The mechanism of enantioselective ketone reduction with Noyori and Noyori–Ikariya bifunctional catalysts
Abstract
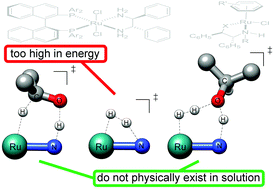
The catalytic hydrogenation of prochiral ketones with second and third-row transition metal complexes bearing chelating chiral ligands containing at least one N–H functionality has achieved unparalleled performance, delivering, in the best cases, chiral alcohols with up to 99.9% ee using extremely small catalyst loadings (∼10−5 mol%). Hence the efficacy of this reaction has closely approached that of natural enzymatic systems and the reaction itself has become one of the most efficient artificial catalytic reactions developed to date. This article describes the current level of understanding of the mechanism of enantioselective hydrogenation and transfer hydrogenation of aromatic ketones with pioneering prototypes of bifunctional catalysts, the Noyori and Noyori–Ikariya complexes. Analysis presented herein expands the concept of “metal–ligand cooperation”, redefines the term “cooperative ligand” and introduces “H–/H+ outer-sphere hydrogenation” as a novel paradigm in outer-sphere hydrogenation.

 Please wait while we load your content...
Please wait while we load your content...