Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Transfer hydrogenation with abnormal dicarbene rhodium(III) complexes containing ancillary and modular poly-pyridine ligands†
Kevin
Farrell
a,
Philipp
Melle
a,
Robert A.
Gossage
bc,
Helge
Müller-Bunz
a and
Martin
Albrecht
*ab
aSchool of Chemistry, University College Dublin, Belfield, Dublin 4, Ireland
bDepartement für Chemie und Biochemie, Universität Bern, Freiestrasse 3, CH-3012 Bern, Switzerland. E-mail: martin.albrecht@dcb.unibe.ch; Tel: +41316314644
cDepartment of Chemistry & Biology, Ryerson University, 350 Victoria St., Toronto, ON M5B 2K3 Canada
First published on 28th January 2016
Abstract
Treatment of an abnormal dicarbene ligated rhodium(III) dimer with 2,2′-bipyridine (bipy), 1,10-phenanthroline (phen) or 2,2′:6′,2′′-terpyridine (terpy) results in coordination of the N-donor ligands and concomitant cleavage of the dimeric structure. Depending on the denticity of the pyridyl ligand, this situation retains one (L = terpy) or two (L = bipy, phen) flexible sites for substrate coordination. In the case of the bipy complexes, modification of the electron density at Rh, without directly affecting the steric environment about the metal centre, was achieved by the incorporation of electron-donating or electron-withdrawing substituents on the bipy backbone. The dicarbene pyridyl complexes were active in transfer hydrogenation catalysis of benzophenone at 0.15 mol% catalyst loading in a iPrOH/KOH mixture. The catalysts displayed a strong characteristic colour change (yellow to purple) after activation which allowed for visual monitoring of the status of the reaction. The colour probe and the robustness of the active catalysts proved useful for catalyst recycling. The catalytic activity sustained over five consecutive substrate batch additions and gave a maximum overall turnover number of 3100.
Introduction
Poly-pyridines and in particular 2,2′-bipyridine (bipy) and 2,2′,6′,2′′-terpyridine (terpy) have been extensively explored as ligands for transition metals, metalloids and main group elements due to the attractive features they impart onto the coordinated centre.1 As ligands, they have numerous applications in catalysis,2 such as C–H borylations,3 alcohol4 and/or water oxidation,5 the aldehyde–water shift reaction,6 and simple hydrogenation chemistry.7 The pyridyl groups can be electronically modified by incorporation of electron-donating (EDGs) or electron-withdrawing substituents (EWGs) at, for example, the 4,4′-position of bipy. Such substitution offers an approach to tune the properties of the metal centre and thus a flexible handle on catalyst optimisation. With the ongoing development of poly-pyridines and their relatives as privileged ligands for homogeneous catalysis, and with the emergence of N-heterocyclic carbenes (NHCs) as a versatile class of strong σ-donating ligands for transition metals,8 a combination of the two scaffolds is a logical field for investigation. For example, the replacement of one or more of the pyridine units with an NHC fragment,9 such as in bipy10 and terpy11 ligand systems, has been investigated. Another strategy involves fusing an NHC onto a pyridyl group such as that of a phenanthroline12 or bipyridine13 unit, which gives inter alia the CNN analogue of a catalytically very active PNN pincer-type ligand. Complexes incorporating these ligands, specifically complexes of Ru, are active catalysts for the hydrogenation of amides to alcohols.14 Much work in ruthenium polypyridine and NHC chemistry has surely been spurred by the advances made in both materials development and photochemistry. This latter aspect is a result of the fact that pyridyl-bound metal centres are, in particular, very useful for the conversion of light to energy and for the fabrication of supramolecular assemblies.15 Some recent reviews have covered the use of NHC ligands in transition metal complexes as components for materials science applications.16 While fused pyridyl-NHC metal complexes have attracted much interest,10,11,17 pyridyl and NHC ligands bound independent of each other to the metal centre have been less investigated.18 Based on the established impact of poly-pyridine ligands, we were interested in exploring pyridyl donors as ligands in conjunction with a chelating C4-diimidazolylidene rhodium(III) scaffold for transfer hydrogenation catalysis.19 Such abnormal or mesoionic ligands20 are strongly donating and have shown to enhance the catalytic activity of the metal center in several cases.21 In the set-up designed here, the chelating dicarbene and bipyridyl ligands bound to Rh leave two flexible sites available for possible hydride ligation and substrate interaction. On the other hand, terpy typically binds in a meridional κ3-N,N′,N′′ coordination mode, which would leave only a single site available for substrate interaction, likely situated trans to one carbene bonding site. The strong trans effect of the carbene offers potential to enhance activity, such as by accelerating the product dissociation step in the catalytic cycle. Thus, we aimed at exploring bipy and terpy as ligands to furnish an active Rh(dicarbene) scaffold for transfer hydrogenation catalysis.Results and discussion
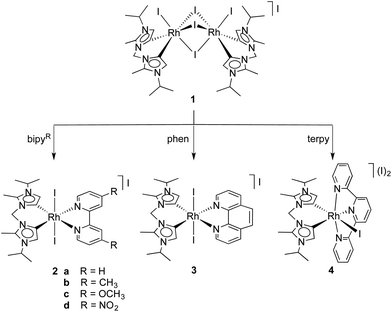
Synthesis of dicarbene pyridyl complexes
Compound 1, a mono-cationic formal [Rh3+]2 dimeric complex, was synthesised as previously described in the literature.22 Coordination of bipy, phen or terpy resulted in cleavage of the dimeric structure of 1 to give the bench-stable monometallic species 2, 3, and 4, respectively (Scheme 1).The 1H NMR spectroscopic data indicated only one set of bipy signals for 2 which suggests a C2v-symmetric complex in solution, in which the bipy N-atoms are in trans position to the carbene carbons. The 1H NMR signals of the bipy display characteristic downfield shifts due to the reduced electron density about bipy when donating electron density to the Rh centre. For example, the protons ortho to the bipy nitrogens shift from δH = 8.66 (free bipy) to 9.35 ppm in 2a (both in CD2Cl2). The NCH2N bridge and the iPr methyl groups of the carbene ligand in complexes 2a–d appear as broad signals at room temperature (RT) indicating slow inversion of the boat-like metallacycle on the NMR timescale. The signals were well resolved on cooling the sample to −20 °C and completely coalesce to a broad singlet upon heating to about 60 °C (CD3CN). From the coalescence temperature (Tc), the free energy of inversion (ΔG‡) for the dicarbene metallacycle was estimated at 63 ± 1 kJ mol−1 for 2a and identical within experimental error at 62 ± 1, 61 ± 1 and 61 ± 1 kJ mol−1 for 2b–d, respectively. Calculating the free energy of inversion based on the Tc of the iPr methyl groups gave values within the standard deviation and are thus identical to the ΔG‡ calculations based on the coalescence of the NCH2N resonances. The substituent on the bipy backbone has a direct effect on the chemical shift of the bipy protons. The ortho bipy protons of 2c (δH = 9.12), with the electron-donating methoxy group, are more shielded than for 2a (δH = 9.38) while those of 2d, with electron-withdrawing –NO2 substituents are significantly more deshielded (δH = 9.70; CD3CN).
