2 solution at 298 K using a Bruker AV II-400 spectrometer and are referenced to the residual CH2Cl2 resonance. IR spectra were recorded as mulls between CsI plates using a Perkin-Elmer Spectrum 100 spectrometer over the range 4000–200 cm−1. Microanalyses were undertaken at London Metropolitan University.
X-ray crystallography
Crystals were obtained as described below. Details of the crystallographic data collection and refinement are in Table 1. Diffractometer: Rigaku AFC12 goniometer equipped with an enhanced sensitivity (HG) Saturn724+ detector mounted at the window of an FR-E+ SuperBright molybdenum rotating anode generator (λ1 = 0.71073 Å) with VHF Varimax optics (70 or 110 μm focus). Cell determination and data collection: CrystalClear-SM Expert 3.1 b27, data reduction, cell refinement, and absorption correction: CrystalClear-SM Expert 2.1.19 Structure solution and refinement were carried out using WinGX and software packages within.20 Disorder in the CF3 groups of the [BArF]− anions was present in all of the structures, which is often observed in compounds containing [BArF]−,10,11 and this was satisfactorily modelled using DFIX, DANG, ISOR, DELU, and SIMU restraints. Positional disorder was also present in the macrocycle ligands and was modelled similarly. The dataset for [Ca([18]aneO4S2)(bipy)][BArF]2 was collected from a poor-quality crystal which was very weakly diffracting at high angles, thus leading to very high Rint values. Repeated attempts at recrystallization of the sample failed to afford better quality crystals. A large amount of residual electron density was located ∼1.05 Å from an Se atom in [Ba([18]aneO4Se2)(acacH)(MeCN)][BArF]2 which could not be satisfactorily modelled as either disordered Se or disordered carbon. H-atoms were placed in geometrically-assigned positions with C–H distances of 0.95 Å (CH), 0.98 Å (CH3) or 0.99 Å (CH2) and refined using a riding model with Uiso(H) = 1.2Ueq(C) (CH, CH2) or 1.5Ueq(C) (CH3). enCIFer was used to prepare material for publication.21 CCDC reference numbers 1455287 [Mg(MeCN)6][BArF]2, 1455288 [Mg(κ3-[18]aneO4S2)(OH2)2(MeCN)][BArF]2, 1455289 [Mg(κ3-[18]aneO4Se2)(OH2)2(MeCN)][BArF]2, 1455290 [Ca(MeCN)8][BArF]2, 1455291 [Ca(18-crown-6)(MeCN)2][BArF]2, 1455292 [Ca([18]aneO2S4)(MeCN)2][BArF]2, 1455293 [Ca([18]aneO4Se2)(MeCN)2][BArF]2, 1455294 [Ca([18]aneO4S2)(bipy)][BArF]2, 1455295 [Sr(MeCN)8][BArF]2, 1455296 [Sr([18]aneO4S2)(MeCN)2][BArF]2, 1455297 [Sr([18]aneO4Se2)(MeCN)2][BArF]2 and 1455298 [Ba([18]aneO4Se2)(acacH)(MeCN)][BArF]2 contain crystallographic data in CIF format.| Compound | [Ca(MeCN)8][BArF]2 | [Ca([18]aneO2S4)(MeCN)2][BArF]2 | [Ca([18]aneO4Se2)(MeCN)2][BArF]2 | [Ca([18]aneO4S2)(bipy)][BArF]2 |
|---|---|---|---|---|
| Formula | C80H48B2CaF48N8 | C80H54B2CaF48N2O2S4 | C80H54B2CaF48N2O4Se2 | C86H56B2CaF48N2O4S2·CH2Cl2 |
| M/g mol−1 | 2094.96 | 2177.19 | 2238.87 | 2304.07 |
| Crystal system | Tetragonal | Monoclinic | Triclinic | Triclinic |
| Space group (no.) | P4/nnc (126) | C2/c (15) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (2) (2) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (2) (2) |
| a/Å | 15.691(3) | 18.674(3) | 13.201(2) | 17.10(1) |
| b/Å | 15.691(3) | 18.249(2) | 13.554(3) | 17.14(1) |
| c/Å | 18.386(5) | 26.280(4) | 14.019(2) | 20.12(2) |
| α/° | 90 | 90 | 77.162(9) | 111.727(9) |
| β/° | 90 | 97.626(2) | 63.267(7) | 94.775(9) |
| γ/° | 90 | 90 | 87.06(1) | 116.229(6) |
