Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Neutral N-donor ligand based flexible metal–organic frameworks
Biplab
Manna†
,
Aamod V.
Desai†
and
Sujit K.
Ghosh
 *
*
Indian Institute of Science Education and Research (IISER), Pune. Dr. Homi Bhabha Road, Pashan, Pune, India 411008. E-mail: sghosh@iiserpune.ac.in; Fax: +91 20 2589 8022; Tel: +91 20 2590 8076
First published on 13th October 2015
Abstract
This short review gives a focussed discussion on metal–organic frameworks (MOFs) made of neutral N-donor ligands which show structural flexibility under various exogenous stimuli. Chemical stimuli such as presence of anions, free guests, coordinated guests and physical stimuli (light, heat and so on) render structural flexibility in MOFs. Single-crystal-to-single-crystal transformation studies have attracted a lot of attention for the understanding of such flexible MOF materials. Such a dynamic structural behavior with proper host–guest interactions gives very interesting functions such as chemical separation, sensing and magnetic properties and so on.
1. Introduction
Metal–organic frameworks (MOFs) or porous coordination polymers (PCPs) have emerged as a rapidly evolving class of porous materials and commanded significant research attention in the materials and solid-state chemistry fields over the last two decades.1 These porous crystalline solids are fabricated from bi- or multi-dentate organic linkers and held by metal-ion nodes in a periodic manner. The coordination bonded, self-assembled polymers are endowed with well-ordered porosity by virtue of a regularized arrangement of the building units in the infinite network. Because of the potential applications in the fields of storage, separation, sensing, and ionic conduction, MOFs have been particularly sought after in recent years.2–5The key distinction of MOFs over congener porous materials is the class of third generation MOFs or soft porous crystals, which are related to the enzymic flexibility in such materials. Several studies over the last decade have demonstrated the advantage of examining molecular motions in crystalline polymeric solids.6–8 The relatively easy access to characterize nano-scale rearrangements unambiguously renders a remarkable additional advantage to these systems. Because of the assigned softness, MOFs have been able to function as guest-responsive host matrices without compromising on the polymeric character.9–12
In pursuit of synthesizing pre-designed architectures in general and dynamic MOFs in particular, several donor groups have been investigated over the years. Among these groups, carboxylate and neutral N-heterocycle terminal ligands have been prominently preferred, with the former type dominating reports in the literature. Neutral N-donor linkers afford formation of the coordination bond leaving the maintenance of ionic equilibrium to the components of metal-salts used in the synthesis. This provides a simplistic route for the preparation of cationic frameworks where the counter-anion is left uncoordinated to the metal centre.13–19 Broadly, dynamic MOFs have relied on the flexibility possessed by the ligand and/or metal-ion with the reorganization primarily driven by desorption/resorption of neutral guest molecules, whereas N-donor ligand-based MOFs provide the additional aspect of enforcing an ionic stimuli (anion).
N-donor based ligands have been previously perceived as only pillars to increase the dimensionality in the carboxylate ligand linked frameworks.20,21 Research in recent years has shown the importance of such donor ligands in the domain of dynamic MOFs both in terms of understanding structural dynamism and probing structure–property correlation. This review focuses on the consolidation of the flexibility aspect in MOFs and the efficacy of N-donor ligands with representative examples concerning the structural aspects and the subsequent properties induced by the reorganization of the frameworks.
