Open Access Article
Open Access ArticleCyclopentadienyl nickel(II) N,C-chelating benzothiazolyl NHC complexes: synthesis, characterization and application in catalytic C–C bond formation reactions†
Wei Jie
Teo
a,
Zhe
Wang
b,
Fei
Xue
a,
T. S.
Andy Hor
*abc and
Jin
Zhao
*ab
aDepartment of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543, Singapore. E-mail: zhaoj@imre.a-star.edu.sg; andyhor@hku.hk
bInstitute of Materials Research and Engineering, Agency for Science, Technology and Research, 2 Fusionopolis Way, Innovis, Singapore 138634, Singapore
cDepartment of Chemistry, The University of Hong Kong, Pokfulam, Hong Kong SAR, China
First published on 11th March 2016
Abstract
Cyclopentadienyl (Cp) Ni(II) complexes [CpNiL][PF6] containing hybrid N,C chelating benzothiazolyl NHC ligands (L1 = 1-(2-benzothiazolyl)-3-methylimidazol-2-ylidene, 3a; L2 = 1-(2-benzothiazolyl)-3-allylimidazol-2-ylidene, 3b; L3 = 1-(2-benzothiazolyl)-3-benzylimidazol-2-ylidene, 3c) have been synthesized and fully characterized. The catalytic activity of 3a–3c in some C–C bond formation reactions has been examined. They are efficient catalysts for the homo-coupling of benzyl bromide in the presence of MeMgCl at r.t. with good functional group tolerance. Complex 3a is active in the catalytic oxidative homo-coupling of Grignard reagents with 1,2-dichloroethane as an oxidant at r.t.
Introduction
Strong σ-electron donating properties, high modularity and ease of handling render N-heterocyclic carbene (NHC) a type of widely used ligand to support transition metal based catalyst systems.1 Nickel complexes containing NHC ligands have been proved to be catalytically active for many organic transformations.2 Due to their ease in synthesis and high thermal stability, a variety of CpNi(II)–NHC complexes have been synthesized3–5 and recently significant interest has been drawn on their catalytic application in different organic reactions.2a–c,4,5 Among them, the use of CpNi(II) complexes containing mono-dentate NHC ligands as pre-catalysts in C–C formation reactions,4i,5 especially Suzuki–Miyaura coupling5b–g has been studied intensively. NHC ligands containing other donor functions can offer metal complexes with enhanced stability and catalytic activity.1g,h Ni complexes with hybrid NHC ligands (with C,N,C, N,C, or P,C coordination mode) are known to be generally good catalysts for C–C bond formation reactions.2a However, CpNi complexes with hybrid NHC ligands are rare in the literature3c,l and their catalytic efficiency is still unknown. Inspired by these recent advances in CpNi–NHC chemistry,2–5 and as part of our continuous interest in hybrid NHC ligand supported catalysis6 and the use of Ni in Grignard reagents involved C–C bond formation reactions,7 we herein report the synthesis and catalytic potential of a series of Ni(II) complexes, [CpNiL][PF6] containing hybrid N,C chelating benzothiazolyl NHC ligands L1–L3 (1-(2-benzothiazolyl)-3-methylimidazol-2-ylidene, L1; 1-(2-benzothiazolyl)-3-allylimidazol-2-ylidene, L2; 1-(2-benzothiazolyl)-3-benzylimidazol-2-ylidene, L3).Results and discussion
Synthesis and characterization of benzothiazolyl imidazolium salts and Ni complexes 3a–3c
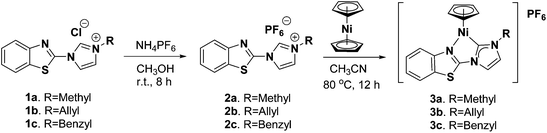
The synthesis of the benzothiazolyl-imidazolium chloride salts 1a–1c was accomplished by a previously described procedure.6c Through an anionic exchange reaction with NH4PF6, 1a–1c were converted to the corresponding hexafluorophosphates 2a–2c (Scheme 1) in 70–84% yield. The 1H- and 13C-NMR spectra of 2a–2c in CD3CN show the resonances of the NCHN protons at δ 9.32, 9.35, and 9.43 ppm and of the NCHN carbon at ca. δ 150.60 ppm, respectively. Their 31P-NMR spectra give a septet signal centered at −143.8 ppm. Complexes 3a–3c with the general formula [CpNiL][PF6] (L = L1, 3a; L = L2, 3b; L = L3, 3c) were prepared through the direct reaction between nickelocene and the corresponding imidazolium salts 2a–2c (Scheme 1).Reactions of nickelocene with 1a–1c resulted in unstable green solid products which turned yellow upon exposure to air and were not characterized in this work. In contrast, use of the PF6− salts resulted in air-stable green solids of complexes 3a–3c in good yield. Their structures were characterized by 1H-, 13C- and 31P-NMR, ESI-MS and single-crystal X-ray diffraction. Their purity was confirmed by elemental analysis. The disappearance of the 1H-NMR signals of NCHC, and the downfield 13C-NMR signals of the NCN carbene carbon at ca. 159 ppm in the 1H- and 13C-NMR spectra of 3a–3c are indicative of Ni–Ccarbene bond formation. The 31P-NMR spectra of 3a–3c give a septet peak with a very similar chemical shift as for 2a–2c, indicating the presence of a non-coordinating [PF6]− ion in 3a–3c. The ESI-MS spectra show the characteristic peaks of the [CpNiL]+ cation at m/z = 338.1 (for 3a), 364.1 (for 3b) and 414.1 (for 3c) (+ve mode) and the [PF6]− anion at m/z 145 (−ve mode), supporting the formation of the ionic Ni(II)–NHC complexes. The carbene carbon signals of 3a–3c are located in the range of those observed in the closely related ionic CpNi(II)–NHC complexes where the Ni center is bound to one mono-dentate NHC and one solvate,3g–i such as [CpNi(IMes)(NCCH3)][PF6] (IMes = 1,3-bis-(2,4,6-trimethylphenyl)imidazol-2-ylidene)3g or two mono-dentate NHC ligands, [CpNi(IMes)(IMe)][PF6] (IMe = 1,3-dimethylimidazol-2-ylidene),5c and the CpNi complexes containing the NHC ligand with allylic N-substitution, [CpNi(η2-IAll)][BF4]3c (IAll = 1-methyl-3-allylimidazolin-2-ylidene or 1,3-diallylimidazolin-2-ylidene) in which one allylic double bond is nickel-coordinated. The singlet 1H-NMR signals of the Cp ligand in 3a–3c at δ 5.97, 5.89 and 5.78 ppm, respectively, locate at the lower field in comparison with those observed in other CpNi(II)–NHC complexes.3–5 Only [CpNi(η2-IAll)][BF4] showed the comparable downfield signals of the Cp ligand (δ 5.8–5.9 ppm in CD2Cl2).3c The 13C-NMR signal of the Cp ligand of 3a–3c is in the range of those observed in other CpNi(II)–NHC complexes (δ 91–96 ppm).3–5 Similar to those in the CpNi(II) complexes with NHC bearing allylic N-substitution,3c,l,4i the protons of the terminal CH2 groups of the allyl group in 3b give rise to two signals at δ 5.38 ppm (cis) and δ 5.15 ppm (trans).