| U/Å3 | 4527(2) | 8876(2) | 2180.7(6) | 4692(6) |
| Z | 2 | 4 | 1 | 2 |
| μ (Mo-Kα)/mm−1 | 0.216 | 0.314 | 1.060 | 1.631 |
| F(000) | 2092 | 4360 | 1110 | 2308 |
| Total reflections | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 064 064 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 599 599 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 936 936 |
58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 691 691 |
| Unique reflections | 2009 | 7839 | 7679 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 180 |
| R int | 0.071 | 0.044 | 0.046 | 0.297 |
| Goodness-of-fit on F2 | 1.065 | 1.043 | 1.032 | 0.917 |
| R 1 [Io > 2σ(Io)] | 0.082 | 0.089 | 0.069 | 0.091 |
| R 1 (all data) | 0.118 | 0.120 | 0.101 | 0.334 |
| wR2 [Io > 2σ(Io)] | 0.263 | 0.240 | 0.168 | 0.146 |
| wR2 (all data) | 0.289 | 0.272 | 0.188 | 0.237 |
| Compound | [Mg(MeCN)6][BArF]2 | [Mg(κ3-[18]aneO4S2)(H2O)2(MeCN)][BArF]2 C6H14 | [Mg(κ3-[18]aneO4Se2)(H2O)2(MeCN)][BArF]2 C6H14 |
|---|---|---|---|
| Formula | C76H42B2F48MgN6 | C78H55B2F48MgNO6S2·C6H14 | C78H55B2F48MgNO6Se2·C6H14 |
| M/g mol−1 | 1997.08 | 2210.45 | 2304.25 |
| Crystal system | Orthorhombic | Triclinic | Triclinic |
| Space group (no.) | Pnn2 (34) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (2) (2) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (2) (2) |
| a/Å | 17.574(4) | 12.778(3) | 12.792(2) |
| b/Å | 14.570(3) | 13.713(4) | 13.802(2) |
| c/Å | 16.836(4) | 14.080(4) | 13.953(2) |
| α/° | 90 | 84.20(1) | 83.273(7) |
| β/° | 90 | 71.851(9) | 73.413(6) |
| γ/° | 90 | 85.42(1) | 84.567(7) |
| U/Å3 | 4311(2) | 2329(1) | 2339.8(6) |
| Z | 2 | 1 | 1 |
| μ (Mo-Kα)/mm−1 | 0.170 | 0.212 | 0.944 |
| F(000) | 1988 | 1114 | 1150 |
| Total reflections | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 977 977 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740 740 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 341 341 |
| Unique reflections | 8528 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 822 822 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 067 067 |
| R int | 0.084 | 0.051 | 0.042 |
| Goodness-of-fit on F2 | 1.014 | 1.017 | 0.940 |
| R 1 [Io > 2σ(Io)] | 0.053 | 0.081 | 0.078 |
| R 1 (all data) | 0.081 | 0.137 | 0.110 |
| wR2 [Io > 2σ(Io)] | 0.131 | 0.191 | 0.196 |
| wR2 (all data) | 0.145 | 0.226 | 0.218 |
| Compound | [Sr([18]aneO4S2)(MeCN)2][BArF]2 | [Sr([18]aneO4Se2)(MeCN)2][BArF]2 | [Ba([18]aneO4Se2)(acacH)(MeCN)][BArF]2 |
|---|---|---|---|
| Common items: T = 100 K, λ1 = 0.71073 Å (Mo Kα), θmax = 27.5°, R1 = ∑||Fo| − |Fc||/∑|Fo|, wR2 = [∑w(Fo2 − Fc2)2/∑wFo2]1/2. | |||
| Formula | C80H54B2F48N2O4S2Sr | C80H54B2F48N2O4Se2Sr | C83H59B2BaF48NO6Se2·0.75CH2Cl2 |
| M/g mol−1 | 2192.61 | 2286.41 | 2455.89 |
| Crystal system | Triclinic | Triclinic | Triclinic |
| Space group (no.) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (2) (2) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (2) (2) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (2) (2) |
| a/Å | 13.1237(7) | 13.181(2) | 12.2148(3) |
| b/Å | 13.7167(6) | 13.588(2) | 18.3147(4) |
| c/Å | 13.8415(8) | 13.965(2) | 21.778(2) |
| α/° | 76.537(4) | 77.134(6) | 85.024(6) |
| β/° | 63.147(5) | 63.202(5) | 82.079(6) |
| γ/° | 87.410(4) | 87.516(7) | 88.732(6) |
| U/Å3 | 2157.0(2) | 2171.8(5) | 4814.2(4) |
| Z | 1 | 1 | 2 |
| μ (Mo-Kα)/mm−1 | 0.829 | 1.613 | 1.354 |
| F(000) | 1092 | 1128 | 2422 |
