Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Transformation of zinc hydroxide chloride monohydrate to crystalline zinc oxide†
Amir
Moezzi
ab,
Michael
Cortie
*b and
Andrew
McDonagh
b
aAvocado Engineering Pty. Ltd., NSW, Australia. E-mail: a.moezzi@avocadoengineering.com; Tel: +61-422088578
bInstitute for Nanoscale Technology, University of Technology Sydney, PO Box 123, Broadway, NSW 2007, Australia. E-mail: Michael.cortie@uts.edu.au; Tel: +61-2-9514-2208
First published on 31st March 2016
Abstract
Thermal decomposition of layered zinc hydroxide double salts provides an interesting alternative synthesis for particles of zinc oxide. Here, we examine the sequence of changes occurring as zinc hydroxide chloride monohydrate (Zn5(OH)8Cl2·H2O) is converted to crystalline ZnO by thermal decomposition. The specific surface area of the resultant ZnO measured by BET was 1.3 m2 g−1. A complicating and important factor in this process is that the thermal decomposition of zinc hydroxide chloride is also accompanied by the formation of volatile zinc-containing species under certain conditions. We show that this volatile compound is anhydrous ZnCl2 and its formation is moisture dependent. Therefore, control of atmospheric moisture is an important consideration that affects the overall efficiency of ZnO production by this process.
Introduction
Zinc oxide is an important industrial chemical that may be synthesized by a very wide variety of means.1 Calcination or pyrolysis of a suitable precursor, for example an alkylzinc alkoxide2 or a basic zinc salt3 are amongst the many options. Here we consider the use of the basic salt ‘zinc hydroxide chloride’ as a precursor in this context. Since it was first reported in 1855,4 the composition and decomposition of ‘zinc hydroxide chloride’ has been controversial. Many compositions and structures have been proposed for this compound, which has the general formula of xZn(OH)2·yZnCl2·zH2O. Among the reported phases, 4Zn(OH)2·ZnCl2·H2O (often described as Zn5(OH)8Cl2·H2O, basic zinc chloride or Simonkolleite, JC-PDF 01-077-2311), ZnO·ZnCl2·2H2O (JC-PDF 00-045-0819) and β-Zn(OH)Cl (JC-PDF 01-072-0525) appear to be the most stable. Zn5(OH)8Cl2·H2O is in the family of layered hydroxide double salts, also called ‘anionic clays’. It possesses hydration/adsorption properties similar to those of natural clays whereby a large interlayer distance (0.79 nm) accommodates H2O/CO2 in the interlayer void sites.The thermal decomposition of Zn5(OH)8Cl2·H2O has been previously studied.5–13 In general it is agreed that the process takes place in a sequence of steps, but there are differing views concerning the actual mechanism.
A two-step decomposition mechanism for Zn5(OH)8Cl2·H2O has been proposed5,6 involving initial decomposition to ZnO and ZnCl2 followed by melting and evaporation of ZnCl2. It was reported that β-Zn(OH)Cl also decomposes to ZnO and ZnCl2 in a similar manner.5,6 In contrast, a stepwise sequence of de-hydration reactions has been suggested;7 in this scenario Zn5(OH)8Cl2·H2O decomposes to ZnO and ZnO·ZnCl2·2H2O at ∼160 °C, loses one water of hydration at ∼230 °C before, finally, the ZnO·ZnCl2·H2O loses its remaining water together with ZnCl2.7 In other studies of Zn5(OH)8Cl2·H2O heated to 1000 °C, Zn(OH)Cl was reported as an intermediate, i.e. as the product of decomposition of Zn5(OH)8Cl2·H2O, which may in turn convert to ZnO, ZnCl2 and water between 210–300 °C. The ZnCl2 formed is volatilised at temperatures above 300 °C and escapes from the system.8 Krunks et al. postulated10 that Zn5(OH)8Cl2·H2O decomposes to ZnO·ZnCl2·2H2O followed by decomposition to ZnO and ZnCl2.