2. Scope of the review
The main focus of this particular review is to give an up-to-date report of flexible MOFs made of neutral N-donor ligands. A flexible MOF can be perturbed by various external stimuli (both chemical and physical) (Scheme 1).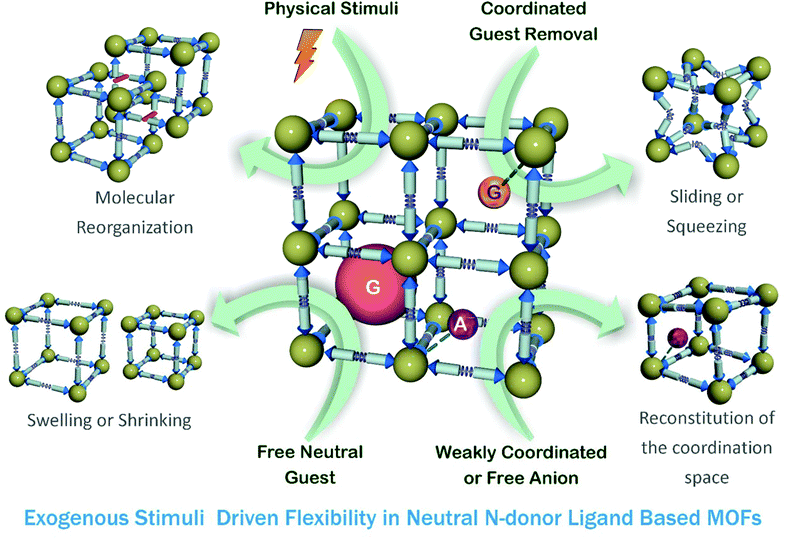 | ||
| Scheme 1 Various exogenous stimuli (chemical and physical) that render flexibility in neutral N-donor ligand based MOFs. | ||
The stimuli which render flexibility in N-donor ligand based MOFs are discussed in detail in this paper. In addition to this, different kinds of structure–property correlations are also thoroughly considered. It is worth noting that the neutral N-donor ligands are widely used classical ligands that can form MOFs upon binding to metal ions. Despite the availability of a few reviews on flexible MOF systems, the current topic of concern lacks such reports in the literature. Several papers and reviews discussed in this article might attract attention for further investigations in such flexible MOF systems for various functions.
3. Synthesis routes to construct flexible frameworks
Generally, the uncontrolled polymeric self-assembly in MOFs makes it a challenging task to pre-design flexible systems. In principle, neutral N-donor ligand based MOFs are relatively well-suited for imparting structural dynamism because of the moderate strength of the primary bond involved. The free solvent molecules occluded during synthesis play the role of principal trigger to modulate the overall structure in MOFs. In particular, use of low boiling solvents facilitates structural dynamism because of the feasibility of the tendencies of such solvents to escape from the frameworks when keeping those MOF crystals away from mother liquor. Although anticipating coordination of guest molecules to metal sites is not possible, use of coordinating solvents and/or moderately interacting anions may afford a greater possibility to provide easily dissociable mono-dentate coordinated guests. The mandatory presence of the mono/multi-dentate anions, on some occasions uncoordinated to the metal centre, within N-donor ligand based MOFs offers the desirable stimulus of the exogenous anions to study solid-state structural transformations.22 In comparison physical stimuli driven investigations, light irradiated transformations in particular, have been found to be simpler in terms of design principles. With further work, the ambiguity in understanding the synthesis strategies of such systems can be significantly overcome.4. Structural dynamism of N-donor ligand based MOFs
Neutral N-donor ligand based MOFs are generally observed to bear guest molecules which remain free or weakly coordinated to the metal centres. Variations of such guests inside the framework can cause alteration in framework structures because of the presence of relatively weaker coordinate bonds (M–N). Apart from such guest induced framework flexibility, dynamism can also be imparted via the perturbation of extra-framework anions.23 Furthermore, inserting proper ligand functionality enhances the chance for molecular reorganization upon shining light (physical stimuli) on such frameworks. The single crystal to single crystal structural transformation method is one of the best direct and key techniques for understanding such molecular level chemical changes in such dynamic frameworks. Various exogenous stimuli (both chemical and physical) that induce structural dynamism will be discussed in detail in the subsequent sections of this paper.4.1. Flexibility driven by chemical stimuli
Because of various exogenous stimuli (chemical) flexible MOFs undergo structural alteration. Such structural variations have often been validated well using single-crystal-to-single-crystal (SCSC) studies. Chemical stimuli (namely, counter anion, free guests, coordinated guests and gaseous guests) have been found to have a profound role in rendering flexibility in such MOFs.24 In the following sections, the different types of chemical stimuli that cause flexibility in such MOF systems are categorized.Yaghi et al. described long back structural transformation via reversible anion exchange.