| Total reflections | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 737 737 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 961 961 |
55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 310 310 |
| Unique reflections | 8828 | 8858 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 971 971 |
| R int | 0.060 | 0.059 | 0.055 |
| Goodness-of-fit on F2 | 1.043 | 1.034 | 1.036 |
| R 1 [Io > 2σ(Io)] | 0.074 | 0.057 | 0.098 |
| R 1 (all data) | 0.102 | 0.081 | 0.122 |
| wR2 [Io > 2σ(Io)] | 0.185 | 0.150 | 0.267 |
| wR2 (all data) | 0.203 | 0.169 | 0.289 |
General method for complex synthesis
The Group 2 [BArF] salt was suspended in CH2Cl2 (5 mL) and a solution of the macrocycle in CH2Cl2 (5 mL) was added. Complete dissolution occurred and the reaction was stirred for 16 h. After this time the solution was filtered, concentrated to ∼3 mL and layered with n-hexane (20 mL) to form crystals. These were isolated by decanting away the supernatant and drying the solid in vacuo.Results and discussion
Metal synthons
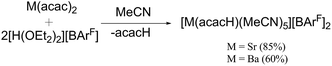
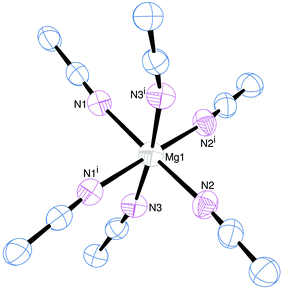
We initially attempted to prepare the MeCN complexes [M(MeCN)x][BArF]2 (M = Mg, x = 6; M = Ca, Sr or Ba, x = 8) by salt metathesis between the appropriate MI2 and Na[BArF] in anhydrous MeCN solution (Scheme 1), since it was anticipated that these compounds would serve as more readily soluble synthons for accessing soft donor macrocyclic complexes for the Group 2 cations, while incorporating weakly coordinating [BArF]− anions. This was successful for M = Mg and Ca, yielding [Mg(MeCN)6][BArF]2 and [Ca(MeCN)8][BArF]2 after purification as described in the Experimental section. However, for M = Sr, salt metathesis resulted in products which retained a large amount of Na[BArF], and although a small number of crystals formed in one reaction which were identified as [Sr(MeCN)8][BArF]2 (see below), this complex could not be obtained in sufficient yield or purity to be a useful synthon. The salt metathesis also failed in the barium case. As an alternative route, the reaction of [M(acac)2] (M = Sr or Ba, acacH = acetylacetone) with [H(OEt2)2][BArF]17 in anhydrous MeCN was used to prepare [M(MeCN)5(acacH)][BArF]2, (M = Sr or Ba) which proved to be readily isolated (Scheme 2). These complexes were characterised by microanalysis, 1H and 13C{1H} NMR spectroscopy. Their IR spectra are consistent with coordination of neutral acacH.22 Colourless, moisture sensitive crystals of [Mg(MeCN)6][BArF]2 were grown from CH2Cl2/n-hexane, and the X-ray crystal structure showed the expected regular octahedral cation (Fig. 1). The cation has been structurally characterised in several salts including [Mg(MeCN)6][MgBr4]7 and [Mg(MeCN)6][AlCl4]2,23 and the dimensions are comparable: the present structure serves to confirm the identity of the reagent.The 1H and 13C{1H} NMR spectra show the [BArF]− anions, with resonances due to the CH3 group of MeCN at δ = 2.16 and δ = 2.18 respectively, shifted from the liquid resonances at δ = 1.96 and δ = 1.79. The IR spectrum (liquid film) shows two resonances at 2252 and 2293 cm−1 assigned as ν(CN) and ν(C–C) + δ(CH3) of similar intensity due to Fermi resonance.24 In [Mg(MeCN)6][BArF]2 both bands are found at higher frequency (2295, 2321 cm−1), consistent with coordination to the metal. Similar patterns were observed for all the complexes in this work, confirming the MeCN is coordinated in all cases.