Tuning of the electron density about Rh, by incorporating electron-donating or electron-withdrawing groups at the para position of the pyridine ring was suggested by NMR spectroscopic analyses of the Cimid–H proton resonances (Table 1). Albeit weak, the observed shift differences are diagnostic. Thus when compared to 2a (δH = 7.29), this signal shifts upfield when an electron-donating substituent is incorporated (methyl; methoxy: δH = 7.26; 7.23), and it is more deshielded in 2d featuring an electron-withdrawing nitro group (δH = 7.32). Similarly, the 13C NMR signals for the metal-bound carbenic nucleus Cimid–Rh were slightly more deshielded in 2b and 2c (δC = 137.6, R = Me, and 137.9, R = OMe, respectively) when compared to 2a (δC = 137.4). This nucleus is considerably more shielded in 2d (δC = 135.3). These data suggest that the electronics of the Rh centre can be finely modulated without affecting the steric environment about the metal center. Interestingly, though, the Rh–C coupling constants were not sensitive to the modulated donor properties of the trans-positioned bipy. Much like the activation energy for metallacycle inversion (see above), no clear trend was apparent in complexes 2a–d for the Rh–C coupling constants (JCRh = 36.9 ± 0.4 Hz).
| Complex | Cimid–H (ppm) | Cimid (ppm) | J CRh (Hz) | ΔGav (kJ mol−1) |
|---|---|---|---|---|
| 2a | 7.29 | 137.4 | 36.5 | 63 ± 1 |
| 2b | 7.26 | 137.6 | 36.8 | 62 ± 1 |
| 2c | 7.23 | 137.9 | 36.8 | 61 ± 1 |
| 2d | 7.32 | 135.3 | 37.3 | 61 ± 1 |
The phen complex 3 showed generally more deshielded 1H NMR resonances compared to those of 2a, including a lower field signal for the Cimid–H protons (δH = 7.42). The NCH2N and iPr methyl proton signals also appeared as broad multiplets at RT indicating slow inversion of the dicarbene metallacycle.
For the terpyridine complex 4, desymmetrisation of the complex was apparent by the two inequivalent sets of 1H and 13C NMR signals for the dicarbene ligand. For example, the two carbene C-atoms for 4 resonate at δC = 141.0 (1JCRh = 38.5 Hz) and 133.2 (1JCRh = 42.1 Hz) ppm. Likewise, in the 1H NMR spectrum, the C–CH3 signals appeared as two singlets at δH = 2.91 and 2.61 each integrating for three protons. The Cimid protons were significantly separated (δH = 7.68, 5.89). The higher field resonance indicates a significant shielding effect of the terpy π-system on the proximal imidazolylidene proton, while the more distant imidazolylidene proton showed a more typical resonance value. Inversion of the metallacycle is more facile in 4 than in complexes 2a–d since the methylene linker appears as a singlet resonance at δH = 6.02.
X-ray crystallography
Structural data was obtained for complexes 2a, 2b and 4 from X-ray diffraction studies (Fig. 1) which unambiguously confirmed that both bipy N-atoms are trans to a carbene in complexes 2a/b. Selected bond lengths and angles are shown in Table 2. For these two compounds, the Rh atom resides in a slightly distorted octahedral geometry comprised of the dicarbene ligand, bipy and two iodido ligands in a mutually trans arrangement. The dicarbene rhodacycle adopts a boat-type geometry in the solid state. The average Rh–C bond length of 2.013 Å is within the expected range,23 and slightly longer than that found in related Rh(III) complexes which bear two acetonitrile ligands instead of a chelating bipy (average Rh–C = 1.985 Å).22 The Rh–N bond lengths for 2b are slightly longer than those of 2a perhaps pointing to an electronic effect of the more electron-donating 4,4′-Me2-bipy ligand. For 4, the meridionally binding terpy ligand restricts coordination of the iodido ligand to the position trans to the carbene. The Rh–I bond length of 2.7464(5) Å is longer than those of 2 (average Rh–I = 2.670 Å), highlighting the strong trans influence of the carbene ligand. The Rh–C bond lengths are identical within standard deviations and thus suggest that the iodine and the pyridine N-atoms have a similar trans influence.24 Meridional binding of the terpy ligand in 4 allows for an almost ideal dicarbene bite angle to Rh (C–Rh–C 87.0(2)°). However, distortion from octahedral geometry is apparent by the N5–Rh–N7 angle of 159.73(19)°. Of note, the proton bound to C10 is rather close to the central pyridyl ring (H10⋯Cgpyr 3.30 Å), which is in agreement with a strong shielding of that proton in the 1H NMR spectrum due to ring current effects.| 2a (X = I2) | 2b (X = I2) | 4 (X = N7) | |
|---|---|---|---|
| Rh–C1 | 2.011(2) | 2.024(5) | 2.026(5) |
| Rh–C9 | 2.006(2) | 2.011(2) | 2.011(5) |
| Rh–N5 | 2.1273(18) | 2.145(4) | 2.062(5) |
| Rh–N6 | 2.1263(17) | 2.131(4) | 1.998(4) |
| Rh–I1 | 2.6543(2) | 2.6505(5) | 2.7464(5) |
| Rh–X | 2.6810(2) | 2.6943(5) | 2.066(5) |
| C1–Rh–C9 | 84.14(8) | 85.31(18) | 87.0 (2) |
| C1–Rh–N6 | 174.95(8) | 175.56(18) | 177.8(2) |
Catalytic transfer hydrogenation
