Crystal phase competition by addition of a second metal cation in solid solution metal–organic frameworks†
Abstract
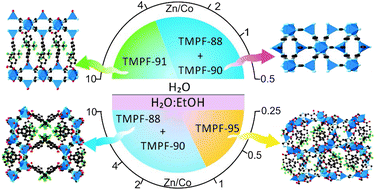
Herein we report a synthetic study focused on the preparation of solid-solution metal–organic frameworks, MOFs, with the use of two kinds of linkers. In particular, we have explored the system composed by zinc, cobalt, 1,2,4-triazole and 4,4′-hexafluoroisopropylidenebisbenzoic acid (H2hfipbb). During this study, four new MOFs have been isolated, denoted TMPF-88 [M3(hfipbb)2(triazole)2(H2O)], TMPF-90 [M2(triazole)3(OCH2CH3)], TMPF-91 [M2(hfipbb)(triazole)2(H2O)] and TMPF-95 [M5(hfipbb)4(triazole)2(H2O)] (TMPF = transition metal polymeric framework, M = Zn, Co, or mixture of them). The study demonstrates that the addition of a second metal element during the MOF synthesis has a major effect in the formation of new phases, even at very high Zn/Co metal ratios. Furthermore, we show that during the MOF formation reaction, there is a competition among different crystal phases, where kinetically favoured phases of various compositions crystallize in short reaction times, precluding the formation of the pure solid-solution phases of other energetically more stable MOFs.
- This article is part of the themed collection: Flexibility and Disorder in Metal-Organic Frameworks


 Please wait while we load your content...
Please wait while we load your content...