Extremely moisture sensitive crystals of [Ca(MeCN)8][BArF]2 were grown from CH2Cl2/n-hexane, but these partially lose MeCN on drying with both microanalysis and integration of the 1H NMR spectrum showing ∼6 MeCN/Ca in the vacuum-dried product.25 The structure (Fig. 2) shows a square antiprismatic cation with no interaction with the [BArF]− anions. This cation has not been reported previously, although the Ca–NCMe bond lengths are similar to those in other calcium complexes including [Ca(18-crown-6)(MeCN)2][SbCl6]2,13 [Ca(18-crown-6)(MeCN)2][BPh4]2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 and [Ca(MeCN)5I2].7
26 and [Ca(MeCN)5I2].7
The spectroscopic properties of [Sr(MeCN)8][BArF]2 are similar to those of the calcium analogue. A few modest quality crystals of this complex were formed as a by-product from the salt metathesis reaction and also deposited from an MeCN solution of [Sr(MeCN)5(acacH)][BArF]2. The X-ray structure showed them to contain a square antiprismatic cation (see Fig. S1, ESI†).
The [M(acacH)(MeCN)5][BArF]2 (M = Sr, Ba) proved to be useful synthons for the heterocrown complexes. The IR spectra show coordinated MeCN and acacH, and although X-ray quality crystals were not obtained, they probably contain seven-coordinate metal centres.
Heterocrown complexes
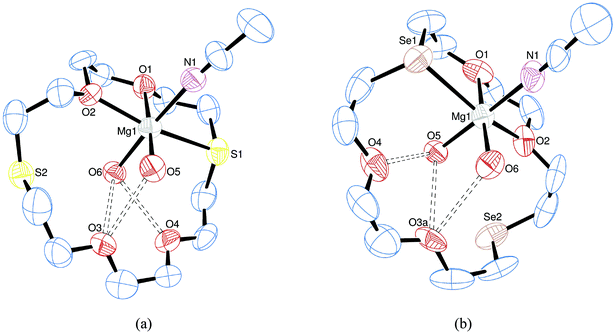
The reaction of [Ca(MeCN)8][BArF]2 with [18]aneO2S4, [18]aneO4S2, [18]aneO4Se2 and 18-crown-6 afforded complexes [Ca(crown)(MeCN)2][BArF]2. In all cases the 1H and 13C{1H} NMR spectra show small high frequency shifts for the CH2O, CH2S/Se and CH3CN groups consistent with coordination. The IR spectra are dominated by the [BArF]− anion vibrations, but show small high frequency shifts in the vibrations due to MeCN. X-ray quality crystals were grown for the adducts with [18]aneO2S4, [18]aneO4Se2 (Fig. 3) and 18-crown-6 (Fig. S2, ESI†).For each complex an eight-coordinate Ca2+ centre sitting in the middle of a hexadentate macrocycle with trans MeCN ligands was observed. Similar structures have been reported for [Ca(18-crown-6)I2],27 [Ca(18-crown-6)(hmpa)2]2+ (hmpa = hexamethylphosphoramide)26 and [Ca(18-crown-6)(MeCN)2][SbCl6]2,13 although in contrast [Ca(heterocrown)I2] (heterocrown = [18]aneO4Se2 or [18]aneO2S4),13 [Ca(18-crown-6)(O3SCF3)2]13 and [Ca([18]aneO4S2)(OH2)2]I2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 contain puckered macrocycles with cis coordinated monodentate ligands. It is probable that the energy difference between the different arrangements is small and the cation geometry may be influenced by intermolecular interactions and crystal packing. Eight coordination, either dodecahedral or square antiprismatic, is relatively common for the heavier alkaline earth metals,2,4 and whilst the geometry is readily established for those containing eight monodentate ligands,28 the distortions resulting with polydentates can make identifying the coordination sphere difficult. Lippard and Russ29 detailed a method for mathematically distinguishing between the two polyhedra, which required the identification of the plane of best fit which encompassed the metal centre and four of the eight coordinating atoms, then calculating the dihedral angle between this plane and the plane defined by the metal centre and the remaining four coordinating atoms. For an ideal dodecahedron this angle will be 90° whereas for an ideal square antiprism this angle will be 77.4°. A “τ8” parameter can be defined as (θ − 77.4)/12.6 where θ is the dihedral angle between the two planes of best fit. A τ8 value of 0 would correspond to an ideal square antiprismatic geometry and a τ8 value of 1 would correspond to an ideal dodecahedral geometry, with intermediate values indicating the degree of distortion away from the two ideals.