In contrast to the above, two recently proposed mechanisms do not involve the formation of ZnCl2 at all.12 Tanaka and Fujioka suggested a decomposition mechanism that included the formation of β-Zn(OH)Cl and Zn(OH)2 followed by complete decomposition of these two intermediates to ZnO and HCl and H2O at 225 °C.12 (However, formation of Zn(OH)2 at such temperatures seems somewhat unlikely as it normally decomposes to ZnO at around 120 to 130 °C).5,14,15 Similarly, Zhang and Yanagisawa suggested11 that Zn5(OH)8Cl2·H2O decomposes to only ZnO and HCl and water with the release of HCl in the last stage of the decomposition.
The proportion of the material that may be volatilised as ZnCl2 has a direct bearing on the mass loss associated with the decomposition. There are lower and upper limits: if all the Cl escapes from the reactor as HCl then 5ZnO is eventually formed for every mole of Zn5(OH)8Cl2·H2O and the mass loss is only 26.3%. This case is equivalent to a situation in which no ZnCl2 is formed or, if ZnCl2 is formed, it is hydrolysed in situ to ZnO. In contrast, if all Cl is converted to ZnCl2, which then all escapes by evaporation, then only 4ZnO remains in the reactor and the mass loss is 41.02%.
A common problem in thermogravimetric analysis (TGA) studies of Zn5(OH)8Cl2·H2O is that the overall mass loss observed after decomposition to ZnO is somewhat variable, with reported values being in the range 25.5 to 37.5%.5,8,9,12,13 These discrepancies are attributed to the partial hydrolysis of ZnCl2 to form ZnO. For example, Garcia-Martinez et al., suggested that β-Zn(OH)Cl and ZnCl2 are formed as intermediate products during the decomposition of Zn5(OH)8Cl2·H2O followed by hydrolysis of a portion of the ZnCl2 to form ZnO and HCl.9 Recently, Kozawa et al., examined the thermal decomposition of Zn5(OH)8Cl2·H2O and examined the hypothesis that water vapour in the calcination atmosphere was the factor responsible for these variable losses. TGA-DTA experiments up to 520 °C were conducted in air, dry N2 and humid N2 with P(H2O) of 4.5 and 10 kPa. They confirmed that mass loss is a function of the partial pressure of water. A 37.5% mass loss under dry-condition experiments was attributed to the formation of volatile ZnCl2, whereas under humid conditions, mass loss was close to the theoretical lower limit of 26.3%.13
Disparities in the suggested mechanisms appear to arise mainly because of the more-than-expected mass loss observed in TGA experiments and confusing DTA data. The observation that Zn5(OH)8Cl2·H2O was almost free of unbound moisture in the studies discussed above led to conclusions that the excess mass loss (relative to the theoretical value of 26.3% for the complete conversion to only ZnO) is related to volatile compounds other than water and HCl, such as ZnCl2. Nevertheless, there is scant direct experimental evidence that ZnCl2 is involved, either as an intermediate or final product.
In the work presented here, we re-examine the phase transformations, crystalline intermediate phases and gases evolved during thermal decomposition of Zn5(OH)8Cl2·H2O using a combination of TGA-DTA (thermogravimetric analysis-differential thermal analysis), TGA-MS (TGA-mass spectrometry) and in situ real-time synchrotron radiation XRD. The evolution of volatile and hydroscopic zinc-containing compounds during the thermal decomposition of Zn5(OH)8Cl2·H2O is confirmed and the identity of the volatile materials is clarified.
Experimental
General
Zinc chloride and zinc oxide powder (AR grade) were purchased from Ajax Chemicals and used as received. MilliQ water (18.2 MΩ cm−1) was used as solvent. Reactions were conducted under atmospheric conditions using a two-neck round bottom flask with a digitally-controlled magnetic heater/stirrer. A magnetic stirrer bar was used at a stirring rate of 500 rpm and isolation of the precipitates was performed by vacuum filtration followed by drying using a rotary evaporator at 70 °C.Synchrotron powder XRD studies were conducted at the Australian Synchrotron using 0.3 mm diameter silica capillaries filled with sample. The X-ray wavelength was set at 0.696603 Å (confirmed by refinement on a LaB6 standard). The time stamp corresponded to the beginning of each two-minute collection interval and the sample was ramped at 5 K min−1. 3D and color-coded contour graphs were generated using MATLAB version 7, which was also used for numerical analysis. Intensity data were corrected by division of the intensity figure by the integrated ion chamber count figure multiplied by 1 × 105 to form an arbitrary unit.