The activity of complexes 2, 3 and 4 in hydrogen transfer catalysis was evaluated using 2-propanol as the formal H2 donor and benzophenone as a model acceptor (Table 3).25 Typically, the active catalyst was formed by heating the precursor complex in basic iPrOH to reflux temperature for 10 min before benzophenone was added. At 0.15 mol% catalyst loading, most imine complexes showed enhanced catalytic activity when compared to dimer 1. The latter gave 50% conversion after 2 h and did not reach full conversion after 24 h (82%, entry 1). Complex 2a and 2b were significantly more active and afforded hydrogenated diphenylmethanol in quantitative yields after 2 and 3 h, respectively (entries 2 and 3). Tuning of the catalytic activity was expected by incorporation of electronically active groups on the bipy backbone. Intriguingly, modification of the bipy by incorporating electron-donating or withdrawing groups resulted in poorer conversions than 2a (which contains a H-atom in the bipy para positions). A methyl group at the para position of the pyridine ring slowed the catalysis compared to 2a which is particularly evident at early onset reaction times (entry 3). Much more detrimental was heteroatom substitution at the 4,4′-position of bipy as shown by 2c and 2d, which both gave only very poor conversion values (entries 4 and 5). The more rigid diimine system 3 led to a catalyst with similar activity to 2a (cf. entry 6). In contrast, complex 4 containing a terpy ligand was slower than 2a and showed essentially the same activity as the imine free dimer 1 and full conversion was only reached at prolonged reaction times (entry 7). Presumably the availability of only one site for substrate coordination decelerates catalytic turnovers. It is worth noting that while the diimine ligand does accelerate the transfer hydrogenation catalysis (cf. entries 1 and 2), the activity is less enhanced than when incorporating diphosphine ligands.26 Related rhodium systems containing a dicarbene ligand scaffold are considerably less active.27| Entry | [Rh] | Mol% [Rh] | Conversion | TON | ||
|---|---|---|---|---|---|---|
| 0.5 h | 2 h | 3 h | ||||
| a General conditions: benzophenone (3 mmol), iPrOH (15 mL), KOH (0.3 mmol), reflux temperature. KOH was added as a 2 M aqueous solution; conversions and yields identical, averages of at least two runs with deviations of max ±3% except entry 5, deviation max 10%. b Conversion after 24 h. | ||||||
| 1 | 1 | 0.15 | 26 | 50 | 82b | 330 |
| 2 | 2a | 0.15 | 26 | 97 | 99 | 660 |
| 3 | 2b | 0.15 | 9 | 70 | 97 | 650 |
| 4 | 2c | 0.15 | 8 | 34 | 50 | 330 |
| 5 | 2d | 0.15 | 17 | 47 | 66 | 440 |
| 6 | 3 | 0.15 | 22 | 97 | 100 | 670 |
| 7 | 4 | 0.15 | 22 | 56 | 93b | 620 |
| 8 | — | — | <2 | <2 | <2 | — |
Interestingly, formation of the catalytically active species during the substrate-free initiation period was indicated by a distinct colour change of the reaction mixture from yellow to deep purple (complexes 2 and 3, Fig. 2) or bright green (for 4). When using the less active complexes 2c and 2d, a colour change to reddish/brown was observed after heating to reflux in the presence of KOH (10 min.). After full conversion of benzophenone, the colour of the reaction mixtures remained purple for some time, and changed back to yellow only after extended periods of stirring. The same colour change was observed upon cooling the reaction mixtures at partial conversion. With further heating (to induce full conversion), the purple colour was again established. These observations suggest that the purple colour is associated with a compound that only persists at elevated temperatures and that is related to the catalytically active species. The yellow colour is thus diagnostic of a dormant state of the catalyst derived from 2a, while the purple species is correlated with catalytic activity. This colour change reveals a useful diagnostic tool to evaluate the status of the catalytic reaction for all the complexes 2–4. Accordingly, the catalyst remains in its active form even after full consumption of the substrate, but gradually deactivates after full conversion, as indicated by the colour change from purple back to yellow after extended periods of stirring (>10 h) when full conversion was reached. Presumably, evaporation of acetone prohibits a steady state hydrogen transfer equilibrium.
 | ||
| Fig. 2 Colour change of solutions containing the dormant catalytically inactive form derived from complex 2a (a; yellow solution), and the catalytically active form (b; purple solution). | ||
To further investigate the nature of the active species, a series of temperature- and solvent-dependent experiments were performed to identify the source of the colour change. The purple species was only observed in solvents such as iPrOH and cyclohexanol which contain a H-atom α to that of the alcohol group. When using tBuOH, with KOH as base, or in 1,2-dichloroethane at reflux temperature, no colour change of 2a nor any catalytic hydrogen transfer was observed. Similarly, no colour change was observed when 2a was refluxed in iPrOH without KOH. Therefore, the colour change is probably not associated with the formation of an alkoxide species and may originate instead from a hydride complex that is formed after β-hydride elimination of the alkoxide.28 Indeed, catalytic transfer hydrogenation with 2a can also be carried out with cyclohexanol as the H2 source. At 160 °C, 24% conversion of benzophenone to diphenylmethanol was reached after 4 h and the solution mixture was again purple in colour. In a parallel run in cyclohexanol at 110 °C (i.e., the typical temperature used for transfer hydrogenation with iPrOH), the mixture remained yellow throughout and was essentially inactive (<5% conversion after 4 h). These temperature dependent studies support the fact that the purple species only forms at vigorous reflux, possibly to advance the β-hydride elimination from the alkoxide by removal of the corresponding ketone.