13 contain puckered macrocycles with cis coordinated monodentate ligands. It is probable that the energy difference between the different arrangements is small and the cation geometry may be influenced by intermolecular interactions and crystal packing. Eight coordination, either dodecahedral or square antiprismatic, is relatively common for the heavier alkaline earth metals,2,4 and whilst the geometry is readily established for those containing eight monodentate ligands,28 the distortions resulting with polydentates can make identifying the coordination sphere difficult. Lippard and Russ29 detailed a method for mathematically distinguishing between the two polyhedra, which required the identification of the plane of best fit which encompassed the metal centre and four of the eight coordinating atoms, then calculating the dihedral angle between this plane and the plane defined by the metal centre and the remaining four coordinating atoms. For an ideal dodecahedron this angle will be 90° whereas for an ideal square antiprism this angle will be 77.4°. A “τ8” parameter can be defined as (θ − 77.4)/12.6 where θ is the dihedral angle between the two planes of best fit. A τ8 value of 0 would correspond to an ideal square antiprismatic geometry and a τ8 value of 1 would correspond to an ideal dodecahedral geometry, with intermediate values indicating the degree of distortion away from the two ideals.
For the [18]aneO2S4 complex the τ8 parameter is calculated to be 0.29 and for the [18]aneO4Se2 complex the τ8 parameter is 0.93. This indicates that the latter complex is very close to an ideal dodecahedral geometry, whereas the former is better described as square antiprismatic, but with a high degree of distortion (Fig. 3(c) and (d)). This contrasts markedly with the [Ca(heterocrown)I2] complexes where the macrocycle conformation was significantly folded because the iodide ligands bound in a cis manner and the geometry of the complexes was close to square antiprismatic. The [Ca(18-crown-6)(MeCN)2]2+ dication (Fig. S2, ESI†) has a τ8 value of 0.90 indicating that it is close to a dodecahedral geometry.
Changes in the macrocyclic coordination geometry do not significantly affect the Ca–S and Ca–Se bond lengths which are very similar between the iodide13 and MeCN complexes, although there are some slight differences in the Ca–O bond lengths. Additionally, a slight shortening of the Ca–NCCH3 bond lengths is observed for the macrocyclic complexes compared to [Ca(MeCN)8][BArF]2, although it should be noted that there was a significant amount of positional disorder present in the [18]aneO2S4 complex (including the MeCN ligands) and care is needed in making the comparisons.
The MeCN ligands can be displaced by neutral donors, for example 2,2′-bipyridyl, which formed [Ca([18]aneO4S2)(bipy)][BArF]2. The 1H and 13C{1H} NMR spectra contained broad resonances indicative of a dissociating system in CD2Cl2 solution at ambient temperatures, but the identity was confirmed by the X-ray crystal structure (Fig. 4).
In order to accommodate the bidentate bipy ligand the [18]aneO4S2 coordination becomes puckered, closer to that previously observed for the [Ca(18-aneO4S2)I2] where the iodide ligands were cis and the geometry is close to dodecahedral, τ8 = 0.82.
Heterocrown complexes of strontium were obtained through reaction of [Sr(acacH)(MeCN)5][BArF]2 with [18]aneO4S2, [18]aneO2S4 and [18]aneO4Se2, forming [Sr(heterocrown)(MeCN)2][BArF]2. Notably the acacH ligand is lost but two MeCN ligands were retained, as shown by the 1H and 13C{1H} NMR data. This was confirmed by the X-ray structures of [Sr([18]aneO4S2)(MeCN)2][BArF]2 and [Sr([18]aneO4Se2)(MeCN)2][BArF]2 (Fig. 5).