X-ray diffraction experiments using powder samples were also performed using a laboratory Siemens D5000 X-ray diffractometer with a graphite post monochromator with the following parameters: wavelength 0.15406 nm (Cu Kα), tube power 1.6 kW (40 kV at 40 mA), step size = 0.02°, time per step = 2 s, divergent slit = 1°, receiving slit = 0.02 mm, scan angle range = 3–80°. Scanning electron microscopy images were obtained using a Zeiss Supra 55VP SEM operating in high vacuum mode. An accelerating voltage of 5–20 kV was used with 10–30 μm aperture and images were obtained using an in-lens secondary detector. Thermogravimetric analysis (TGA) experiments were performed using TA Instruments-SDT 2960 with simultaneous DTA-TGA. A heating rate of 5 K min−1 was used in air atmosphere.
Simultaneous thermogravimetric analysis-mass spectrometry (TGA-MS) experiments were conducted using a Quadrupole mass spectrometer (model: Thermostar QMS 200 M3) from Balzers Instruments in a platinum crucible. Heating rates of 3 and 4 K min−1 and environments of air and argon were applied. The ion intensities associated with the following masses were examined: H2O: 18, HCl: 36, CO2: 44 and I136 for ZnCl2: 136. BET specific surface area measurement was performed using Autosorb-1 from Quantachrome Instrument by the precise vacuum volumetric method for nitrogen chemisorption. Multi-point (5 points) measurement was conducted on the sample.
Synthesis of zinc hydroxide chloride hydrate (Zn5(OH)8Cl2·H2O)
An aqueous solution of ZnCl2 (24.42 g in 30 mL, 6 M, pH = 3) was added to a suspension of ZnO (14.65 g, 0.18 mol) in water (70 mL) in a single step at 90 °C. The mixture was stirred for 2 hours after which time the pH was 7. The resultant white suspension was filtered, washed with water and dried. The sample was stable during storage for over a year under ambient conditions (ESI, Fig. S1†).Synthesis of zinc oxide from zinc hydroxide chloride hydrate
Zinc hydroxide chloride hydrate was calcined in a preheated oven. Samples were examined by XRD after each of the following three stages: (1) at 400 °C for 2 hours; (2) at 400 °C for an extra 4 hours and (3) at 600 °C for 4 hours. After each stage, the product was cooled to room temperature in air. The ZnO sample produced by calcining at 600 °C was stable during storage for over a year under ambient conditions (ESI, Fig. S2†).Sublimation experiments were performed using a cold finger sublimation apparatus heated to ∼450 °C in vacuo. Zn5(OH)8Cl2·H2O was first dehydrated in a preheated oven at 300 °C for 1 hour and the resultant white powder transferred to the sublimation apparatus and heated to 450 °C. The white powder became green-yellow in colour. A condensate formed on the water-cooled core but disappeared after a short time while off-white crystals formed on the wall of the vessel. These crystals could be melted by additional heat (heat gun) and reformed by removing the additional heat. After cooling, the crystalline sublimate, which was quite hygroscopic, was collected.