The colour–activity relationship and the robustness of the active species derived from 2a were utilised in a recycling experiment. To this end, a reaction was launched with the same substrate/base/catalyst ratio (6 mmol benzophenone, 0.15 mol% 2a, 30 mL iPrOH) and every 2.5 hours, conversions were determined and a fresh batch of benzophenone (6 mmol) was added (Fig. 3). The conversions, were high for each addition of benzophenone (typically >90%) and the reaction mixture remained active and purple in colour over five consecutive additions of substrate. The fifth batch was stirred for 14 rather than just 2.5 h, and while this extended reaction time did not affect yields (overall >92%), the colour of the solution changed back to yellow during this period. Addition of a sixth batch of benzophenone did not lead to significant conversions anymore and the catalytic activity effectively ceased. Thus, an overall yield of 92% (30 mmol benzophenone) and a turnover number (TON) of 3100 over five batches of benzophenone was achieved with 2a. Similarly high turnovers have been obtained with rhodium complexes containing specific carbene spectator ligands.29
Typically, the status of catalytic reactions can rarely be determined visually by simply monitoring the reaction mixture. For example, the imine-free complex 1 or related dicarbene Rh(III) complexes containing diphosphine spectator ligands displayed no colour changes throughout the same transfer hydrogenation catalysis.26 The colour change observed in this work appears to be specific to complexes 2, 3 and 4 and probably involves in some way the pyridyl ligand, perhaps by a hydride migration process. Chirik and co-workers have observed ligand-centred radicals and migration of hydrides and alkyl groups to the 4-position of a metal-bound pyridine unit in a cobalt complex.30 The lower conversions of the catalysis when incorporating substituents, whether electron-donating or -withdrawing, on the 4,4′-positions of the bipy ligand (2b–d) is unexpected and may hint at a similar involvement of the pyridine unit in complexes 2a–d. Hence, hydride migration from the Rh centre to the bipy may occur and may be more sterically hindered in the case of 2b–c when compared to that of 2a. In an attempt to probe such a migration, a catalytic run with the deuterated hydrogen donor (CD3)2CDOH as solvent was carried out. Approximately 91% deuterium incorporation was noted at the carbinol carbon of the formed diphenylmethanol, thus indicating a monohydride mechanism for the hydrogen transfer.31 The Rh species recovered at the end of the process did not reveal any deuterium incorporation into the aromatic region (2H NMR spectroscopy). In another attempt to determine if migration to the 4-position of bipy was occurring, a standard catalytic run was set up with 2a and using 1,1-diphenylethylene as substrate which may migrate to the pyridyl ligand.30 While full conversion to the corresponding alkane was observed after 14 h, no migrated pyridyl-bound alkyl species was detected by either NMR spectroscopy or mass spectrometry. Even though these experiments thus do not provide any further mechanistic insights, they demonstrate that complex 2a is also an efficient catalyst for transfer hydrogenation of hydrocarbon olefins (Scheme 2).32 Hence, bipy coordination is an attractive extension for Rh-catalysed hydrogen transfer catalysis as this ligand enhances the catalytic performance of the metal centre and offers a convenient visual indicator of the catalytic activity.
Conclusions
A series of air-stable pyridyl coordinated formally Rh(III) dicarbene complexes, bearing bipy, phen or terpy, have been synthesised in high yields and utilised for transfer hydrogenation in iPrOH/KOH. Pyridyl ligand incorporation enhances the activity of the dicarbene rhodium(III) system for this hydrogenation involving benzophenone at low catalyst loadings (0.15 mol%). The active species display a visual signature for determining the status of the reaction based on a vivid colour change (yellow to purple or green) upon activation. Temperature and solvent-dependent studies indicate that the colour change is probably associated with a Rh–H species formed following β-hydride elimination of a coordinated alkoxide at elevated temperatures during the catalytic cycle. The correlation between the colour of the reaction and the catalytically active species is unusual and offers further potential in catalysis investigations. For example, sampling of the catalytic reaction is often disruptive to the reaction rate and may be circumvented using the UV-Vis properties rather than intrusive sampling. The colour of the reaction enables a visual determination of whether the catalytically active species still persists and is thus particularly useful for recycling experiments. Incorporation of aliphatic diamines or diimines to the dicarbene Rh(III) platform, rather than aromatic diimines reported here, may shed further light on the source of the colour change on catalyst activation.Experimental section
General comments
All syntheses were carried out under ambient conditions unless otherwise stated and all reagents and solvents were used as received from commercial suppliers. The diimidazolium salt and complex 1 were prepared according to literature procedures.22 Unless specified, 1H and 13C{1H} NMR spectra were recorded at 25 °C on Varian spectrometers operating at 300, 400, 500 or 600 MHz (1H NMR) and 101, 126 or 151 MHz (13C NMR) respectively. Chemical shifts (δ in ppm, coupling constants J in Hz) were referenced to residual solvent signals (1H, 13C). Assignments are based on homo- and heteronuclear shift correlation spectroscopy. Elemental analyses were performed at UCD Microanalytic Laboratory using an Exeter Analytical CE-440 elemental analyser. High-resolution mass spectrometry was carried out with a Micromass/Waters Corp. USA liquid chromatography with an electrospray source. RT is 18 °C unless otherwise stated.General procedure for the synthesis of complexes 2a–d, 3 and 4
The dimeric complex 1 was dissolved in CH2Cl2 (20 mL) and the relevant pyridyl compound was added (2.2 equiv.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 equiv. per Rh atom). The solution was stirred for 16 h at RT. The resulting suspension was added to a centrifuge vessel with Et2O (80 mL) and the precipitate was then isolated by centrifugation and decantation. The orange residue was re-dissolved (CH2Cl2: 10 mL) and precipitated again with Et2O (80 mL). Following centrifugation and decantation of the supernatant, the recovered residue was dried under reduced pressure to yield the pure product.
1.1 equiv. per Rh atom). The solution was stirred for 16 h at RT. The resulting suspension was added to a centrifuge vessel with Et2O (80 mL) and the precipitate was then isolated by centrifugation and decantation. The orange residue was re-dissolved (CH2Cl2: 10 mL) and precipitated again with Et2O (80 mL). Following centrifugation and decantation of the supernatant, the recovered residue was dried under reduced pressure to yield the pure product.
1H NMR (500 MHz, CD3CN): δ = 9.73 (dd, 2H, 3JHH = 5.1 Hz, 4JHH = 1.4 Hz, Hphen), 8.70 (dd, 2H, 3JHH = 8.2 Hz, 4JHH = 1.4 Hz, Hphen), 8.21 (s, 2H, Hphen), 8.09 (dd, 2H, 3JHH = 8.2 Hz, 4JHH = 5.1 Hz, Hphen), 7.42 (s, 2H, Himid), 7.11, 6.24 (br, 1H, NCH2N), 4.63 (septet, 3JHH = 6.7 Hz, CH(Me)2), 2.77 (s, 6H, Cimid–CH3), 1.69, 1.48 (br, 6H, NCH(CH3)2). 13C NMR (253 K, 151 MHz, CD3CN): δ = 152.9 (Cphen–H), 147.5 (Cphen), 142.3 (NCimidN), 138.6 (Cphen–H), 137.6 (d, 1JCRh = 37.2 Hz, Cimid–Rh), 131.6 (Cphen), 128.4 (Cphen–H), 126.4 (Cphen–H), 124.4 (Cimid–H), 61.9 (NCH2N), 51.2 (NCH(Me)2), 22.6 (NCH(CH3)2), 11.0 (Cimid–CH3). ESI-MS (m/z): 796.9936, calcd for [M − I]+ 796.9959. Anal. calcd for C27H32I3N6Rh (924.20): C, 35.09; H, 3.49; N, 9.09. Found: C, 34.60; H, 3.19; N, 8.79.