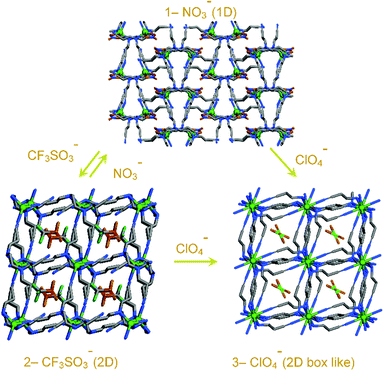
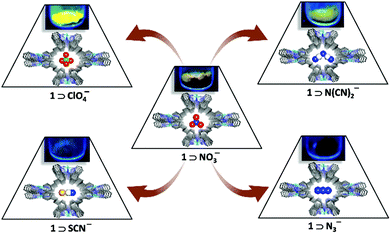
For example, [Ag(4,4′-bpy)(NO3)]n (bpy = bipyridyl) transforms to an exchanged solid upon addition of an excess amount of potassium hexafluorophosphate.28 On addition of excess potassium nitrate onto the exchanged solid, the original network is restored. Min and Suh showed anion dependent structural transformation in a cationic polynitrile-based network.29 During the course of this anion exchange, one-dimensional (1D) [Ag(edtpn)(NO3)]n (edtpn = ethylenediaminetetrapropionitrile) transforms to a two-dimensional (2D) layer structure [Ag(edtpn)(CF3SO3)]n, 2D box like network [Ag(edtpn)(ClO4)]n in a reversible and irreversible manner, respectively. [Ag(edtpn)(ClO4)]n can also be obtained from [Ag(edtpn)(CF3SO3)]n in an irreversible manner (Fig. 1). The helical pitch of cationic infinite solids can be tuned by varying the extra framework anions. Jung et al. described a cationic coordination polymer (CP) {[Ag(Py2O)]·X}n (X = NO3−, BF4−, ClO4− or PF6−) (Py2O = 3,3′-oxybispyridine) as a smart helical spring30 and this can stretch reversibly because of the counter anion exchange which can be correlated to the volume of anion (Fig. 2). Anion induced framework engineering has been shown in a 2D-interpenetrated cationic CP coordination polymer based on Cu(II) and 4,4′-bpy by Noro et al.31 The exchange of anion in an interpenetrated framework leads to an increase in the pore size of the overall framework.32 Replacement of free N(CN)2− in the framework {[Ni(bpe)2(N(CN)2)](N(CN)2)}n (bpe = 1,2-bis(4-pyridyl)ethane) by the smaller N3− causes mutual dislocations of the interpenetrating nets for enlargement in the effective porous area in the framework (Fig. 3). Such enhancement in the porous area in the framework affects the gas uptake property of the material. An N3− exchanged solid with a larger porous aperture shows a greater uptake of gas than the original framework. Our group devised a dynamic luminescent cationic framework [{Zn(L1)(MeOH)2}·(NO3)2·xG]n (L1-4,4′-(ethane-1,2-diyl)bis(N-(pyridin-2-ylmethylene)aniline which showed an interesting anion dependent structural dynamism depending on the size, shape and coordinating tendencies of various anions (NO3−, N3−, SCN−, ClO4− and N(CN)2−).33 Li et al. showed acetate anion driven structural change in two isostructural three-dimensional (3D) cationic frameworks {[M(L2)2] (ClO4)2}n (M = Co(II) or Zn(II); L2 = 4-(4-pyridyl)-3,5-bis(2-pyridyl)-1,2,4-triazole).34 Hou et al. reported spherical halide anions (Cl− and Br−) dependent structural changes in a Cd(II)-based cationic framework Cd(L3)2(NO3)2·2THF (L3 = 3,5-bis(3-pyridyl-3-(3′-methylphenyl)-1,3,4-oxadiazole).35
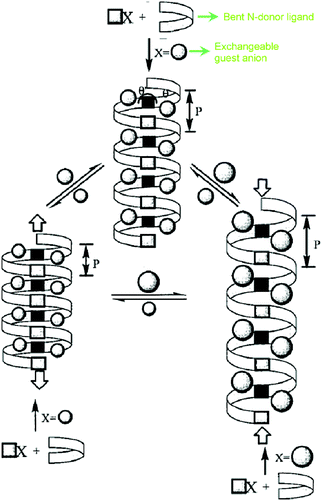 | ||
| Fig. 2 Helical pitch tuning by anion exchange in a cationic solid. Reproduced with permission from ref. 30, Copyright (2000) American Chemical Society. | ||
 | ||
| Fig. 3 Schematic illustration of mutual sliding of nets induced by anion exchange in a cationic MOF. Reprinted with permission from ref. 32, Copyright (2007) Macmillan Publishers Ltd. | ||
In an early work, our group devised two MOF-based supramolecular isomers [{Cd(L4)2(ClO4)2}·toluene·MeOH]n and [{Cd2(L4)4(ClO4)4}·3(mesitylene)]n (L4 = 1,4-bis(4-pyridyl)-2,3-diaza-1,3-butadiene) which show anion induced structural changes for incoming strongly coordinating N3− and SCN− anions because of the replacement of weakly coordinated ClO4− anions in the original frameworks.36 Anion driven structural transformation has also been exploited for visual colorimetric detection of anions.