Molecular structures of 3a–3c determined by single-crystal X-ray diffraction
The green single crystals of 3a–3c, suitable for X-ray diffraction, were obtained by cooling their saturated CH3CN solution at −19 °C. The X-ray diffraction structural analysis confirms 3a–3c are ionic complexes. In the [CpNiL]+ cation, the benzothiazolyl imidazol-2-ylidene ligand coordinates to the Ni(II) center in a bidentate N,C chelating mode, forming a five-membered ring as shown in Fig. 1–3. The allyl donor in 3b is dangling. The key bond lengths and angles of 3a–3c are given in Table 1. | ||
| Fig. 1 ORTEP diagram of the [CpNiL1]+ cation of 3a (50% probability ellipsoids). Hydrogen atoms are omitted. | ||
 | ||
| Fig. 2 ORTEP diagram of the [CpNiL2]+ cation of 3b (50% probability ellipsoids). Hydrogen atoms are omitted. | ||
 | ||
| Fig. 3 ORTEP diagram of the [CpNiL3]+ cation of 3c (50% probability ellipsoids). Hydrogen atoms are omitted. | ||
| Bond length (Å) or angle (deg) | 3a | 3b | 3c |
|---|---|---|---|
| Ni–Cpcent | 1.740 | 1.738 | 1.730 |
| Ni–Ccarbene | 1.868(2) | 1.874(2) | 1.866(3) |
| Ni–Nbenzothiazole | 1.929(18) | 1.9274(19) | 1.925(2) |
| Ccarbene–Ni–Cpcent | 138.04 | 139.89 | 138.87 |
| Ccarbene–Ni–Nbenzothiazole | 83.83(9) | 83.82(9) | 83.92(10) |
| Cpcent–Ni–Nbenzothiazole | 138.12 | 136.23 | 137.16 |
The Ni center adopts a distorted pseudo-trigonal geometry. The sum of the angles with Ni as the vertex is 360° in all complexes with the CcarbeneNi–Nbenzothiazole angle of ca. 84° being significantly smaller than the idealized angle. In the other neutral and ionic CpNi(II)–NHC complexes, the Ccarbene–Ni–X or Ccarbene–Ni–L angles (X = halides or SR−; L = a neutral donor) are in the range of 92°–98°.3–5 The smaller bite angle observed in 3a–3c can be attributed to the chelating effect. The Ni–Ccarbene bond lengths of 3a–3c (1.866–1.874 Å) are at the short end of the range (1.85–1.92 Å) of the Ni–Ccarbene distance found in CpNi(II)–NHC complexes3–5 due to the chelating effect. Similar shortening of the M–Ccarbene bond has also been found in their CpMo analogues.6c
Catalytic application of 3a–3c in C–C bond formation reactions
In transition metal catalysed cross-coupling reactions, the homo-coupling products of the organometallic reagents are unwanted by-products. However, the selective oxidative homo-coupling of aryl metal reagents has been receiving significant attention because this type of reaction provides a straightforward protocol to symmetrical biaryls and polyaromatic conjugated compounds.9 The oxidative coupling reactions of aryl Grignard reagents have been actively investigated using different transition metal based catalyst systems,10 such as Fe,10a–f Cu,10g,h Mn,10d,i Co10f,j,k and Ru.10l The use of Ni catalysts is less common in comparison with their use in other types of C–C bond formation reactions.10m The results shown in Scheme 2 prompted us to explore the activity of 3a in the oxidative homo-coupling product of the aryl Grignard reagent. A preliminary condition screening (Table S2 in ESI†) showed 3a (3 mol%) gave 81% yield of the homo-coupling product of p-tolylmagnesium bromide at r.t. after 1 h in the presence of 1,2-dichloroethane as an oxidant (Scheme 3), suggesting that CpNi(II)–NHC complexes can be used as catalysts for this type of reaction. The results also indicate that the oxidative addition of organo halide to the reduced Ni center could occur during the reaction.
Catalytic application of 3a–3c in the homo-coupling of benzylic halides
Bibenzyl compounds and their derivatives can be found in a wide array of naturally occurring products and pharmaceuticals.11 It is, thus, important to have a facile entry to symmetrical bibenzyl derivatives through the catalytic homo-coupling of benzylic halides.12 Some Ni-based catalyst systems supported by different ligands are known to be active for this type of reaction.12d–h For example, POCOP-nickel(II) pincer complexes could catalyse the homo-coupling reactions of benzyl halides in the presence of Zn, giving the coupled products in high yields at 115 °C after 15 h.12g NiCl2(PPh3)2 catalysed the reaction with moderate to good activity in the presence of Mg, Mn or Zn at r.t. for 18 h.12h As shown in Table 2, under conditions similar to the Kumada–Tamao–Corriu cross-coupling reactions shown in Scheme 2, 3a–3c (1 mol%) could catalyse the reaction between benzylic bromide and MeMgCl at r.t. affording bibenzyl in 70–80% yield after 1 h with 100% conversion of benzylic bromide (entries 1–3). The reduction product and the cross-coupling product were observed in GC-MS as side products. No reaction occurred in the absence of a catalyst (entry 4). A stoichiometric amount of Grignard reagents was required for the reaction. No bibenzyl was detected when only 0.2 equivalent of MeMgCl was used (entry 5). When the complex CpNi(IMes)Cl was used as the catalyst (entry 6), the yield of bibenzyl was significantly lower and the reaction was incomplete.| Entry | Catalyst | Yield of bibenzylb (%) | Recovered benzyl bromideb (%) |
|---|---|---|---|
| a Reaction conditions: benzyl bromide (0.5 mmol), MeMgCl (0.6 mmol, 1.0 M in THF), catalyst (1 mol%) in THF (2 mL), r.t. 1 h. b Isolated yields and NMR yields. The isolated yields are given in parentheses. c n.d. = not detected. d 0.1 mmol of MeMgCl used. | |||
| 1 | 3a | 71 (71) | 0 |
| 2 | 3b | 81 (78) | 0 |
| 3 | 3c | 76 (74) | 0 |
| 4 | — | n.d.c | 100 |
| 5d | 3b | n.d. | 86 |
| 6 | CpNi(IMes)Cl | 51 | 37 |
The scope of the benzylic halide substrates was studied using 3b as a catalyst and the results are summarized in Table 3. The electron-donating groups (entries 1 and 2) and electron withdrawing groups (entries 3–5) on the para-position did not influence the activity of the catalyst 3b giving similar high yields of bibenzyl products. It is peculiar that the reaction was tolerant to the nitrile and ester functional groups (entries 4 and 5). The homo-coupling reaction also proceeded selectively on the sp3 halide instead of the sp2 halide (entries 6 and 7). The homo-coupling of (1-bromoethyl)benzene led to a parallel amount of diastereomeric products (entry 8) with 77% yield. The reaction of a sterically hindered substrate (entry 9) required an additional hour of reaction to reach full conversion and gave a yield comparable to the less sterically hindered substrates. When the less reactive chlorinated substrates were used (entries 10–13), the reactions required 24 h to achieve full conversion and the yield of bibenzyl products was generally low as expected.