1H NMR (400 MHz, (CD3)2SO): δ = 8.90 (d, 2H, 3JHH = 8.1 Hz, Hterpy), 8.78 (d, 2H, 3JHH = 7.9 Hz, Hterpy), 8.66 (t, 1H, 3JHH = 8.1 Hz, Hterpy), 8.43 (d, 2H, 3JHH = 5.7 Hz, Hterpy), 8.28 (td, 2H, 3JHH = 7.9 Hz, 4JHH = 1.2 Hz, Hterpy), 7.68 (s, 1H, Himid), 7.64–7.59 (m, 2H, Hterpy), 6.02 (s, 2H, NCH2N), 5.89 (s, 1H, Himid), 4.83 (septet, 1H, 3JHH = 6.7 Hz, NCH(Me)2), 4.34 (septet, 1H, 3JHH = 6.6 Hz, NCH(Me)2), 2.91 (s, 3H, Cimid–CH3), 2.61 (s, 3H, Cimid–CH3), 1.60, 1.01 (d, 6H, 3JHH = 6.6 Hz, NCH(CH3)2). 13C NMR (101 MHz, (CD3)2SO): δ = 157.4 (C Cterpy), 154.5 (Cterpy–H), 152.7 (Cterpy), 143.33 (NCimidN), 143.27 (NCimidN), 141.2 (C–Rh, 1JCRh = 38.5 Hz), 140.3 (Cterpy–H), 139.9 (Cterpy–H), 133.2 (C–Rh, 1JCRh = 42.1 Hz), 128.1 (Cterpy–H), 125.9 (Cterpy–H), 125.2 (Cimid–H), 124.7 (Cterpy–H), 114.2 (Cimid–H), 57.7 (NCH2N), 49.6, 49.5 (CH(Me)2), 22.3, 21.5 (CH(CH3)2), 10.5, 10.2 (Cimid–CH3). ESI-MS (m/z): 361.5526, calcd for [M − 2I]2+ 361.5527. Anal. calcd for C30H35I3N7Rh (977.26)·1CH2Cl2: C, 35.05; H, 3.51; N, 9.23. Found: C, 34.77; H, 3.45; N, 9.20.
Typical procedure for catalytic transfer hydrogenation
The precatalyst (4.5 μmol, 0.15 mol%) was weighed directly into the reaction vessel. To the vessel, iPrOH (15 mL) and KOH (0.15 mL of 2 M solution in H2O: 0.3 mmol) were added and the mixture was then heated to reflux temperature for 10 min. in an oil bath (110 °C). Subsequently, the substrate (3 mmol) was added directly and the reaction was sampled at set time intervals. The samples (0.2 mL) were quenched with cyclohexane (2 mL) and filtered through a short pad of Celite, which was then washed with Et2O (3 × 2 mL). The combined organic phases were evaporated to dryness (40 °C, 100 mbar) and then analysed by 1H NMR spectroscopy. Selected runs with hexamethylbenzene as internal standard revealed that spectroscopic conversion of benzophenone and yield of diphenylmethanol were identical under these conditions.Crystal structure determinations
Crystal data for 2a, 2b and 4 were collected using a Rigaku (former Agilent Technologies) Oxford Diffraction SuperNova A diffractometer fitted with an Atlas detector and using monochromated Mo-Kα radiation (0.71073 Å) (2a) or Cu-Kα (1.54184 Å) (2b, 4). A complete dataset was collected, assuming that the Friedel pairs are not equivalent. The structures were solved by direct methods using SHELXS-97 and refined by full-matrix least squares fitting on F2 for all data using SHELXL-97.33 Hydrogen atoms were added at calculated positions and refined by using a riding model. Anisotropic thermal displacement parameters were used for all nonhydrogen atoms except for the disordered isopropyl group in 2b. The solvents in 4 could not be modelled in terms of atomic size and the SQUEEZE option as incorporated in PLATON was used to compensate for the spread electron density.34 Crystallographic details are compiled in Tables S3 and S4.† Crystallographic data (excluding structure factors) for all three complexes have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC 1437163 (2a), 1437165 (2b), and 1437164 (4).Acknowledgements
This work has been financially supported by a NUI Traveling Scheme (K.F.), by the European Research Council (CoG 615653) and Science Foundation Ireland (RFP/2010/CHS2844).References
- (a) A. P. Sadimenko, in Advances in Heterocyclic Chemistry, ed. A. R. Katritzky, Elsevier, Amsterdam, 2009, p. 45 Search PubMed; (b) A. P. Smith and C. L. Fraser, in Comprehensive Coordination Chemistry II, ed. J. A. McCleverty and T. J. Meyer, Elsevier, Amsterdam, 2003, p. 1 Search PubMed; (c) R. P. Thummel, in Comprehensive Coordination Chemistry II, ed. T. J. Meyer and J. A. McCleverty, Elsevier, Amsterdam, 2003, p. 41 Search PubMed; (d) U. S. Schubert, A. Winter and G. R. Newkome, Terpyridine-Based Materials: for Catalytic, Optoelectronic and Life Science Applications, Wiley-VCH, Weinheim, 2011 Search PubMed; (e) U. S. Schubert, H. Hofmeier and G. R. Newkome, Modern Terpyridine Chemistry, Wiley-VCH, Weinheim, 2006 Search PubMed.
- G. Chelucci and R. P. Thummel, Chem. Rev., 2002, 102, 3129 CrossRef CAS PubMed.
- (a) T. Ishiyama, J. Takagi, K. Ishida, N. Miyaura, N. R. Anastasi and J. F. Hartwig, J. Am. Chem. Soc., 2002, 124, 390 CrossRef CAS PubMed; (b) I. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy and J. F. Hartwig, Chem. Rev., 2010, 110, 890 CrossRef CAS PubMed; (c) T. Ishiyama, J. Takagi, J. F. Hartwig and N. Miyaura, Angew. Chem., Int. Ed., 2002, 41, 3056 CrossRef CAS.
- R. Kawahara, K.-I. Fujita and R. Yamaguchi, J. Am. Chem. Soc., 2012, 134, 3643 CrossRef CAS PubMed.