Results and discussion
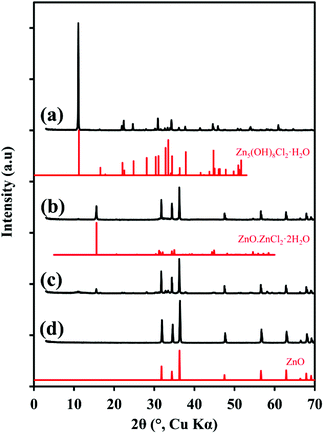
The powder XRD pattern of the Zn5(OH)8Cl2·H2O is shown in Fig. 1(a). The peaks associated with the as-synthesized material correspond to those previously reported for this compound (JC-PDF: 01-077-2311). Calcination of Zn5(OH)8Cl2·H2O in air at 400 °C yielded ZnO as well as ZnO·ZnCl2·2H2O (JC-PDF: 00-045-0819), Fig. 1(b). The intensity of the peaks associated with ZnO·ZnCl2·2H2O decreased after 6 hours of calcination at 400 °C, but conversion to ZnO was still incomplete after this time with small peaks associated with Zn5(OH)8Cl2·H2O still visible, Fig. 1(c). This behaviour has been reported by others although the intermediate phase in those instances was identified as β-Zn(OH)Cl.13 After calcination at 600 °C for 4 hours, only peaks assigned to ZnO (JC-PDF: 01-075-0576) were evident in the XRD data, Fig. 1(d).The BET specific surface area of the ZnO produced by calcination of the Zn5(OH)8Cl2·H2O to 600 °C was 1.3 m2 g−1, which is lower than that of ZnO prepared by the common industrial French process (∼3–5 m2 g−1)1 or by direct wet-chemistry precipitation techniques (∼10–33 m2 g−1)15 or from decomposition of zinc hydroxide acetate (15.4 m2 g−1)16 or from decomposition of zinc hydroxide carbonate (47–65 m2 g−1),1 but is similar to that reported for the grade of ZnO produced by the ‘American’ process1 and to ZnO made by thermal decomposition of zinc hydroxide sulfate (∼0.7 m2 g−1).17 SEM images of Zn5(OH)8Cl2·H2O and the ZnO particles prepared from it are shown in Fig. 2 (additional images are shown in the ESI, Fig. S3†). The Zn5(OH)8Cl2·H2O morphology is plate-like with micron-sized crystals whereas the subsequent ZnO crystal size is 200 nm to 1 μm and more spherical in shape.
TGA-DTA was performed on samples of Zn5(OH)8Cl2·H2O prepared in the same batch but measured on separate occasions. In both cases a heating rate of 5 K min−1 was used in air atmosphere but the results, shown in Fig. 3, are significantly different. The total mass loss of sample (I) is ∼26.5% and that of sample (II) is 31.5%. At temperatures less than 100 °C, almost no mass loss occurs in either sample indicating that the compound is free of unbound moisture. To aid discussion, the TGA data are examined in four zones. Mass losses in Zones A and B for both samples were similar, but mass losses in Zone C and particularly in Zone D increased significantly in sample (II) relative to sample (I). As we show below, the difference between mass loss values for this material can be assigned to the atmospheric moisture content, which changes the decomposition mechanism.
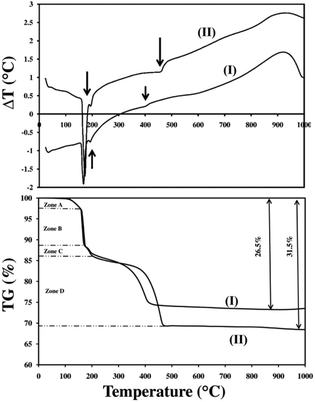 | ||
| Fig. 3 TGA-DTA of Zn5(OH)8Cl2·H2O. (I) freshly made Zn5(OH)8Cl2·H2O, (II) Zn5(OH)8Cl2·H2O tested after 13 months of aging. | ||
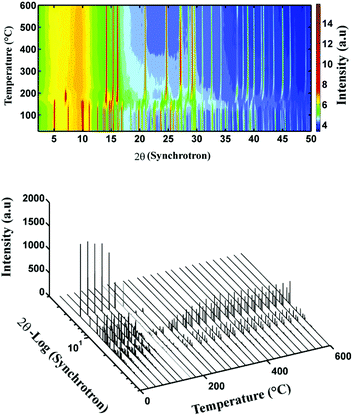
To uncover the processes that occur during the transformation of Zn5(OH)8Cl2·H2O to ZnO, in situ real-time synchrotron radiation XRD studies were undertaken. Data were collected while heating Zn5(OH)8Cl2·H2O from 26 to 600 °C, which revealed the crystalline intermediate phases, Fig. 4 (see also ESI Fig. S4 and S5†).
The first transformation occurred from 100 to 161 °C, where the intensities of the initial diffraction pattern decrease. This process is assigned to a dehydration process, eqn (1), and is consistent with Zone A in the TGA data.