For example, a 1D amide functionalized Cu(II)-based framework [{CuL52(NO3)2}·o-xylene·DMF]n changes its structure to a 2D framework [{CuL5(SCN)2}·xG]n (L5 = 5-tert-butyl-N1,N3-di(pyridin-4-yl)isophthalamide) upon treatment with SCN− and this is accompanied by a color change from blue to green which can be seen by the naked eye (Fig. 4).37
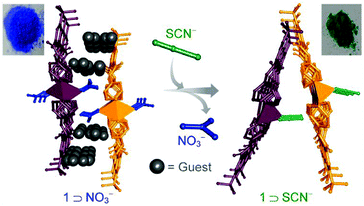 | ||
| Fig. 4 Visual colorimetric anion sensing in a dynamic cationic MOF. Reproduced with permission from ref. 37, Copyright (2015) Wiley-VCH. | ||
Carlucci et al. showed sponge like behavior in a Cu(II)-based framework [Cu5(bpp)8(SO4)4(EtOH)(H2O)5](SO4)·EtOH·25.5H2O. (bpp = 1,3-bis(4-pyridyl)propane) The compound exhibits guest dependent reversible structural transformation towards desolvation and resolvation.39 Noro et al. reported a 3D framework {[Cu(AF6)(4,4′-bpy)2]·xH2O}n that changes to a 2D interpenetrating network {[Cu(4,4′-bpy)2(H2O)2]·AF6}n (A = Si, Ge or Ti) in an aqueous environment.31 Guest driven sliding of 2D layers in a MOF has been demonstrated by Biradha et al.40 Upon guest exchange, the 2D framework [Ni(L6)2(NO3)2·4(o-xylene)]n transformed to [{Ni(L6)2(NO3)2·1.7(mesitylene)}n] (L6 = 2,4,6-tris(4-pyridyl)triazine) with a significant increase in the channel dimensions (Fig. 5).