- (a) J. D. Blakemore, N. D. Schley, D. Balcells, J. F. Hull, G. W. Olack, C. D. Incarvito, O. Eisenstein, G. W. Brudvig and R. H. Crabtree, J. Am. Chem. Soc., 2010, 132, 16017 CrossRef CAS PubMed; (b) J. DePasquale, I. Nieto, L. E. Reuther, C. J. Herbst-Gervasoni, J. J. Paul, V. Mochalin, M. Zeller, C. M. Thomas, A. W. Addison and E. T. Papish, Inorg. Chem., 2013, 52, 9175 CrossRef CAS PubMed; (c) D. C. Marelius, S. Bhagan, D. J. Charboneau, K. M. Schroeder, J. M. Kamdar, A. R. McGettigan, B. J. Freeman, C. E. Moore, A. L. Rheingold, A. L. Cooksy, D. K. Smith, J. J. Paul, E. T. Papish and D. B. Grotjahn, Eur. J. Inorg. Chem., 2014, 676 CrossRef CAS; (d) J. L. Cape, S. V. Lymar, T. Lightbody and J. K. Hurst, Inorg. Chem., 2009, 48, 4400 CrossRef CAS PubMed; (e) J. K. Hurst, J. L. Cape, A. E. Clark, S. Das and C. Qin, Inorg. Chem., 2008, 47, 1753 CrossRef CAS PubMed; (f) H. Yamada, W. F. Siems, T. Koike and J. K. Hurst, J. Am. Chem. Soc., 2004, 126, 9786 CrossRef CAS PubMed; (g) R. A. Binstead, C. W. Chronister, J. Ni, C. M. Hartshorn and T. J. Meyer, J. Am. Chem. Soc., 2000, 122, 8464 CrossRef CAS; (h) J. J. Concepcion, J. W. Jurss, M. K. Brennaman, P. G. Hoertz, A. O. T. Patrocinio, N. Y. Murakami Iha, J. L. Templeton and T. J. Meyer, Acc. Chem. Res., 2009, 42, 1954 CrossRef CAS PubMed; (i) F. Liu, J. J. Concepcion, J. W. Jurss, T. Cardolaccia, J. L. Templeton and T. J. Meyer, Inorg. Chem., 2008, 47, 1727 CrossRef CAS PubMed.
- T. P. Brewster, W. C. Ou, J. C. Tran, K. I. Goldberg, S. K. Hanson, T. R. Cundari and D. M. Heinekey, ACS Catal., 2014, 4, 3034 CrossRef CAS.
- (a) W.-H. Wang, Y. Suna, Y. Himeda, J. T. Muckerman and E. Fujita, Dalton Trans., 2013, 42, 9628 RSC; (b) W. Li, J.-H. Xie, M.-L. Yuan and Q.-L. Zhou, Green Chem., 2014, 16, 4081 RSC; (c) P. Frediani, A. Salvini, M. Bessi, L. Rosi and C. Giannelli, Inorg. Chem. Commun., 2005, 8, 94 CrossRef CAS; (d) P. Frediani, L. Rosi, L. Cetarini and M. Frediani, Inorg. Chim. Acta, 2006, 359, 2650 CrossRef CAS; (e) O. Dayan, S. Dayan, İ. Kani and B. Çetinkaya, Appl. Organomet. Chem., 2012, 26, 663 CrossRef CAS.
- (a) F. E. Hahn and M. C. Jahnke, Angew. Chem., Int. Ed., 2008, 47, 3122 CrossRef CAS PubMed; (b) A. J. Arduengo and G. Bertrand, Chem. Rev., 2009, 109, 3209 CrossRef CAS PubMed; (c) D. Bourissou, O. Guerret, F. P. Gabbaï and G. Bertrand, Chem. Rev., 2000, 100, 39 CrossRef CAS PubMed; (d) N-Heterocyclic Carbenes in Transition Metal Catalysis, ed. F. Glorius, Topics in Organometallic Chemistry, Springer, Berlin, 2007 Search PubMed; (e) M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485 CrossRef CAS PubMed; (f) S. Díez-González, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612 CrossRef PubMed; (g) C. M. Crudden and D. P. Allen, Coord. Chem. Rev., 2004, 248, 2247 CrossRef CAS; (h) R. H. Crabtree, Coord. Chem. Rev., 2007, 251, 595 CrossRef CAS; (i) N-Heterocyclic Carbenes: from Laboratory Curiosities to Efficient Synthetic Tools, ed. S. Díez-González, RSC Publishing, Cambridge, 2011 Search PubMed; (j) N-Heterocyclic Carbenes in Transition Metal Catalysis and Organocatalysis, ed. C. S. J. Cazin, Springer, Berlin, 2011 Search PubMed.
- (a) K. Farrell and M. Albrecht, in The Privileged Pincer-Metal Platform: Coordination Chemistry & Applications, Topics in Organometallic Chemistry, ed. G. van Koten and R. A. Gossage, Springer, Berlin, 2016, p. 45 Search PubMed; (b) K. F. Donnelly, A. Petronilho and M. Albrecht, Chem. Commun., 2013, 49, 1145 RSC; (c) O. Schuster, L. Yang, H. G. Raubenheimer and M. Albrecht, Chem. Rev., 2009, 109, 3445 CrossRef CAS PubMed; (d) A. T. Normand and K. J. Cavell, Eur. J. Inorg. Chem., 2008, 2008, 2781 CrossRef.
- (a) V. Leigh, W. Ghattas, R. Lalrempuia, H. Müller-Bunz, M. T. Pryce and M. Albrecht, Inorg. Chem., 2013, 52, 5395 CrossRef CAS PubMed; (b) M. Delgado-Rebollo, D. Canseco-Gonzalez, M. Hollering, H. Mueller-Bunz and M. Albrecht, Dalton Trans., 2014, 43, 4462 RSC; (c) W.-C. Chang, H.-S. Chen, T.-Y. Li, N.-M. Hsu, Y. S. Tingare, C.-Y. Li, Y.-C. Liu, C. Su and W.-R. Li, Angew. Chem., Int. Ed., 2010, 49, 8161 CrossRef CAS PubMed; (d) O. Kaufhold, F. E. Hahn, T. Pape and A. Hepp, J. Organomet. Chem., 2008, 693, 3435 CrossRef CAS.