The structures revealed that both complexes adopted 8-coordinate geometries with a hexadentate macrocycle and two trans MeCN ligands. The τ8 parameter for the [18]aneO4S2 complex was 0.73 and for the [18]aneO4Se2 complex it was 0.89 indicating that distorted dodecahedral is the best description, albeit severely distorted for the former. The Sr–S and Sr–Se bond lengths are very similar to those reported for the 9-coordinate [Sr(macrocycle)(OH2)3]I2 complexes,13 although the Sr–O bond lengths are slightly shorter than those previously reported which may be a result of lower coordination number (8 vs. 9). No evidence for any Sr⋯[BArF] interactions was observed, although such interactions were found with the larger alkali metals.11
The only example of an oxathia-crown complex of barium is in the polymer [Ba{Cu(SCN)3([18]aneO4S2)}],15 and previous attempts to use BaI2 as a metal source failed to result in any complexes.13 The reaction of [Ba(acacH)(MeCN)5][BArF]2 with [18]aneO2S4 and [18]aneO4Se2 in MeCN produced the complexes [Ba(heterocrown)(acacH)(MeCN)][BArF]2, in which the acacH ligand was retained, contrasting with the strontium complexes. This was confirmed by the crystal structure of [Ba([18]aneO4Se2)(acacH)(MeCN)][BArF]2 (Fig. 6) the first barium complex containing neutral selenoether coordination. The larger Ba2+ (1.43 Å) compared to 1.27 Å for Sr2+ (ref. 30) results in an increase in coordination number to nine, composed of the six donor atoms from the heterocrown, two oxygens from the neutral acacH and one MeCN, in a geometry that can be described as distorted tricapped trigonal prismatic. Coordination numbers of 8–10 have been found in complexes of 18-crown-6 with Ba2+ with similar bond lengths and irregular geometries.15,26,27,31–33
The complexes of these heterocrowns with the much smaller magnesium ion (r2+ = 0.78 Å) were expected to differ from those described above. The reaction of [Mg(MeCN)6][BArF]2 with 18-crown-6, [18]aneO2S4, [18]aneO4S2 and [18]aneO4Se2 in anhydrous CH2Cl2 gave yellow or off-white solids of composition [Mg(crown)(MeCN)2][BArF]2. Repeated attempts to obtain X-ray quality crystals were unsuccessful, although structures of two hydrolysis products were obtained (Fig. 6). The spectroscopic data on [Mg(crown)(MeCN)2][BArF]2 (crown = 18-crown-6, [18]aneO4S2 and [18]aneO4Se2) show the presence of coordinated MeCN and the absence of water. The 1H NMR resonances of the crown ligands show small high frequency shifts relative to the unbound ligands,18 but are rather broad. The CH2O and CH2S resonances of [Mg([18]aneO2S4)(MeCN)2][BArF]2 are very broad both in the 1H NMR and 13C{1H} spectra which probably indicates substantial solution lability of the heterocrown, which contains four soft thioether donors on the hard magnesium centre. Since the steric demands of the linear MeCN groups are small, it is possible that the [Mg(crown)(MeCN)2]2+ cations contain eight-coordinate magnesium in the solid state, although the coordination number could be lower, whilst the ring undergoes a fast dynamic process in solution. Seven-coordination is known with κ5-18-crown-6 in [Mg(18-crown-6)(HCl2)2]34 and [Mg(κ5-18-crown-6)Cl2],35 which contain near planar MgO5 units with trans coordinated anions above and below the plane.
During attempts to grow crystals of [Mg(crown)(MeCN)2][BArF]2 from CH2Cl2/n-hexane in a freezer, crystals of [Mg(crown)(MeCN)(OH2)2][BArF]2 (crown = [18]aneO4S2 or [18]aneO4Se2) were obtained, no doubt due to ingress of adventitious water. The structures are isomorphous (Fig. 7) and contain κ3-crown, one MeCN and two water ligands. The crown is coordinated O2S/Se in a mer geometry – providing extremely rare examples of Mg2+ coordinated to neutral thio- and seleno-ether functions, with cis-diaquo ligands. Hydrogen-bonding is present between the coordinated H2O ligands and the remaining O-atoms of the crown. κ3-Coordination of 18-crown-6 is present in the five-coordinate [Mg(18-crown-6)(iBu)(2,6-tBu2C6H4O)].36
Adventitious hydrolysis of some [M(heterocrown)I2] (M = Ca or Sr) complexes displaced the iodide ligands to give [M(heterocrown)(OH2)2]I2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 but κ6-coordination of the crown remained. The different behaviour of Mg versus Ca and Sr in these systems may be largely attributable to the smaller size of the magnesium. We note that mixed aqua-crown complexes are also well established for some 3d metals, e.g. [Cr(κ2-18-crown-6)(OH2)Cl3] or [V(κ2-18-crown-6)(OH2)Cl3].37,38