| Entry | R1 | R2 | X | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
a Reaction conditions: benzylic halide (0.5 mmol), MeMgCl (0.6 mmol, 1.0 M in THF), 3b (1 mol%), THF (2 mL) at r.t. for 1 h.
b Isolated yields; complete conversion for all reactions.
c NMR yield.
d Diastereomeric ratio [meso![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dl] based on NMR.
e 87% conversion after 1 h.
f 3 equiv. of MeMgCl was used. dl] based on NMR.
e 87% conversion after 1 h.
f 3 equiv. of MeMgCl was used.
|
|||||
| 1 | 4-iPr | H | Br | 1 | 83 |
| 2 | 4-tBu | H | Br | 1 | 88 |
| 3 | 4-CF3 | H | Br | 1 | 77 |
| 4 | 4-CN | H | Br | 1 | 85c |
| 5 | 4-COOMe | H | Br | 1 | 70c |
| 6 | 4-Br | H | Br | 1 | 81 |
| 7 | 2-Br | H | Br | 1 | 83 |
| 8 | H | Me | Br | 1 | 77c [1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1]d 1]d |
| 9 | 2-Me | H | Br | 2 | 87e |
| 10f | H | H | Cl | 24 | 44 |
| 11f | 2-Me | H | Cl | 24 | 65 |
| 12f | 4-Me | H | Cl | 24 | 24 |
| 13f | 4-CF3 | H | Cl | 24 | 16 |
In CpNi(II)–NHC complex catalysed Buchwald–Hartwig arylaminations and Suzuki–Miyaura coupling reactions, low valent Ni(0) and Ni(I) species stabilized by the NHC ligand have been proposed to be the active species or intermediates.4a,b,5c Although neither Ni(0)–NHC nor Ni(I)–NHC species have been isolated by using CpNi(II)–NHC complexes as precursors, they could have been obtained through other synthetic routes and their application in C–C bond formation reactions has been studied.13 It has been reported recently that CpNi(I)–NHC complexes, CpNi(IPr) and CpNi(SIPr) (IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene; SIPr = 1,3-bis(2,6-diisopropylphenyl)imidazolidin-2-ylidene), isolated from the reaction between NaCp and [(μ-Cl)Ni(IPr)]2 or [(μ-Cl)Ni(SIPr)]2, could be used as pre-catalysts for Suzuki–Miyaura cross-coupling reactions.13h T-shaped three-coordinate nickel(I) halide bearing two bulky NHC ligands, Ni(IPr)2Cl, has been proved to be a reaction intermediate in Ni(IPr)2Cl2 catalysed Kumada–Tamao–Corriu cross-coupling reactions.13i The analogous complex Ni(IMes)2Br has been found to be active for both Kumada–Tamao–Corriu and Suzuki–Miyaura cross-coupling reactions.13j For both reactions, Ni(I)(IMes)nAr formed through transmetalation was proposed to be the active species capable of reacting with aryl halide to form a Ni(III) diaryl halide.13j Reaction of [(μ-Cl)Ni(IPr)]2 with {CH(SiMe3)2}MgCl could afford a two coordinate nickel(I) alkyl complex (IPr)Ni{CH(SiMe3)2}.13k The use of sterically encumbered Grignard reagents prevents the formation of the Ni(0) dimer. (IPr)Ni{CH(SiMe3)2} could react with benzyl bromide to give Ni(II) alkyl halide, bibenzyl and 1,1-bis(trimethylsilyl)-2-phenylethane through a radical oxidative addition mechanism and a Ni(III) dialkyl bromide complex has been proposed to be the intermediate.13k Although the catalytic reaction mechanism has not been investigated in this work, on the basis of the mechanistic studies for Ni-catalysed homo- and cross-coupling reactions of organic halides involving Ni(I) and Ni(III) species14 and the results mentioned above,13 a tentative reaction mechanism for the homo-coupling of benzylic halides catalysed by 3a–3c has been proposed (Scheme 4). Before the beginning of the catalytic cycle, Ni(I) methyl species A supported by the bidentate hybrid NHC ligand could be generated from the reactions between 3a–3c with MeMgCl. Oxidative addition of benzyl halide onto A gives the Ni(III) species B. Subsequently reacting with MeMgCl, B is converted to another Ni(III) species C. The elimination of ethane from C gives a Ni(I)-benzyl intermediate D. Through another oxidation addition process of the benzyl halide, D is converted to a Ni(III) bisbenzyl species E. E undergoes reductive elimination to give the bibenzyl product and a Ni(I) halide F. F reacts further with MeMgCl to regenerate A. The higher efficiency of 3a–3c compared with CpNi(IMes)Cl indicates the beneficial effect of the chelating ligand on catalyst performance which has been observed in many Ni–NHC catalysed C–C formation reactions.2a In this system, the bidentate hybrid NHC ligand could stabilize the highly reactive Ni(I) and Ni(III) species more efficiently. Furthermore, the presence of N donors in the hybrid NHC ligand could result in a more facile oxidation addition, while the steric pressure caused by the bidentate ligand could favour the reduction elimination process. When PhMgBr was used to replace MeMgCl, a mixture containing bibenzyl, the cross-coupling product, and the homo-coupling product of PhMgBr could be observed in GC-MS after 1 h using 3a as a catalyst. The proposed catalytic cycle is also consistent with this observation.
Conclusions
A series of air-stable CpNi(II) complexes 3a–3c with bidentate hybrid NHC ligands have been synthesized and fully characterized. A preliminary survey of their application in C–C bond formation reactions shows 3a is active for the catalytic oxidative homo-coupling of Grignard reagents. In the presence of a Grignard reagent, complexes 3a–3c are efficient catalysts for the homo-coupling of benzyl bromides under facile conditions (r.t., 1 h and 1 mol% of catalyst loading) and with good functional groups tolerance. Further exploration of the catalytic applications of CpNi–NHC complexes towards C–C bond formation reactions is currently underway.Experimental
Materials and physical measurements
All commercial chemicals were used as purchased. All preparations and manipulations were performed using standard Schlenk techniques under an argon atmosphere. Solvents were dried by standard procedures. Nickelocene,3d CpNiCl(IMes)Cl3d and benzothiazolyl-imidazolium chloride salts 1a–1c6c were prepared according to the literature methods. Elemental analyses for C, H, and N were performed on a Perkin-Elmer PE 2400 CHNS elemental analyzer and an Elementar vario MICRO Cube. 1H and 13C NMR were recorded at r.t. with Bruker ACF300 300 MHz and AMX500 500 MHz FT NMR spectrometers. Electrospray ionisation mass spectrometric (ESI-MS) analysis was performed on a Finnigan LCQ quadrupole ion trap mass spectrometer and a Bruker amaZon X ion trap mass spectrometer.General procedure for the synthesis of benzothiazolyl-imidazolium hexafluorophosphates (2a–2c)
To a solution of benzothiazolyl-imidazolium chloride salts 1a–1c (1.0 mmol) in methanol (40 mL) was added NH4PF6 (173 mg, 1.1 mmol). The solution was stirred at r.t. for 8 h. The precipitate was collected and washed with methanol (2 × 20 mL). The characterization data for 2a–2c are as follows.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHN(CH3) and Hd), 7.67 (dt, J = 8.0 Hz, 1.0 Hz, 1H, Hc), 7.62–7.59 (m, 2H,
CHN(CH3) and Hd), 7.67 (dt, J = 8.0 Hz, 1.0 Hz, 1H, Hc), 7.62–7.59 (m, 2H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHN(benzothiazole) and Hb), 3.99 (s, 3H, N(CH3)). 13C NMR (125.77 MHz, CD3CN): δ (ppm) = 154.91 (NCS), 150.60 (NCN), 136.90, 134.58, 128.97, 128.23, 126.31, 124.51, 123.77, 121.72, 37.82 (N–CH3). 31P NMR (202.44 MHz, CD3CN): δ (ppm) = −143.78 (sep, PF6). ESI-MS (in CH3CN: m/z): [L1H]+ = 216.2, [PF6]− = 145.1. Anal. Calcd for C11H10F6N3PS: C, 36.57; H 2.79; N, 11.63; S, 8.88. Found: C, 36.16; H, 3.03; N, 11.58; S, 9.10.