- (a) H.-J. Park, K. H. Kim, S. Y. Choi, H.-M. Kim, W. I. Lee, Y. K. Kang and Y. K. Chung, Inorg. Chem., 2010, 49, 7340 CrossRef CAS PubMed; (b) D. G. Brown, N. Sanguantrakun, B. Schulze, U. S. Schubert and C. P. Berlinguette, J. Am. Chem. Soc., 2012, 134, 12354 CrossRef CAS PubMed; (c) B. Schulze, D. Escudero, C. Friebe, R. Siebert, H. Goerls, U. Koehn, E. Altuntas, A. Baumgaertel, M. D. Hager, A. Winter, B. Dietzek, J. Popp, L. Gonzalez and U. S. Schubert, Chem. – Eur. J., 2011, 17, 5494 CrossRef CAS PubMed; (d) S. Sinn, B. Schulze, C. Friebe, D. G. Brown, M. Jäger, E. Altuntaş, J. Kübel, O. Guntner, C. P. Berlinguette, B. Dietzek and U. S. Schubert, Inorg. Chem., 2014, 53, 2083 CrossRef CAS PubMed; (e) D. G. Brown, P. A. Schauer, J. Borau-Garcia, B. R. Fancy and C. P. Berlinguette, J. Am. Chem. Soc., 2013, 135, 1692 CrossRef CAS PubMed; (f) T.-A. Koizumi, T. Tomon and K. Tanaka, Organometallics, 2003, 22, 970 CrossRef CAS; (g) L.-H. Chung, K.-S. Cho, J. England, S.-C. Chan, K. Wieghardt and C.-Y. Wong, Inorg. Chem., 2013, 52, 9885 CrossRef CAS PubMed; (h) C.-Y. Wong, L.-M. Lai, P.-K. Pat and L.-H. Chung, Organometallics, 2010, 29, 2533 CrossRef CAS.
- (a) S. Gu and W. Chen, Organometallics, 2009, 28, 909 CrossRef CAS; (b) S. Gu, B. Liu, J. Chen, H. Wu and W. Chen, Dalton Trans., 2012, 41, 962 RSC.
- (a) E. Balaraman, E. Fogler and D. Milstein, Chem. Commun., 2012, 48, 1111 RSC; (b) E. Fogler, E. Balaraman, Y. Ben-David, G. Leitus, L. J. W. Shimon and D. Milstein, Organometallics, 2011, 30, 3826 CrossRef CAS.
- E. Balaraman, B. Gnanaprakasam, L. J. W. Shimon and D. Milstein, J. Am. Chem. Soc., 2010, 132, 16756 CrossRef CAS PubMed.
- (a) A. Juris, V. Balzani, F. Barigelletti, S. Campagna, P. Belser and A. von Zelewsky, Coord. Chem. Rev., 1988, 84, 85 CrossRef CAS; (b) M. D. Purugganan, C. V. Kumar, N. J. Turro and J. K. Barton, Science, 1988, 241, 1645 CAS; (c) V. Balzani and A. Juris, Coord. Chem. Rev., 2001, 211, 97 CrossRef CAS; (d) L. Spiccia, G. B. Deacon and C. M. Kepert, Coord. Chem. Rev., 2004, 248, 1329 CrossRef CAS; (e) M. M. Richter, Chem. Rev., 2004, 104, 3003 CrossRef CAS PubMed; (f) E. C. Constable, Chem. Soc. Rev., 2007, 36, 246 RSC; (g) M. D. Ward, Chem. Commun., 2009, 4487 RSC; (h) W. J. Youngblood, S.-H. A. Lee, K. Maeda and T. E. Mallouk, Acc. Chem. Res., 2009, 42, 1966 CrossRef CAS PubMed; (i) A. J. McConnell, M. H. Lim, E. D. Olmon, H. Song, E. E. Dervan and J. K. Barton, Inorg. Chem., 2012, 51, 12511 CrossRef CAS PubMed; (j) L. Flamigni, J.-P. Collin and J.-P. Sauvage, Acc. Chem. Res., 2008, 41, 857 CrossRef CAS PubMed; (k) M. K. Nazeeruddin, E. Baranoff and M. Grätzel, Solar Energy, 2011, 85, 1172 CrossRef CAS; (l) M. K. Nazeeruddin and M. Grätzel, in Comprehensive Coordination Chemistry II, ed. T. J. Meyer and J. A. McCleverty, Elsevier, Amsterdam, 2003, p. 719 Search PubMed.
- (a) R. Visbal and M. C. Gimeno, Chem. Soc. Rev., 2014, 43, 3551 RSC; (b) L. Mercs and M. Albrecht, Chem. Soc. Rev., 2010, 39, 1903 RSC.
- A. T. Normand and K. J. Cavell, Eur. J. Inorg. Chem., 2008, 2781 CrossRef CAS.
- For selected recent examples, see: (a) L.-H. Chung, Inorg. Chem., 2012, 51, 8693 CrossRef CAS PubMed; (b) S. Conejero, P. Lara, M. Paneque, A. Petronilho, M. L. Poveda, O. Serrano, F. Vattier, E. Álvarez, C. Maya, V. Salazar and E. Carmona, Angew. Chem., Int. Ed., 2008, 47, 4380 CrossRef CAS PubMed; (c) L.-H. Chung, K.-S. Chot, J. England, S.-C. Chan, K. Wieghardt and C.-Y. Wong, Inorg. Chem., 2013, 52, 9885 CrossRef CAS PubMed; (d) M. Schmidtendorf, T. Pape and F. E. Hahn, Dalton Trans., 2013, 42, 16128 RSC; (e) R. Staehle, L. Tong, L. Wang, L. Duan, A. Fischer, M. S. G. Ahlquist, L. Sun and S. Rau, Inorg. Chem., 2014, 53, 1307 CrossRef CAS PubMed; (f) T. Duchanois, T. Etienne, M. Beley, X. Assfeld, E. A. Perpete, A. Monari and P. C. Gros, Eur. J. Inorg. Chem., 2014, 3747 CrossRef CAS; (g) M. A. Larsen, C. V. Wilson and J. F. Hartwig, J. Am. Chem. Soc., 2015, 137, 8633 CrossRef CAS PubMed; (h) S. A. Hauser, R. Tonner and A. B. Chaplin, Organometallics, 2015, 34, 4419 CrossRef CAS.
- (a) A. Krüger, A. Neels and M. Albrecht, Chem. Commun., 2010, 46, 315 RSC; (b) A. Krüger, L. J. L. Häller, H. Müller-Bunz, O. Serada, A. Neels, S. A. Macgregor and M. Albrecht, Dalton Trans., 2011, 40, 9911 RSC.
- For reviews, see: (a) P. L. Arnold and J. Pearson, Coord. Chem. Rev., 2007, 251, 596 CrossRef CAS; (b) O. Schuster, L. Yang, H. G. Raubenheimer and M. Albrecht, Chem. Rev., 2009, 109, 3445 CrossRef CAS PubMed; (c) M. Melaimi, M. Soleilhavoup and G. Bertrand, Angew. Chem., Int. Ed., 2010, 49, 8810 CrossRef CAS PubMed; (d) K. F. Donnelly, A. Petronilho and M. Albrecht, Chem. Commun., 2013, 49, 1145 RSC; (e) R. H. Crabtree, Coord. Chem. Rev., 2013, 257, 755 CrossRef CAS; (f) M. Albrecht, Adv. Organomet. Chem., 2014, 62, 111 CrossRef CAS.