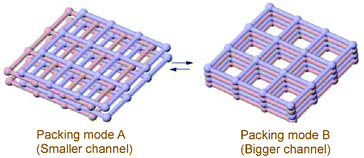 | ||
| Fig. 5 Guest induced sliding of 2D layers in a reversible way. Reproduced with permission from ref. 40, Copyright (2002) Wiley-VCH. | ||
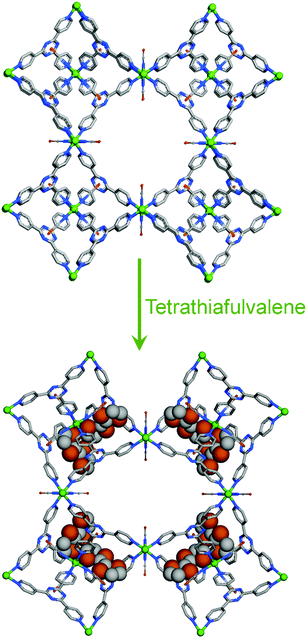
In continuation, Biradha et al. also exhibited a dynamic MOF which remarkably shrinks or swells upon desorption or adsorption of the guest molecule.41 When the crystals of the framework [{(ZnI2)3(L6)2·6C6H5NO2}n] were kept in the open atmosphere for one day, they changed to a new phase with a remarkable compression of the network without losing their single crystalline nature. Interestingly, upon immersing the crystals of the new phase into nitrobenzene, the parent crystals are restored (Fig. 6). A reversible crystal structure to amorphous structural transformation has been examined in an amide functionalized 3D MOF by Uemura et al. The 3D framework {[Co(NCS)2(4-peia)2]·4Me2CO}n (4-peia = N-(2-pyridin-4-yl-ethyl)-isonicotinamide) transformed to an amorphous state upon complete removal of the guest acetone molecules and changed back to their original state when the amorphous phase was exposed to acetone vapour.42 Guest dependent structural flexibility has also been shown in a cage-based coordination network.43 For example [Co3(SCN)6(TPT)4]n transformed to [Co3(SCN)6(TPT)4·(TTF)]n (TPT = 2,4,6-tris(4-pyridyl)triazine) while taking up guests (tetrathiafulvalene) with a change of the crystal system from cubic to tetragonal (Fig. 7).
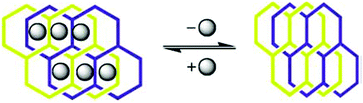 | ||
| Fig. 6 Schematic representation of guest dependent shrinking or swelling in a dynamic MOF. Reproduced with permission from ref. 41, Copyright (2002) Wiley-VCH. | ||
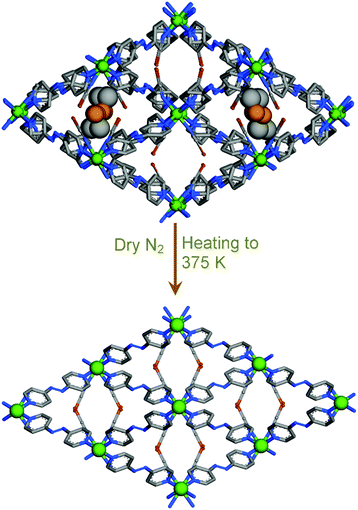
Recently, Bloch and Sumby demonstrated guest induced crystal to crystal expansion and contraction in a Ag(I)-based 3D MOF {[Ag(dpzm)]ClO4·1.1(DMSO)·0.9(ETOAC)}n (dpzm = di-2-pyrazinylmethane)(1 As-made).44
Upon guest exchange with dichloromethane (DCM), 1 As-made changed to 1 DCM in a crystal to crystal manner accompanied by a dramatic contraction of 3.09 Å along the c axis. But 1 DCM expanded to 1 acetone in a reversible fashion after soaking in acetone for few days (Fig. 8). In continuation, the authors reported a similar series of MOFs which exhibited similar breathing transformations upon guest exchange.45 In a very recent report our group showed a 3D interpenetrated cationic MOF [{Zn(L7)2}(NO3)2·xG]n (L7 = [(E)-N′-[1-(pyridin-4-yl)ethyidene]hydrazine carbohydrazide] which upon dynamic structural transformation transformed to a 2D MOF (Fig. 9).46
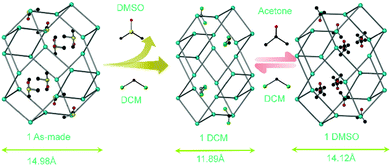 | ||
| Fig. 8 Guest mediated crystal expansion and contraction in a cationic MOF. Reproduced from ref. 44, Copyright (2012) The Royal Society of Chemistry. | ||
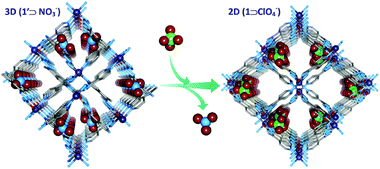 | ||
| Fig. 9 Anion induced structural transformation from a 3D framework to 2D sheets. Reproduced with permission from ref. 46, Copyright (2014) American Chemical Society. | ||
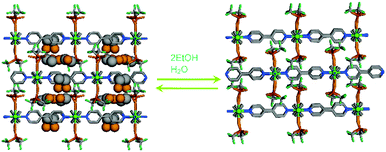
In continuation, guest driven inherent dynamic behaviour of a Cd(II)-based cationic MOF has been shown in research by us.47 Upon air-drying, the as-made MOF [{Cd(L4)3·(ClO4)2}·xG]n transforms to a 2D MOF [{Cd(L4)2(OH2)2(ClO4)2}·THF)]nvia the loss of a coordinated ligand molecule (Fig. 10).