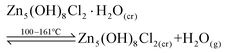 | (1) |
The next transformation occurred in the region of 161–197 °C where the peaks associated with Zn5(OH)8Cl2 disappear and peaks assigned to ZnO appear together with those of ZnO·ZnCl2·2H2O, a lower order zinc hydroxide chloride. This process is described in eqn (2). Although others have reported on the probable formation of β-Zn(OH)Cl,8,9,12 the XRD data collected in the present work indicate that ZnO·ZnCl2·2H2O is the intermediate crystalline phase in this temperature range. The TGA-DTA data, Fig. 3, reveal an endothermic peak corresponding to a decomposition at ∼164 °C with the associated 3 moles of water released up to a temperature of ∼ 200 °C (Zones A and B in the TGA graph) consistent with the combination of eqn (1) and (2).
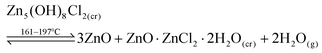 | (2) |
At higher temperatures, only one more transformation in the synchrotron XRD data was evident: all the peaks except for those assigned to ZnO disappear. This transformation occurs in the temperature range 197–225 °C, and coincides with the mass loss in Zone C. Up to 400 °C, the intensity of the ZnO diffraction peaks increases whereupon no further changes are observed up to the final measurement temperature of 600 °C. Importantly, while the XRD data indicate that ZnO is the only crystalline material present at temperatures greater than 225 °C, TGA indicates that significant mass is lost beyond this temperature, Zone D (ESI, Fig. S6 and S7†). When considered in conjunction with mass spectrometry data (discussed below), we can assign this mass loss to decomposition of an amorphous phase, Zn(OH)2·ZnCl2, formed by rearrangement and dehydration of the crystalline intermediate ZnO·ZnCl2·2H2O according to eqn (3). We note that examples of zinc compounds (e.g., zinc borate, xZnO·yB2O3·zH2O) exist with either crystalline or amorphous structures based on the synthesis method and temperature. Such compounds can retain water up to temperatures of ∼400 °C.18,19 In addition, amorphous materials have been reported when other layered hydroxide salts are calcined at temperatures of 300–500 °C.20–22 We discount the possibility of the formation and melting of ZnCl2 as this would imply that no further release of water should occur, which is in contrast to our mass spectrometry data (see below).
 | (3) |
Interpretation of the last stage of decomposition, after which only ZnO remains, is not straightforward and, as shown in Fig. 3, mass loss can be variable. The major reaction involved is the decomposition of amorphous Zn(OH)2·ZnCl2 to ZnO with the release of HCl, eqn (4). DTA shows an endothermic peak corresponding to this process at ∼400 °C.
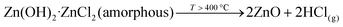 | (4) |
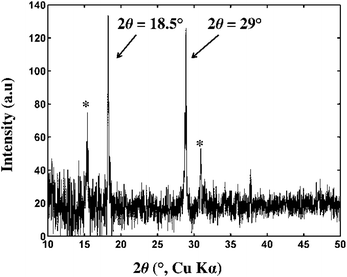
Considering the previous work of Kozawa et al.13 (discussed above) the variable thermal decomposition behaviour of the Zn5(OH)8Cl2·H2O is a consequence of variable amounts of volatile zinc-containing species being formed which are, in turn, dependent on the moisture content of the atmosphere under which the material is decomposed. In particular, anhydrous zinc chloride is implicated in this process. Our attempts to identify zinc chloride released from Zn5(OH)8Cl2·H2O upon heating using TGA-MS experiments (ESI Fig. S8–S10†) were inconclusive due to instrumental limitations although experiments confirmed that water was released at temperatures up to ∼230 °C with MS peaks associated with the TGA zones A, B and C, and that hydrogen chloride was evolved at temperatures corresponding to Zone D. However, anhydrous zinc chloride was unambiguously identified as a volatile reaction product in experiments where Zn5(OH)8Cl2·H2O was heated in a sublimation apparatus (ESI, Fig. S11a and b†). Powder XRD patterns were collected for the sublimate using a diffractometer stage that was heated at 100–120 °C to avoid hydration of the moisture-sensitive material. The diffraction pattern revealed that the sublimate is a mixture of α-ZnCl2 (JC-PDF: 01-074-0519) and β-ZnCl2 (JC-PDF: 01-072-1284), Fig. 5. No peak associated with β-Zn(OH)Cl was detected.