CHN(benzothiazole) and Hb), 3.99 (s, 3H, N(CH3)). 13C NMR (125.77 MHz, CD3CN): δ (ppm) = 154.91 (NCS), 150.60 (NCN), 136.90, 134.58, 128.97, 128.23, 126.31, 124.51, 123.77, 121.72, 37.82 (N–CH3). 31P NMR (202.44 MHz, CD3CN): δ (ppm) = −143.78 (sep, PF6). ESI-MS (in CH3CN: m/z): [L1H]+ = 216.2, [PF6]− = 145.1. Anal. Calcd for C11H10F6N3PS: C, 36.57; H 2.79; N, 11.63; S, 8.88. Found: C, 36.16; H, 3.03; N, 11.58; S, 9.10.
For 1-(2-benzothiazolyl)-3-allylimidazolium hexafluorophosphate (2b). Off-white solid (yield: 325 mg, 84%). 1H NMR (500 MHz, CD3CN): δ (ppm) = 9.35 (s, 1H, NCHN), 8.13–8.12 (m, 2H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHN(Allyl) and Ha), 8.07 (d, J = 8.5 Hz, 1H, Hd), 7.69–7.64 (m, 2H,
CHN(Allyl) and Ha), 8.07 (d, J = 8.5 Hz, 1H, Hd), 7.69–7.64 (m, 2H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHN(benzothiazole) and Hc), 7.61 (dt, J = 8.0 Hz, 1.0 Hz, 1H, Hb), 6.10 (ddt, J = 7.0 Hz, 6.5 Hz, 6.0 Hz, 1H, –CH2CH
CHN(benzothiazole) and Hc), 7.61 (dt, J = 8.0 Hz, 1.0 Hz, 1H, Hb), 6.10 (ddt, J = 7.0 Hz, 6.5 Hz, 6.0 Hz, 1H, –CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.54–5.51 (m, 2H, –CH2CH
CH2), 5.54–5.51 (m, 2H, –CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.92 (d, J = 6.5 Hz, 2H, –CH2CH
CH2), 4.92 (d, J = 6.5 Hz, 2H, –CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2). 13C NMR (125.77 MHz, CD3CN): δ (ppm) = 154.91 (NCS), 150.59 (NCN), 136.39, 134.65, 130.72, 128.99, 128.28, 124.98, 124.54, 123.79, 123.17, 122.14, 53.55. 31P NMR (202.44 MHz, CD3CN): δ (ppm) = −143.77 (sep, PF6). ESI-MS (in CH3CN: m/z): [L2H]+ = 242.2, [PF6]− = 145.1. Anal. Calcd for C13H12F6N3PS: C, 40.31; H, 3.12; N, 10.85; S, 8.28. Found: C, 40.06; H, 3.35; N, 11.02; S, 8.52.
CH2). 13C NMR (125.77 MHz, CD3CN): δ (ppm) = 154.91 (NCS), 150.59 (NCN), 136.39, 134.65, 130.72, 128.99, 128.28, 124.98, 124.54, 123.79, 123.17, 122.14, 53.55. 31P NMR (202.44 MHz, CD3CN): δ (ppm) = −143.77 (sep, PF6). ESI-MS (in CH3CN: m/z): [L2H]+ = 242.2, [PF6]− = 145.1. Anal. Calcd for C13H12F6N3PS: C, 40.31; H, 3.12; N, 10.85; S, 8.28. Found: C, 40.06; H, 3.35; N, 11.02; S, 8.52.
For 1-(2-benzothiazolyl)-3-benzylimidazolium hexafluorophosphate (2c). White solid (yield: 354 mg, 81%). 1H NMR (500 MHz, CD3CN): δ (ppm) = 9.43 (s, 1H, NCHN), 8.13–8.10 (m, 2H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHN(Bn) and Ha), 8.06 (d, J = 8.0 Hz, 1H, Hd), 7.67 (t, J = 8.5 Hz, 1H, Hc), 7.63–7.59 (m, 2H,
CHN(Bn) and Ha), 8.06 (d, J = 8.0 Hz, 1H, Hd), 7.67 (t, J = 8.5 Hz, 1H, Hc), 7.63–7.59 (m, 2H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHN(benzothiazole) and Hb), 7.52–7.45 (m, 5H, Ph), 5.48 (s, 2H, –CH2Ph). 13C NMR (125.77 MHz, CD3CN): δ (ppm) = 154.91 (NCS), 150.56 (NCN), 136.39, 134.64, 133.74, 130.61, 130.37, 130.02, 129.00, 128.29, 124.88, 124.53, 123.78, 122.34, 54.87. 31P NMR (202.44 MHz, CD3CN): δ (ppm) = −143.78 (sep, PF6). ESI-MS (in CH3CN: m/z): [L3H]+ = 292.2, [PF6]− = 145.1. Anal. Calcd for C17H14F6N3PS: C, 46.69; H, 3.23; N, 9.61; S, 7.33. Found: C, 46.74; H, 3.40; N, 9.60; S, 7.74.
CHN(benzothiazole) and Hb), 7.52–7.45 (m, 5H, Ph), 5.48 (s, 2H, –CH2Ph). 13C NMR (125.77 MHz, CD3CN): δ (ppm) = 154.91 (NCS), 150.56 (NCN), 136.39, 134.64, 133.74, 130.61, 130.37, 130.02, 129.00, 128.29, 124.88, 124.53, 123.78, 122.34, 54.87. 31P NMR (202.44 MHz, CD3CN): δ (ppm) = −143.78 (sep, PF6). ESI-MS (in CH3CN: m/z): [L3H]+ = 292.2, [PF6]− = 145.1. Anal. Calcd for C17H14F6N3PS: C, 46.69; H, 3.23; N, 9.61; S, 7.33. Found: C, 46.74; H, 3.40; N, 9.60; S, 7.74.