- For selected examples, see: (a) M. Heckenroth, E. Kluser, A. Neels and M. Albrecht, Angew. Chem., Int. Ed., 2007, 46, 6293 CrossRef CAS PubMed; (b) L. Bernet, R. Lalrempuia, W. Ghattas, H. Müller-Bunz, L. Vigara, A. Llobet and M. Albrecht, Chem. Commun., 2011, 47, 8058 RSC; (c) D. Canseco-Gonzalez, A. Petronilho, H. Müller-Bunz, K. Ohmatsu, T. Ooi and M. Albrecht, J. Am. Chem. Soc., 2013, 135, 13193 CrossRef CAS PubMed; (d) J. A. Woods, R. Lalrempuia, A. Petronilho, N. D. McDaniel, H. Müller-Bunz, M. Albrecht and S. Bernhard, Energy Environ. Sci., 2014, 7, 2316 RSC; (e) B. Bagh, A. M. McKinty, A. J. Lough and D. W. Stephan, Dalton Trans., 2014, 43, 12842 RSC; (f) M. Delgado-Rebollo, D. Canseco-Gonzalez, M. Hollering, H. Müller-Bunz and M. Albrecht, Dalton Trans., 2014, 43, 4462 RSC; (g) P. K. Hota, G. Vijaykumar, A. Pariyar, S. C. Sau, T. K. Sen and S. K. Mandal, Adv. Synth. Catal., 2015, 357, 3162 CrossRef CAS; (h) P. Daw, R. Petakamsetty, A. Sarbajna, S. Laha, R. Ramapanicker and J. K. Bera, J. Am. Chem. Soc., 2014, 136, 13987 CrossRef CAS PubMed. See also: (i) E. Aldeco-Perez, A. J. Rosenthal, B. Donnadieu, P. Parameswaran, G. Frenking and G. Bertrand, Science, 2009, 326, 556 CrossRef CAS PubMed.
- L. Yang, A. Krüger, A. Neels and M. Albrecht, Organometallics, 2008, 27, 3161 CrossRef CAS.
- See for examples: (a) J. A. Mata, A. R. Chianese, J. R. Miecznikowski, M. Poyatos, E. Peris, J. W. Faller and R. H. Crabtree, Organometallics, 2004, 23, 1253 CrossRef CAS; (b) F. E. Hahn, C. Holtgrewe, T. Pape, M. Martin, E. Sola and L. A. Oro, Organometallics, 2005, 24, 2203 CrossRef CAS; (c) C. H. Leung, C. D. Incarvito and R. H. Crabtree, Organometallics, 2006, 25, 6099 CrossRef CAS; (d) A. R. Chianese, B. M. Zeglis and R. H. Crabtree, Chem. Commun., 2004, 2176 RSC; (e) M. Viciano, M. Feliz, R. Corberan, J. A. Mata, E. Clot and E. Peris, Organometallics, 2007, 26, 5304 CrossRef CAS.
- (a) J. Hartwig, Organotransition Metal Chemistry, University Science Books, Mill Valley, CA, 2010 Search PubMed; (b) R. H. Crabtree, The Organometallic Chemistry of the Transition Metals, Wiley-VCH, Weinheim, Germany, 6th edn, 2014 Search PubMed.
- (a) G. Zassinovich, G. Mestroni and S. Gladiali, Chem. Rev., 1992, 92, 1051 CrossRef CAS; (b) D. Klomp, U. Hanefeld and J. A. Peters, in The Handbook of Homogeneous Hydrogenation, ed. J. G. de Vries and C. J. Elsevier, Wiley-VCH, Weinheim, Germany, 2007, p. 585 Search PubMed; (c) S. Gladiali and E. Alberico, Chem. Soc. Rev., 2006, 35, 226 RSC; (d) D. Wang and D. Astruc, Chem. Rev., 2015, 115, 6621 CrossRef CAS PubMed.
- K. Farrell, H. Müller-Bunz and M. Albrecht, Organometallics, 2015, 34, 5723 CrossRef CAS.
- (a) M. Poyatos, W. McNamara, C. Incarvito, E. Clot, E. Peris and R. H. Crabtree, Organometallics, 2008, 27, 2128 CrossRef CAS; (b) N. B. Jokić, M. Zhang-Presse, S. L. M. Goh, C. S. Straubinger, B. Bechlars, W. A. Herrmann and F. E. Kühn, J. Organomet. Chem., 2011, 696, 3900 CrossRef; (c) V. Gierz, A. Urbanaite, A. Seyboldt and D. Kunz, Organometallics, 2012, 31, 7532 CrossRef CAS; (d) S. N. Sluijter and C. J. Elsevier, Organometallics, 2014, 33, 6389 CrossRef CAS.
- (a) S. E. Clapham, A. Hadzovic and R. H. Morris, Coord. Chem. Rev., 2004, 248, 2201 CrossRef CAS; (b) J. S. M. Samec, J.-E. Bäckvall, P. G. Andersson and P. Brandt, Chem. Soc. Rev., 2006, 35, 237 RSC.
- (a) M. Poyatos, E. Mas-Marza, J. A. Mata, M. Sanau and E. Peris, Eur. J. Inorg. Chem., 2003, 1215 CrossRef CAS; (b) A. Binobaid, M. Iglesias, D. Beetstra, A. Dervisi, I. Fallis and K. J. Cavell, Eur. J. Inorg. Chem., 2010, 5426 CrossRef CAS.
- R. P. Yu, J. M. Darmon, C. Milsmann, G. W. Margulieux, S. C. E. Stieber, S. DeBeer and P. J. Chirik, J. Am. Chem. Soc., 2013, 135, 13168 CrossRef CAS PubMed.
- O. Pamies and J.-E. Bäckvall, Chem. – Eur. J., 2001, 7, 5052 CrossRef CAS.
- S. Horn and M. Albrecht, Chem. Commun., 2011, 47, 8802 RSC.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details on variable temperature measurements, specific catalytic runs, and crystal structure determinations. CCDC 1437163 (2a), 1437165 (2b) and 1437164 (4). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5dt04656d |
| This journal is © The Royal Society of Chemistry 2016 |