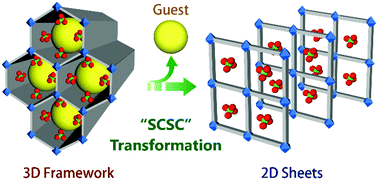 | ||
| Fig. 10 SCSC transformation from a 3D porous framework to 2D non porous sheets. Reproduced from ref. 47, Copyright (2015) The Royal Society of Chemistry. | ||
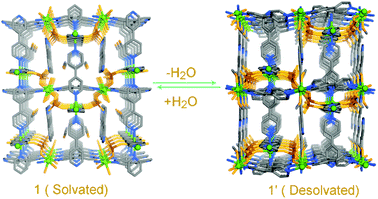
Bradshaw et al. demonstrated the flexibility of a MOF by regulating the coordinated neutral guest via thermal stimuli.50 The authors synthesized a neutral compound [Co2(bipy)3(SO4)2(H2O)2](bipy)(CH3OH), (bipy-4,4′-bipyridyl) bearing an uncoordinated ligand and a solvent molecule. Upon heating the coordinated water molecules were replaced by the uncoordinated species in the parent compound. This change was accompanied by alteration in the structure of the compound to give a new phase. The reversibility of this experiment and the hypothesis was validated by controlled cooling in the presence and absence of atmospheric moisture (Fig. 11).
In a similar report, Aslani and Morsali showed that drastic structural changes of a coordination polymer occurred by removal of a coordinated solvent upon heating.51 A 1D coordination polymer [Pb2(8-Quin)2(NO3)2(MeOH)], (8-Quin = 8-hydroxyquinoline) was synthesized which was converted to a 2D structure [Pb(8-Quin)-(NO3)] when the compound was heated at 165–170 °C. The Pb–O bond with MeOH was replaced by a Pb–O bond from the NO3 anion. This transition was also accompanied by a visual change in the colour of the crystal.
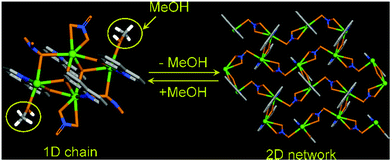
The resolvation of the compound to its parent phase was achieved when this was dipped in MeOH and the authors observed such multiple reversible transformations (Fig. 12). Zhuang et al. extended this concept through fabrication of a Cd(II) based MOF [(Cd(ImBNN)2(CF3SO3)2)·guest]n, (ImBNN = 2,5-bis[4′-(imidazol-1-yl)phenyl]-3,4-diaza-2,4-hexadiene) bearing weakly coordinated triflate anions and free toluene molecules.52 Upon removal of the toluene molecules by heating, the metal–anion bond is weakened. When the compound was cooled under atmospheric conditions, one water molecule replaced the triflate anion leading to slight structural changes.
The original phase of the compound was restored by heating the water coordinated phase in toluene at 110 °C. Toluene molecules occupied the free space of the MOF and both the anions were coordinated to the metal centre. This report yields an example of neutral guest molecules competing with charged anions for metal-coordination leading to structural changes (Fig. 13).