General procedure for the synthesis of nickel complexes (3a–3c)
A solution of 2a, 2b or 2c (0.1 mmol) in acetonitrile (5 mL) was added to nickelocene (23 mg, 0.12 mmol). The solution was stirred at 80 °C for 12 h. The solution slowly turned from dark green to brownish-green. The solvent was then removed under vacuum. The residue was washed with ethanol (3 × 5 mL) and then redissolved in acetonitrile (10 mL) and filtered. The solvent of the filtrate was evaporated to yield a green solid.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHN(CH3)), 7.66 (t, J = 7.5 Hz, 1H, Hc), 7.58–7.55 (m, 2H, Hb and Hd), 7.25 (d, J = 2.0 Hz, 1H,
CHN(CH3)), 7.66 (t, J = 7.5 Hz, 1H, Hc), 7.58–7.55 (m, 2H, Hb and Hd), 7.25 (d, J = 2.0 Hz, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHN(benzothiazole)), 5.97 (s, 5H, Cp), 3.77 (s, 3H, N(CH3)). 13C NMR (125.77 MHz, CD3CN): δ (ppm) = 175.71 (NCS), 158.61 (NCN), 149.67, 131.19, 129.44, 128.45, 127.66, 125.14, 121.34, 118.97, 93.44 (Cp), 39.04 (N–CH3). 31P NMR (202.44 MHz, CD3CN): δ (ppm) = −143.76 (sep, PF6). ESI-MS (in CH3CN: m/z): [CpNiL1]+ = 338.1, [PF6]− = 145.1. Anal. Calcd for C16H14F6N3PSNi: C, 39.70; H, 2.92; N, 8.68; S, 6.62. Found: C, 39.50; H, 3.33; N, 8.66; S, 6.50.
CHN(benzothiazole)), 5.97 (s, 5H, Cp), 3.77 (s, 3H, N(CH3)). 13C NMR (125.77 MHz, CD3CN): δ (ppm) = 175.71 (NCS), 158.61 (NCN), 149.67, 131.19, 129.44, 128.45, 127.66, 125.14, 121.34, 118.97, 93.44 (Cp), 39.04 (N–CH3). 31P NMR (202.44 MHz, CD3CN): δ (ppm) = −143.76 (sep, PF6). ESI-MS (in CH3CN: m/z): [CpNiL1]+ = 338.1, [PF6]− = 145.1. Anal. Calcd for C16H14F6N3PSNi: C, 39.70; H, 2.92; N, 8.68; S, 6.62. Found: C, 39.50; H, 3.33; N, 8.66; S, 6.50.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHN(Allyl)), 7.66 (t, J = 7.5 Hz, 1H, Hc), 7.58–7.55 (m, 2H, Hb and Hd), 7.27 (s, 1H,
CHN(Allyl)), 7.66 (t, J = 7.5 Hz, 1H, Hc), 7.58–7.55 (m, 2H, Hb and Hd), 7.27 (s, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHN(benzothiazole)), 6.11–6.02 (m, 1H, –CH2CH
CHN(benzothiazole)), 6.11–6.02 (m, 1H, –CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.89 (s, 5H, Cp), 5.38 (d, J = 10.5 Hz, 1H, –CH2CH
CH2), 5.89 (s, 5H, Cp), 5.38 (d, J = 10.5 Hz, 1H, –CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.15 (d, J = 17 Hz, 1H, –CH2CH
CH2), 5.15 (d, J = 17 Hz, 1H, –CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.66 (s, 2H, –CH2CH
CH2), 4.66 (s, 2H, –CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2). 13C NMR (125.77 MHz, CD3CN): δ (ppm) = 175.86 (NCS), 158.71 (NCN), 149.68, 132.89, 131.19, 129.46, 127.69, 127.27, 125.17, 121.32, 119.54, 118.88, 93.76 (Cp), 53.56 (N–C–CH
CH2). 13C NMR (125.77 MHz, CD3CN): δ (ppm) = 175.86 (NCS), 158.71 (NCN), 149.68, 132.89, 131.19, 129.46, 127.69, 127.27, 125.17, 121.32, 119.54, 118.88, 93.76 (Cp), 53.56 (N–C–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2). 31P NMR (202.44 MHz, CD3CN): δ (ppm) = −143.77 (sep, PF6). ESI-MS (in CH3CN: m/z): [CpNiL2]+ = 364.1, [PF6]− = 145.1. Anal. Calcd for C18H16F6N3PSNi: C, 42.39; H, 3.16; N, 8.24; S, 6.29. Found: C, 42.50; H, 3.28; N, 8.11; S, 6.12.
CH2). 31P NMR (202.44 MHz, CD3CN): δ (ppm) = −143.77 (sep, PF6). ESI-MS (in CH3CN: m/z): [CpNiL2]+ = 364.1, [PF6]− = 145.1. Anal. Calcd for C18H16F6N3PSNi: C, 42.39; H, 3.16; N, 8.24; S, 6.29. Found: C, 42.50; H, 3.28; N, 8.11; S, 6.12.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHN(Bn)), 7.66 (t, J = 7.5 Hz, 1H, Hc), 7.60–7.51 (m, 2H, Hb and Hd), 7.46–7.26 (m, 6H,
CHN(Bn)), 7.66 (t, J = 7.5 Hz, 1H, Hc), 7.60–7.51 (m, 2H, Hb and Hd), 7.46–7.26 (m, 6H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHN(benzothiazole) and Ph), 5.78 (s, 5H, Cp), 5.29 (s, 2H, –CH2Ph). 13C NMR (125.77 MHz, CD3CN): δ (ppm) = 176.34 (NCS), 158.77 (NCN), 149.71, 136.15, 131.21, 130.15, 129.47, 127.82, 127.72, 127.54, 125.19, 121.33 119.80, 93.79 (Cp), 54.87 (N–C-Ph). 31P NMR (202.44 MHz, CD3CN): δ (ppm) = −143.77 (sep, PF6). ESI-MS (in CH3CN: m/z): [CpNiL3]+ = 414.1, [L3H]+ = 292.1, [PF6]− = 145.1. Anal. Calcd for C22H18F6N3PSNi: C, 47.17; H, 3.24; N, 7.50; S, 5.72. Found: C, 47.26; H, 3.48; N, 7.19; S, 5.45.
CHN(benzothiazole) and Ph), 5.78 (s, 5H, Cp), 5.29 (s, 2H, –CH2Ph). 13C NMR (125.77 MHz, CD3CN): δ (ppm) = 176.34 (NCS), 158.77 (NCN), 149.71, 136.15, 131.21, 130.15, 129.47, 127.82, 127.72, 127.54, 125.19, 121.33 119.80, 93.79 (Cp), 54.87 (N–C-Ph). 31P NMR (202.44 MHz, CD3CN): δ (ppm) = −143.77 (sep, PF6). ESI-MS (in CH3CN: m/z): [CpNiL3]+ = 414.1, [L3H]+ = 292.1, [PF6]− = 145.1. Anal. Calcd for C22H18F6N3PSNi: C, 47.17; H, 3.24; N, 7.50; S, 5.72. Found: C, 47.26; H, 3.48; N, 7.19; S, 5.45.