13 but κ6-coordination of the crown remained. The different behaviour of Mg versus Ca and Sr in these systems may be largely attributable to the smaller size of the magnesium. We note that mixed aqua-crown complexes are also well established for some 3d metals, e.g. [Cr(κ2-18-crown-6)(OH2)Cl3] or [V(κ2-18-crown-6)(OH2)Cl3].37,38
Conclusions
A range of cationic complexes of the Group 2 metals Mg–Ba with heterocrown ethers have been prepared from acetonitrile synthons taking advantage of the solubility in weak donor solvents conferred by the [BArF]− anions. The new complexes have been fully characterised spectroscopically and in many cases by X-ray crystallography. They include the first example of coordination of a neutral selenoether function to Ba2+, and rare examples with seleno- and thio-ether coordination to the other hard metal centres. The heterocrown-nitrile complexes should prove to be useful synthons for incorporation of other donor types to the metal centre, as demonstrated by the characterisation of [Ca([18]aneO4S2)(bipy)][BArF]2, whilst more generally, the [M(MeCN)x][BArF]2 (M = Mg, x = 6; M = Ca, x = 8) or [M′(acacH)(MeCN)5][BArF]2 (M′ = Sr, Ba) may offer a route into other Group 2 complexes with soft donor atoms. Further work to explore these possibilities is currently underway.Acknowledgements
We thank the EPSRC for support via the SCFED project through a Programme Grant (EP/1033394/1), and also through EP/K039466/1. The SCFED Project (http://www.scfed.net) is a multidisciplinary collaboration of British universities investigating the fundamental and applied aspects of supercritical fluids. Additional Data Available: Original IR and NMR spectra for all complexes are available to download viahttp://dx.doi.org/10.5258/SOTON/391234.References
- N. N. Greenwood and A. Earnshaw, Chemistry of the Elements, Butterworth, Oxford, 2nd edn, 1997 Search PubMed; F. A. Cotton, G. Wilkinson, C. A. Murillo and M. Bochmann, Advanced Inorganic Chemistry, Wiley, NY, 1999 Search PubMed.
- D. E. Fenton, in Comprehensive Coordination Chemistry, ed. G. Wilkinson, R. D. Gillard and J. A. McCleverty, Pergamon Press, Oxford, 1987, vol. 3, p. 25 Search PubMed.
- W. E. Lindsell, in Comprehensive Organometallic Chemistry II, ed. G. Wilkinson, F. G. A. Stone and E. W. Abel, Elsevier, Oxford, 1995, vol. 1, p. 57 Search PubMed.
- T. P. Hanusa, Coord. Chem. Rev., 2000, 31, 143 Search PubMed; T. P. Hanusa, in Comprehensive Coordination Chemistry II, ed. J. A. McCleverty and T. J. Meyer, Elsevier, Oxford, 2004, vol. 3, p. 1 CrossRef CAS; A. Torvisco, A. Y. O'Brien and K. Ruhlandt-Senge, Coord. Chem. Rev., 2011, 255, 1268 CrossRef CAS; M. Westerhausen, S. Krieck, J. Langer, T. M. A. Al-Shboul and H. Gorls, Coord. Chem. Rev., 2013, 257, 1049 CrossRef; S. Harder, Chem. Rev., 2010, 110, 3852 CrossRef PubMed.
- T. P. Hanusa, in Comprehensive Organometallic Chemistry III, ed. R. H. Crabtree and D. M. P. Mingos, Elsevier, Oxford, 2007, vol. 2, p. 68 CrossRef CAS; A. Torvisco and K. Ruhlandt-Senge, Top. Organomet. Chem., 2013, 45, 1 CrossRef CAS.
- Handbook of Grignard Reagents, ed. G. S. Silverman and P. E. Rakita, Marcel Dekker, New York, 1996 Search PubMed; H. G. Richey, Grignard Reagents: New Developments, Wiley, New York, 2000 Search PubMed.
- A. F. Waters and A. H. White, Aust. J. Chem., 1996, 49, 27 CAS.
- M. Carravetta, M. Concistre, W. Levason, G. Reid and W. Zhang, Chem. Commun., 2015, 51, 9555 RSC.
- M. J. D. Champion, J. M. Dyke, W. Levason, M. E. Light, D. Pugh, H. Bhakhoa, L. Rhyman, P. Ramasami and G. Reid, Inorg. Chem., 2015, 54, 2497 CrossRef CAS PubMed.
- M. Everett, A. Jolleys, W. Levason, D. Pugh and G. Reid, Chem. Commun., 2014, 50, 5843 RSC; J. M. Dyke, W. Levason, M. E. Light, D. Pugh, G. Reid, H. Bhakhoa, P. Ramasami and L. Rhyman, Dalton Trans., 2015, 44, 13853 RSC.
- M. J. D. Champion, W. Levason, D. Pugh and G. Reid, Dalton Trans., 2015, 43, 18748 RSC.
- W. Levason and G. Reid, in Supramolecular Chemistry: From molecules to nanomaterials, ed. P. A. Gale and J. W. Steed, Wiley, New York, 2012, vol. 3, p. 785 Search PubMed.