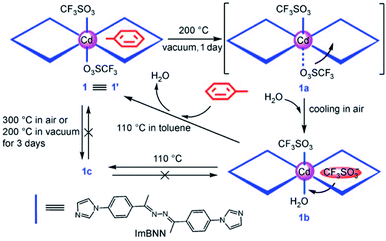 | ||
| Fig. 13 Schematic illustration of structural transformation upon loss and gain of coordinated guests. Reproduced with permission from ref. 52, Copyright (2009) Wiley-VCH. | ||
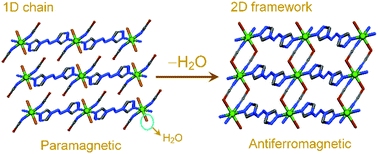
Liu et al. observed the structural changes which accompanied the promising magnetic regulation in a MOF-based material.53 The authors synthesized a Co(II) centred 1D coordination polymer [Co(SCN)2(bta)(H2O)2], (bta = 1,2-di(4H-1,2,4-triazol-4-yl)diazene) comprising water molecules in the coordination sphere of the metal ion. Upon desolvation at 100 °C the authors observed structural changes together with an increase in dimensionality of the packing. Interestingly, a magnetic variation was noticed during this transition from the simple paramagnetic behaviour of the parent compound to the antiferromagnetic nature of the desolvated phase (Fig. 14). As discussed in the previous section, a MOF exhibiting anion and guest dependent structural changes has been reported.33 The as-synthesized compound on air-drying underwent noticeable structural modifications, via SCSC characterization, and accompanied with a change in the coordination environment of the metal centre. The coordinated methanol molecules were replaced by H2O from atmospheric moisture.
The affinity of the framework towards several hydrophilic adsorbates was studied further, and this yielded size selective uptake (Fig. 15).
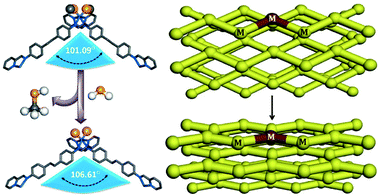 | ||
| Fig. 15 Guest triggered dynamic structural transformation in a flexible cationic MOF. Reproduced with permission from ref. 33, Copyright (2013) Wiley-VCH. | ||
In an early work Li and Kaneko studied the adsorption behaviour for a Cu(II) MOF [Cu(bpy)(BF4)2(H2O)2·(bpy)]n.56 A similar response to different gases such as N2, Ar, and CO2 was observed in which the relatively non-porous packed compound was able to adsorb a significant amount of these gases after tuning of the gate pressures. The authors attribute the breach of hydrogen bond assisted packing of the compound (because of the presence of BF4− anions) to the respective gate pressures of the adsorbed gases (Fig. 16).
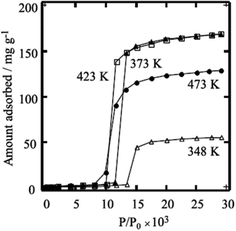 | ||
| Fig. 16 Gate opening behaviour for CO2 adsorption in a Cu(II) MOF. Reprinted with permission from ref. 56, Copyright (2001) Elsevier. | ||
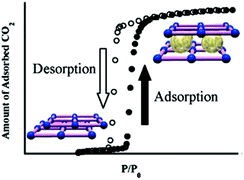
Kondo et al. reported a dual flexible MOF system which underwent structural alterations upon guest removal and later upon adsorption of gas molecules.57 The authors synthesized a 3D MOF {[Cu(bpy)(BF4)2(H2O)2]·(bpy)}, which underwent a dimensionality reduction to form a 2D framework having the formula [Cu(BF4)2(bpy)2] (Fig. 17).
 | ||
| Fig. 17 Schematic representation of clathrate formation during CO2 adsorption. Reproduced with permission from ref. 57, Copyright (2006) American Chemical Society. | ||
Upon studying CO2 adsorption for the 2D compound, a notable change in the interactions between two 2D sheets was found to happen, which was ascribed to the clathrate formation between the gaseous molecules and the framework in the adsorbed state.
In a continuation of the research, Kondo et al. demonstrated the flexibility of dynamic frameworks upon inclusion of gaseous molecules by constructing a Cu(II) based 2D MOF [Cu(bpy)2(OTf)2]·2EtOH·H2O.58 An isostructural MOF with MeOH occluded guests has also been used in this work to study the effect of free guest molecules. Upon desolvation the parent compound was subjected to low temperature N2 adsorption, where the authors observed the typical gate-opening behaviour at P/P0 = 0.16–0.22. Powder X-ray diffraction (PXRD) measurements at the first step of gate-opening showed the structural changes corresponding to the expansion of the 2D layers in the compound (Fig. 18).