General procedure for the catalytic cross-coupling of 4-bromoanisole with aryl Grignard reagents
A Schlenk tube, placed under an argon atmosphere, was charged with 4-bromoanisole (0.5 mmol), 3a (0.005 mmol, 1 mol%), and toluene (1 mL) at r.t. under stirring. An aryl Grignard reagent (0.6 mmol, 1.0 M in THF) was added dropwise within 30 min and the resulting reaction mixture was stirred at r.t. for another 30 min. The reaction was quenched with a drop of HCl (aq., 2 M) and the solvent removed under reduced pressure. The product was isolated by flash chromatography on silica gel with hexane as the eluent.General procedure for catalytic homo-coupling of aryl Grignard reagents
A Schlenk tube, placed under an argon atmosphere, was charged with 3a (0.005 mmol, 1 mol%), 1,2-dichloroethane (0.62 mmol) and THF (2 mL) at r.t. under stirring. An aryl Grignard reagent (0.5 mmol, 1.0 M in THF) was added and the resulting reaction mixture was stirred at r.t. for 1 h. The reaction was quenched with a drop of HCl (aq., 2 M) and the solvent removed under reduced pressure. The product was isolated by flash chromatography on silica gel with hexane as the eluent.General procedure for catalytic homo-coupling of benzyl halides
In a typical example, after standard cycles of evacuation and back-fill with pure argon, complex 3b (2.5 mg, 0.005 mmol) was introduced into a 25 mL-Schlenk tube equipped with a magnetic stir bar. To the catalyst were added THF (2 mL), internal standard 1,3,5-trimethoxybenzene (42 mg, 0.25 mmol) and benzyl bromide (59.5 μL, 0.5 mmol) at r.t. under stirring. A methylmagnesium chloride solution (3.0 M in THF, 0.2 mL, 0.6 mmol) was added and the resulted reaction mixture was stirred at r.t. for 1 h. The reaction was quenched with a drop of HCl (aq., 2 M) and the solvent removed under reduced pressure. The product was isolated by flash chromatography on silica gel with hexane as the eluent. NMR yields were calculated using 1,3,5-trimethoxybenzene as the internal standard.X-ray crystallography
Diffraction measurements were conducted at 100 K on a Bruker D8 Venture X-Ray diffractometer by using Mo Kα radiation (λ = 0.71073 Å) and a Photon 100 detector. The data were corrected for Lorentz and polarization effects with the Apex 2 suite of programs and for absorption effects with SADABS.15 Structure solutions and refinements were performed by using the program Bruker Apex 2. The structures were solved by direct methods to locate the heavy atoms, followed by difference maps for the light non-hydrogen atoms. Anisotropic thermal parameters were refined for the rest of the non-hydrogen atoms. Hydrogen atoms were placed geometrically and refined isotropically. Crystal data and structure refinement for complexes 3a–3c are given in Table S3 in the ESI.†Acknowledgements
We acknowledge the Agency for Science, Technology and Research (A*Star) of Singapore for financial support (WBS no. R-143-000-566-305). We thank Ms G. K. Tan for X-ray diffractometry assistance.Notes and references
- (a) S. Kaufhold, L. Petermann, R. Staehle and S. Rau, Coord. Chem. Rev., 2015, 304, 73–87 CrossRef; (b) M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485–496 CrossRef CAS PubMed; (c) D. J. Nelson and S. P. Nolan, Chem. Soc. Rev., 2013, 42, 6723–6753 RSC; (d) L.-A. Scharper, S. J. Hock, W. A. Herrmann and F. E. Kuehn, Angew. Chem., Int. Ed., 2013, 52, 270–289 CrossRef PubMed; (e) G. C. Fortman and S. P. Nolan, Chem. Soc. Rev., 2011, 40, 5151–5169 RSC; (f) M. Poyatos, J. A. Mata and E. Peris, Chem. Rev., 2009, 109, 3677–3707 CrossRef CAS PubMed; (g) S. Díez-González, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612–3676 CrossRef PubMed; (h) L. Yang, O. Schuster, H. G. Raubenheimer and M. Albrecht, Chem. Rev., 2009, 109, 3445–3478 CrossRef PubMed; (i) R. Corberan, E. Mas-Marza and E. Peris, Eur. J. Inorg. Chem., 2009, 1700–1716 CrossRef CAS; (j) N. Marion and S. P. Nolan, Chem. Soc. Rev., 2008, 37, 1776–1782 RSC; (k) E. Peris and R. H. Crabtree, Coord. Chem. Rev., 2004, 248, 2239–2246 CrossRef CAS; (l) V. César, S. Bellemin-Laponnaz and L. H. Gade, Chem. Soc. Rev., 2004, 33, 619–636 RSC; (m) W. A. Herrmann, Angew. Chem., Int. Ed., 2002, 41, 1290–1309 CrossRef CAS.
- (a) M. Henrion, V. Ritleng and M. J. Chetcuti, ACS Catal., 2015, 5, 1283–1302 CrossRef CAS; (b) A. P. Prakasham and P. Ghosh, Inorg. Chim. Acta, 2015, 431, 61–100 CrossRef CAS; (c) D. J. Nelson, Eur. J. Inorg. Chem., 2015, 2012–2027 CrossRef CAS; (d) A. Thakur and J. Louie, Acc. Chem. Res., 2015, 48, 2354–2365 CrossRef CAS PubMed; (e) M. Tobisu and N. Chatani, Acc. Chem. Res., 2015, 48, 1717–1726 CrossRef CAS PubMed; (f) M. T. Haynes II, E. P. Jackson and J. Montgomery, Nickel Complexes of N-Heterocyclic Carbenes, in N-Heterocyclic Carbenes: Effective Tools for Organometallic Synthesis, ed. S. P. Nolan, Wiley-VCH, Weinheim, 2014, pp. 371–396 Search PubMed; (g) Y. Fort and C. Comoy, NHC-Nickel and Platinum complexes, in N-Heterocyclic Carbenes: From Laboratory Curiosities to Efficient Synthetic Tools, ed. S. Díez-González, RSC Publishing, Cambridge, 2011, pp. 284–326 Search PubMed; (h) S. Gu, P. Ni and W. Chen, Chin. J. Catal., 2010, 31, 875–886 CrossRef CAS; (i) J. Louie, Ni-NHC mediated catalysis, in N-Heterocyclic Carbenes in Synthesis, ed. S. P. Nolan, Wiley-VCH, Weinheim, 2006, pp. 163–182 Search PubMed.