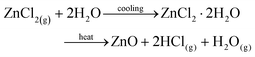
TGA-DTA of the obtained ZnCl2 recorded under a flow of air revealed a mass gain of ∼1% from room temperature up to ∼50 °C (in agreement with the hygroscopic nature of ZnCl2), and a subsequent mass loss of ∼99.3% upon heating to 1000 °C. TGA-DTA of zinc chloride dihydrate was conducted for comparison and the data are significantly different to those of the collected ZnCl2 (ESI Fig S12†). It is apparent that greater mass loss occurs with the more dehydrated zinc chloride species. Examination of the mechanism of decomposition of hydrated zinc chloride from previous work9,23 is illustrative in this context. Heating hydrated zinc chloride does not directly form dehydrated zinc chloride but rather ZnO, eqn (5), or Zn(OH)Cl, eqn (6) that may subsequently decompose to ZnO or ZnCl2. On the other hand anhydrous zinc chloride can volatilise when heated, eqn (7).
 | (5) |
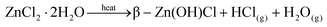 | (6) |
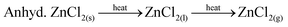 | (7) |
It is apparent that anhydrous ZnCl2 can be formed by heating Zn5(OH)8Cl2·H2O, eqn (8), but the amount of ZnCl2 formed will depend on the moisture content of the atmosphere under which Zn5(OH)8Cl2·H2O is thermally decomposed. If the moisture content of the atmosphere in which reaction occurs increases, the amount of volatile ZnCl2 decreases. That is, higher atmospheric moisture favours eqn (4) compared to eqn (8), and vice versa. Therefore, higher atmospheric moisture favours ZnO production (closer to the theoretical 5 moles of ZnO for every mole of Zn5(OH)8Cl2·H2O decomposed). On the other hand, in dry atmospheres some zinc chloride will be volatilised and the yield of ZnO will be reduced. These findings are reflected in the variable TGA mass loss values.
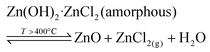 | (8) |
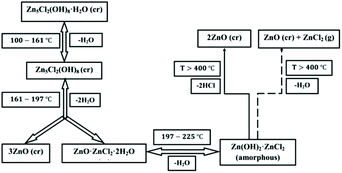
From the data obtained in the experiments described above, we can now propose a mechanism for the thermal decomposition of Zn5(OH)8Cl2·H2O to ZnO, Scheme 1. Importantly, this mechanism accounts for the moisture dependent pathway that may lead to sub-optimal yields of ZnO.
Incidentally, in the current work the presence or absence of the zinc oxychloride species Zn2OCl2 was not confirmed. This compound had been reported by others24 and thus we cannot discount a side reaction involved in the system according to reaction (9).
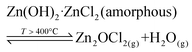 | (9) |
Conclusions
In this work, we examined the thermal decomposition of zinc hydroxide chloride monohydrate, Zn5(OH)8Cl2·H2O, into ZnO and the sequence of changes occurring in the process. Crystalline zinc oxide particles with BET specific surface area of 1.3 m2 g−1 were formed by thermal decomposition of zinc hydroxide chloride monohydrate sheets. The specific surface area of the resultant zinc oxide is lower than that of the commercially available French process ZnO (∼3–5 m2 g−1). The mechanism of decomposition of zinc hydroxide chloride monohydrate was examined in detail and found to proceed via the formation of ZnO·ZnCl2·2H2O and ZnCl2 intermediates. The formation of anhydrous zinc chloride under moisture-free conditions was demonstrated by collection and post analysis of material deposited on cooler areas of the reaction apparatus. Therefore, the discrepancies in the literature can be resolved by noting that the mechanism of conversion of zinc hydroxide chloride monohydrate to zinc oxide is dependent on the relative humidity of the atmosphere under which the process occurs and the degree to which the ZnCl2 intermediate is hydrolysed to ZnO.Acknowledgements
This work was supported by PT. Indo Lysaght (Indonesia). We also thank Mr Jean-Pierre Guerbois (TGA-MS), Dr R. Wuhrer (scanning electron microscopy) Ms Verena Schrameyer (translation of German papers) and the Australian Synchrotron, Melbourne, Victoria (Powder Diffraction beamline).Notes and references
- A. Moezzi, A. M. McDonagh and M. B. Cortie, Chem. Eng. J., 2012, 185–186, 1–22 CrossRef CAS.