- P. Farina, W. Levason and G. Reid, Dalton Trans., 2013, 42, 89 RSC.
- I.-H. Park, K.-M. Park and S. S. Lee, Dalton Trans., 2010, 39, 9696 RSC.
- T. Roettgers and W. S. Sheldrick, Z. Anorg. Allg. Chem., 2001, 627, 1976 CrossRef.
- M. V. Alfimov, A. V. Churakov, Y. V. Fedorov, O. A. Fedorova, S. P. Gromov, J. A. K. Howard, L. G. Kuz'mina, D. Mobius, T. I. Sergeevad, A. I. Vedernikov, O. V. Yescheulova and S. Y. Zaitsev, New J. Chem., 2002, 26, 543 RSC.
- M. Brookhart, B. Grant and A. F. Volpe Jr., Organometallics, 1992, 11, 30866 Search PubMed.
- P. Farina, T. Latter, W. Levason and G. Reid, Dalton Trans., 2013, 42, 4714 RSC.
- CrystalClear-SM Expert 3.1 b27, Rigaku Corporation, Tokyo, Japan, 2012 Search PubMed; CrystalClear-SM Expert 2.1 b29, Rigaku Corporation, Tokyo, Japan, 2013 Search PubMed.
- L. J. Farrugia, J. Appl. Crystallogr., 2012, 45, 849 CrossRef CAS.
- F. H. Allen, O. Johnson, G. P. Shields, B. R. Smith and M. Towler, J. Appl. Crystallogr., 2004, 37, 335 CrossRef CAS.
- Y. Nakamura, K. Isobe, H. Morita, S. Yamazaki and S. Kawaguchi, Inorg. Chem., 1972, 7, 1573 CrossRef.
- B. A. Stork-Blaisse, G. C. Verschoor and C. Romers, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1972, 8, 2445 CrossRef.
- B. Swanson and D. F. Shriver, Inorg. Chem., 1970, 9, 1406 CrossRef CAS.
- Elemental analyses show that [M(MeCN)5I2] (M = Ca, Sr) also lose some nitrile ligands on drying – see ref. 7.
- A. Verma, M. Guino-o, M. Gillett-Kunnath, W. Teng and K. Ruhlandt-Senge, Z. Anorg. Allg. Chem., 2009, 635, 903 CrossRef CAS.
- J. Langer, S. Krieck, R. Fischer, H. Goerls and M. Westerhausen, Z. Anorg. Allg. Chem., 2010, 636, 1190 CrossRef CAS.
- D. L. Kepert, Prog. Inorg. Chem., 1978, 24, 179 CrossRef CAS.
- S. J. Lippard and B. J. Russ, Inorg. Chem., 1968, 7, 1686 CrossRef CAS.
- J. Emsley, The Elements, OUP, Oxford, 1989 Search PubMed.
- P. C. Junk and J. W. Steed, J. Coord. Chem., 2007, 60, 1017 CrossRef.
- M.-M. Zhao, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2012, 68, m286 CAS.
- M. D. Brown, M. F. Davis, J. M. Dyke, F. Ferranti, W. Levason, J. S. Ogden and M. Webster, Chem. – Eur. J., 2008, 14, 2615 CrossRef CAS PubMed.
- J. L. Atwood, S. G. Bott, C. M. Means, A. W. Coleman, H. Zhang and J. T. May, Inorg. Chem., 1999, 29, 467 CrossRef.
- N. R. Streltsova, L. V. Ivakina, P. A. Storozhenko, B. M. Bulychev and V. K. Bel'ski, Dokl. Akad. Nauk SSSR, 1986, 291, 1373 CAS.
- A. D. Parjerski, E. P. Squiller, M. Parvez, R. D. Whittle and H. G. Richey Jr., Organometallics, 2005, 24, 809 CrossRef.
- U. Kynast, S. G. Bott and J. L. Atwood, J. Coord. Chem., 1988, 17, 53 CrossRef CAS.
- C. D. Beard, L. Carr, M. F. Davis, J. Evans, W. Levason, L. D. Norman, G. Reid and M. Webster, Eur. J. Inorg. Chem., 2006, 4399 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray data on [Sr(MeCN)8][BArF]2 and [Ca(18-crown-6)(MeCN)2][BArF]2. Spectroscopic data on the complexes. CCDC 1455287–1455298. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt00808a |
| This journal is © The Royal Society of Chemistry 2016 |