- (a) C. D. Abernethy, J. C. Clyburne, A. H. Cowley and R. A. Jones, J. Am. Chem. Soc., 1999, 121, 2329–2330 CrossRef CAS; (b) C. D. Abernethy, A. H. Cowley and R. A. Jones, J. Organomet. Chem., 2000, 596, 3–5 CrossRef CAS; (c) E. F. Hahn, B. Heidrich, A. Hepp and T. Pape, J. Organomet. Chem., 2007, 692, 4630–4638 CrossRef CAS; (d) V. Ritleng, E. Brenner and M. J. Chetcuti, J. Chem. Educ., 2008, 85, 1646–1648 CrossRef CAS; (e) V. Ritleng, C. Barth, E. Brenner, S. Milosevic and M. J. Chetcuti, Organometallics, 2008, 27, 4223–4228 CrossRef CAS; (f) F. E. Hahn, C. Radloff, T. Pape and A. Hepp, Organometallics, 2008, 27, 6408–6410 CrossRef CAS; (g) A. M. Oertel, V. Ritleng, M. J. Chetcuti and L. F. Veiros, J. Am. Chem. Soc., 2010, 132, 13588–13589 CrossRef CAS PubMed; (h) A. M. Oertel, V. Ritleng, A. Busiah, L. F. Veiros and M. J. Chetcuti, Organometallics, 2011, 30, 6495–6498 CrossRef CAS; (i) A. M. Oertel, J. Freudenreich, J. Gein, V. Ritleng, L. F. Veiros and M. J. Chetcuti, Organometallics, 2011, 30, 3400–3411 CrossRef CAS; (j) O. R. Luca, B. A. Thompson, M. K. Takase and R. H. Crabtree, J. Organomet. Chem., 2013, 730, 79–83 CrossRef CAS; (k) O. R. Luca, D. L. Huang, M. K. Takase and R. H. Crabtree, New J. Chem., 2013, 37, 3402–3405 RSC; (l) A. Włodarska, A. Kozioł, M. Dranka, A. Gryff-Keller, P. Szczeciński, J. Jurkowski and A. Pietrzykowski, Organometallics, 2015, 34, 577–581 CrossRef.
- Catalytic application in dehalogenation of aryl halides and aryl amination: (a) R. A. Kelly III, N. M. Scott, S. Díez-González, E. D. Stevens and S. P. Nolan, Organometallics, 2005, 24, 3442–3447 CrossRef; (b) A. R. Martin, Y. Makida, S. Meiries, A. M. Z. Slawin and S. P. Nolan, Organometallics, 2013, 32, 6265–6270 CrossRef CAS. Catalytic application in hydrothiolation of alkynes: (c) D. A. Malyshev, N. M. Scott, N. Marion, E. D. Stevens, V. P. Ananikov, I. P. Beletskaya and S. P. Nolan, Organometallics, 2006, 25, 4462–4470 CrossRef CAS. Catalytic application in hydrosilylation of carbonyls: (d) L. Postigo and B. Royo, Adv. Synth. Catal., 2012, 354, 2613–2618 CrossRef CAS; (e) L. P. Bheeter, M. Henrion, L. Brelot, C. Darcel, M. J. Chetcuti, J.-B. Sortais and V. Ritleng, Adv. Synth. Catal., 2012, 354, 2619–2624 CrossRef CAS. Catalytic application in α-arylation of acyclic ketones: (f) M. Henrion, M. J. Chetcuti and V. Ritleng, Chem. Commun., 2014, 50, 4624–4627 RSC. Catalytic application in polymerization reactions: (g) W. Buchowicz, A. Kozioł, L. B. Jerzykiewicz, T. Lis, S. Pasynkiewicz, A. Pecherzewska and A. Pietrzykowski, J. Mol. Catal. A: Chem., 2006, 257, 118–123 CrossRef CAS; (h) W. Buchowicz, W. Wojtczak, A. Pietrzykowski, A. Lupa, L. B. Jerzykiewicz, A. Makal and K. Woźniak, Eur. J. Inorg. Chem., 2010, 648–656 CrossRef CAS; (i) A. Włodarska, A. Kozioł, M. Dranka, J. Jurkowski and A. Pietrzykowski, J. Mol. Catal. A: Chem., 2014, 395, 481–485 CrossRef; (j) Ł. Banach, P. A. Guńka, D. Górska, M. Podlewska, J. Zachara and W. Buchowicz, Eur. J. Inorg. Chem., 2015, 5677–5686 CrossRef.
- Catalytic application in C–C cross-coupling reactions: Kumada–Tamao–Corriu cross-coupling reactions: (a) T. K. Macklin and V. Snieckus, Org. Lett., 2005, 7, 2519–2522 CrossRef CAS PubMed. Suzuki–Miyaura cross-coupling reactions: (b) V. Ritleng, A. M. Oertel and M. J. Chetcuti, Dalton Trans., 2010, 39, 8153–8160 RSC; (c) A. M. Oertel, V. Ritleng, L. Burr and M. J. Chetcuti, Organometallics, 2011, 30, 6685–6691 CrossRef CAS; (d) A. M. Oertel, V. Ritleng and M. J. Chetcuti, Organometallics, 2012, 31, 2829–2840 CrossRef CAS; (e) Y. Wei, A. Petronilho, H. Mueller-Bunz and M. Albrecht, Organometallics, 2014, 33, 5834–5844 CrossRef CAS; (f) W. Buchowicz, Ł. Banach, J. Conder, P. A. Guńka, D. Kubicki and P. Buchalski, Dalton Trans., 2014, 43, 5847–5857 RSC; (g) J. Yau, K. E. Hunt, L. McDougall, A. R. Kennedy and D. J. Nelson, Beilstein J. Org. Chem., 2015, 11, 2171–2178 CrossRef CAS PubMed.
- (a) F. Li, S.-Q. Bai and T. S. A. Hor, Organometallics, 2008, 27, 672–677 CrossRef CAS; (b) F. Li, J. J. Hu, L. L. Koh and T. S. A. Hor, Dalton Trans., 2010, 39, 5231–5241 RSC; (c) Z. Wang, S. W. B. Ng, L. Jiang, W. J. Leong, J. Zhao and T. S. A. Hor, Organometallics, 2014, 33, 2457–2466 CrossRef CAS.
- (a) F. Xue, J. Zhao, T. S. A. Hor and T. Hayashi, J. Am. Chem. Soc., 2015, 137, 3189–3192 CrossRef CAS PubMed; (b) F. Xue, J. Zhao and T. S. A. Hor, Dalton Trans., 2013, 42, 5150–5158 RSC; (c) F. Xue, J. Zhao and T. S. A. Hor, Chem. Commun., 2013, 49, 10121–10123 RSC.
- (a) G. Cahiez, A. Moyeux and J. Cossy, Adv. Synth. Catal., 2015, 357, 1983–1989 CrossRef CAS; (b) R. L.-Y. Bao, R. Zhao and L. Shi, Chem. Commun., 2015, 51, 6884–6900 RSC; (c) C. E. I. Knappke and A. J. von Wangelin, Chem. Soc. Rev., 2011, 40, 4948–4962 RSC.
- (a) C. Liu, H. Zhang, W. Shi and A. Lei, Chem. Rev., 2011, 111, 1780–1824 CrossRef CAS PubMed; (b) J. Hassan, M. Sévignon, C. Gozzi, E. Schulz and M. Lemaire, Chem. Rev., 2002, 102, 1359–1470 CrossRef CAS PubMed; (c) T. Korenaga, K. Nitatori, H. Muraoka, S. Ogawa and K. Shimada, Org. Lett., 2015, 17, 5500–5503 CrossRef CAS PubMed.