- K. Sokołowski, I. Justyniak, W. Bury, J. Grzonka, Z. Kaszkur, Ł. Ma˛kolski, M. Dutkiewicz, A. Lewalska, E. b. Krajewska, D. Kubicki, K. Wûjcik, K. J. Kurzydłowski and J. Lewinski, Chem. – Eur. J., 2015, 21, 5488–5495 CrossRef PubMed.
- A. Moezzi, M. Cortie, A. Dowd and A. McDonagh, J. Nanopart. Res., 2014, 16, 2344 CrossRef.
- M. Sorel, Comptes rendus hebdomadaires des séances de l'Académie des sciences, 1855, 41, 784–785 Search PubMed.
- O. K. Srivastava and E. A. Secco, Can. J. Chem., 1967, 45, 579–583 CrossRef CAS.
- J. W. Hoffman and I. Lauder, Aust. J. Chem., 1968, 21, 1439–1443 CrossRef CAS.
- C. A. Sorrell, J. Am. Ceram. Soc., 1977, 60, 217–220 CrossRef CAS.
- I. Rasines and J. I. Morales de Setién, Thermochim. Acta, 1980, 37, 239–246 CrossRef CAS.
- O. Garcia-Martinez, E. Vila, J. L. Martin de Vidales, R. M. Rojas and K. Petrov, J. Mater. Sci., 1994, 29, 5429–5434 CrossRef CAS.
- M. Krunks, J. Madarász, T. Leskelä, A. Mere, L. Niinistö and G. Pokol, J. Therm. Anal. Calorim., 2003, 72, 497–506 CrossRef CAS.
- W. Zhang and K. Yanagisawa, Chem. Mater., 2007, 19, 2329–2334 CrossRef CAS.
- H. Tanaka and A. Fujioka, Mater. Res. Bull., 2010, 45, 46–51 CrossRef CAS.
- T. Kozawa, A. Onda, K. Yanagisawa, A. Kishi and Y. Masuda, J. Solid State Chem., 2011, 184, 589–596 CrossRef CAS.
- A. Shaporev, V. Ivanov, A. Baranchikov, O. Polezhaeva and Y. Tret'yakov, Russ. J. Inorg. Chem., 2007, 52, 1811–1816 CrossRef.
- A. Moezzi, M. Cortie and A. McDonagh, Dalton Trans., 2011, 40, 4871–4878 RSC.
- A. Moezzi, A. McDonagh, A. Dowd and M. Cortie, Inorg. Chem., 2013, 52, 95–102 CrossRef CAS PubMed.
- A. Moezzi, M. B. Cortie and A. M. McDonagh, Dalton Trans., 2013, 42, 4432–14437 RSC.
- D. M. Schubert, US Patent, 5l342553, 1994 Search PubMed.
- Y.-H. Gao, Z.-H. Liu and X.-L. Wang, J. Chem. Thermodyn., 2009, 41, 775–778 CrossRef CAS.
- V. Rives, Mater. Chem. Phys., 2002, 75, 19–25 CrossRef CAS.
- F. M. Labajos, V. Rives and M. A. Ulibarri, J. Mater. Sci., 1992, 27, 1546–1552 CrossRef CAS.
- M. K. Ram Reddy, Z. P. Xu, G. Q. Lu and J. C. Diniz da Costa, Ind. Eng. Chem. Res., 2006, 45, 7504–7509 CrossRef CAS.
- J. E. House and K. A. House, Descriptive Inorganic Chemistry, Academic Press, London, 2010 Search PubMed.
- S. H. Son and F. Tsukihashi, J. Phys. Chem. Solids, 2005, 66, 392–395 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD and TGA data, additional SEM images, a schematic of the sublimation apparatus. See DOI: 10.1039/c5dt04864h |
| This journal is © The Royal Society of Chemistry 2016 |