- (a) T. Nagano and T. Hayashi, Org. Lett., 2005, 7, 491–493 CrossRef CAS PubMed; (b) G. Cahiez, C. Chaboche, F. Mahuteau-Betzer and M. Ahr, Org. Lett., 2005, 7, 1943–1946 CrossRef CAS PubMed; (c) K. Kude, S. Hayase, M. Kawatsura and T. Itoh, Heteroat. Chem., 2011, 22, 397–404 CrossRef CAS; (d) G. Cahiez, A. Moyeux, J. Buendia and C. Duplais, J. Am. Chem. Soc., 2007, 129, 13788–13789 CrossRef CAS PubMed; (e) W. Liu and A. Lei, Tetrahedron Lett., 2008, 49, 610–613 CrossRef CAS; (f) G. Kiefer, L. Jeanbourquin and K. Severin, Angew. Chem., Int. Ed., 2013, 52, 6302–6305 CrossRef CAS PubMed; (g) S.-K. Hua, Q.-P. Hu, J. Ren and B.-B. Zeng, Synthesis, 2013, 518–526 CAS; (h) Y. Zhu, T. Xiong, W. Han and Y. Shi, Org. Lett., 2014, 16, 6144–6147 CrossRef CAS PubMed; (i) Z. Zhou and W. Xue, J. Organomet. Chem., 2009, 694, 599–603 CrossRef CAS; (j) S.-Y. Chen, J. Zhang, Y.-H. Li, J. Wen, S.-Q. Bian and X.-Q. Yu, Tetrahedron Lett., 2009, 50, 6795–6797 CrossRef CAS; (k) M. Mayer, W. M. Czaplik and A. J. von Wangelin, Synlett, 2009, 2919–2923 CAS; (l) P. I. Aparna and B. R. Bhat, J. Mol. Catal. A: Chem., 2012, 358, 73–78 CrossRef CAS; (m) A. P. I. Bhat, F. Inam and B. R. Bhat, Eur. J. Org. Chem., 2013, 7139–7144 CrossRef CAS.
- (a) A. Cirla and J. Mann, Nat. Prod. Rep., 2003, 20, 558–564 RSC; (b) J. A. Baur and D. A. Sinclair, Nat. Rev. Drug Discovery, 2006, 5, 493–506 CrossRef CAS PubMed; (c) S.-H. Baek, R. K. Phipps and N. B. Perry, J. Nat. Prod., 2004, 67, 718–720 CrossRef CAS PubMed; (d) C. Labbé, F. Faini, C. Villagrán, J. Coll and D. S. Rycroft, J. Nat. Prod., 2007, 70, 2019–2021 CrossRef PubMed; (e) Y. Asakawa and A. Ludwiczuk, Heterocycles, 2012, 86, 891–917 CrossRef CAS; (f) R. A. Aitken, J. Bouquet, J. Frank, A. L. G. Gidlow, Y. L. Powder, R. S. Ramsewak and W. F. Reynolds, RSC Adv., 2013, 3, 7230–7232 RSC.
- (a) A. F. Barrero, M. M. Herrador, J. F. Quílez del Moral, P. Arteaga, M. Akssira, F. E. Hanbali, J. F. Arteaga, H. R. Diéguez and E. M. Sánchez, J. Org. Chem., 2007, 72, 2251–2254 CrossRef CAS PubMed; (b) K. Sato, Y. Inoue, T. Mori, A. Sakaue, A. Tarui, M. Omote, I. Kumadaki and A. Ando, Org. Lett., 2014, 16, 3756–3759 CrossRef CAS PubMed; (c) Y.-L. Hu, F. Li, G.-L. Gu and M. Lu, Catal. Lett., 2011, 141, 467–473 CrossRef CAS; (d) S. M. Goldup, D. A. Leigh, R. T. McBurney, P. R. McGonigal and A. Plant, Chem. Sci., 2010, 1, 383–386 RSC; (e) M. R. Prinsell, D. A. Everson and D. J. Weix, Chem. Commun., 2010, 46, 5743–5745 RSC; (f) Y. Peng, L. Luo, C.-S. Yan, J.-J. Zhang and Y.-W. Wang, J. Org. Chem., 2013, 78, 10960–10967 CrossRef CAS PubMed; (g) T. Chen, L. Yang, L. Li and K.-W. Huang, Tetrahedron, 2012, 68, 6152–6157 CrossRef CAS; (h) C. D. Mboyi, M. D. Mabaye, N. Pannetier and J.-L. Renaud, Tetrahedron, 2013, 69, 4875–4882 CrossRef CAS.
- Ni(0)–NHC complexes: (a) A. J. Arduengo, S. F. Gamper, J. C. Calabrese and F. Davidson, J. Am. Chem. Soc., 1994, 116, 4391–4394 CrossRef CAS; (b) D. S. McGuinness, K. J. Cavell, B. W. Skelton and A. H. White, Organometallics, 1999, 18, 1596–1605 CrossRef CAS; (c) T. Schaub and U. Radius, Chem. – Eur. J., 2005, 11, 5024–5030 CrossRef CAS PubMed; (d) S. Kuhl, Y. Fort and R. Schneider, J. Organomet. Chem., 2005, 690, 6169–6177 CrossRef CAS; (e) T. Schaub, M. Backes and U. Radius, J. Am. Chem. Soc., 2006, 128, 15964–15965 CrossRef CAS PubMed; (f) Y. Zhang, K. C. Ngeow and J. Y. Ying, Org. Lett., 2007, 9, 3495–3498 CrossRef CAS PubMed; (g) K. Matsubara, S. Miyazaki, Y. Koga, Y. Nibu, T. Hashimura and T. Matsumoto, Organometallics, 2008, 27, 6020–6024 CrossRef CAS. Ni(I)–NHC complexes: (h) J. Wu, A. Nova, D. Balcells, G. W. Brudvig, W. Dai, L. M. Guard, N. Hazari, P.-H. Lin, R. Pokhrel and M. K. Takase, Chem. – Eur. J., 2014, 20, 5327–5337 CrossRef CAS PubMed; (i) S. Miyazaki, Y. Koga, T. Matsumoto and K. Matsubara, Chem. Commun., 2010, 46, 1932–1934 RSC; (j) K. Zhang, M. Conda-Sheridan, S. R. Cooke and J. Louie, Organometallics, 2011, 30, 2546–2552 CrossRef CAS PubMed; (k) C. A. Laskowski, D. J. Bungum, S. M. Baldwin, S. A. Del Ciello, V. M. Iluc and G. L. Hillhouse, J. Am. Chem. Soc., 2014, 135, 18272–18275 CrossRef PubMed.
- (a) F.-S. Han, Chem. Soc. Rev., 2013, 42, 5270–5298 RSC; (b) S. Z. Tasker, E. A. Standley and T. F. Jamison, Nature, 2014, 509, 299–309 CrossRef CAS PubMed; (c) V. P. Ananikov, ACS Catal., 2015, 5, 1964–1971 CrossRef CAS; (d) D. L. Weix, Acc. Chem. Res., 2015, 48, 1767–1775 CrossRef CAS PubMed.
- SADABS: Area-Detection Absorption Correction, Bruker AXS Inc., Madison, WI, 1995 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: For the NMR spectra of compounds 2a–2c and 3a–3c, the catalytic reaction results, the characterization data and the NMR spectra of the bibenzyl products, the crystallographic data of 3a–3c, and the crystal data. CCDC 1441071 (3b), 1441072 (3c) and 1441073 (3a). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt00252h |
| This journal is © The Royal Society of Chemistry 2016 |