Kondo et al. expanded their previous work by fabricating a series of MOFs having the same composition, namely, [Cu(bpy)2(OTf)2]n, in different solvent combinations.59 In the 2D architectures the authors observed stepwise adsorption for gases such as N2, CO2, Ar, CH4. This behaviour was not observed for the 3D compounds where a Type-I adsorption profile was seen in all cases and the uptake followed a molecular sieving effect. The layered 2D structures have been thought to undergo sliding to accommodate the respective adsorbents (Fig. 19).
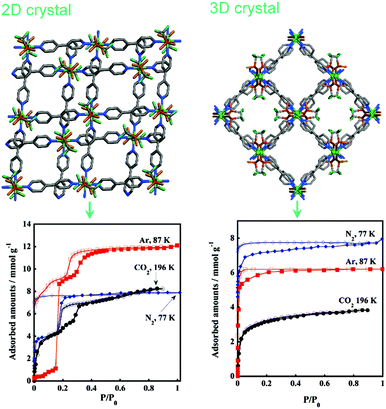 | ||
| Fig. 19 Differential adsorption behaviours in two MOFs having the same composition. Reproduced with permission from ref. 59, Copyright (2011) American Chemical Society. | ||
In a similar work, Kotani et al. synthesized a Cu(II) based 1D coordination polymer, namely, Cu(bpp)2(BF4)2, which underwent structural changes upon desolvation.60 Low temperature N2 adsorption was performed on the guest-free phase, which did not show any uptake. When CO2 adsorption was performed on the same phase under moderate conditions, the compound exhibited a gate-opening adsorption profile.
4.2. Flexibility arising from physical stimuli
MOFs are known to be responsive to physical stimuli because of the dual advantages bestowed by the metal ions and organic ligands. Among them, light irradiated structural changes have commanded significant attention among other physical stimuli. Park et al. have pioneered the domain of cycloaddition reaction based structural changes in olefin-bearing ligands.61 Because of the lack of chemical stability in neutral N-donor ligand based MOFs, solid-state reactions are more favoured for investigation of structural subtleties. The prominent reports among such class of studies are discussed next.In an early study on this aspect, Nagarathinam and Vittal reported anisotropic movements within a CP structure.62 The authors synthesized a CP [Ag(μ-bpe)(H2O)](CF3CO2)·CH3CN, (bpe-4,4′-bipyridylethylene) which upon desolvation was subjected to photo-irradiation. The resultant compound was found to bear the desired cyclobutane rings, as confirmed using nuclear magnetic resonance (NMR) studies. Although crystallographic evidence could not be provided for the desolvated structure, the authors crystallized a similar compound bearing coordinated anions [{(μ-O2CCF3)Ag}2(μ-bpe)2]·H2O which supported their claim of the formation of a ladder-like CP. The authors hypothesized the reorganization of the compound after desolvation to align the ethylene groups making them suitable for a cycloaddition reaction.
Using this knowledge, Nagarathinam and Vittal used a photochemical cycloaddition reaction to show the formation of the intermediate upon dehydration of a 1D coordination polymer [Cd(bpe)(CH3COO)2(H2O)]n.63 The synthesized compound upon dehydration was subjected to ultraviolet (UV) irradiation to achieve 100% formation of the cyclobutane ring. Compared to this result, only 33% conversion was noted when the parent compound was irradiated. Thus, among the two possible intermediates, the authors could conclusively infer the formation of the 1D ladder-like structure formation upon dehydration.
In continuation, Peedikakkal and Vittal synthesized a triple-stranded ladder CP [Pb3(bpe)3(O2CCF3)4(O2CCH3)2], which underwent a solid state cycloaddition reaction.64 A two-step reaction was observed which occurred because of the anisotropic movements among the adjacent strands of the CP. In the first step ∼67% photodimerization was noted which corresponded to the reaction between a pair of bpe ligands with a triple-stranded structure. The second step involved the remaining 33% reaction with cooperative movements in the polymeric structure to align the unreacted olefin for completing the dimerization (Fig. 20).
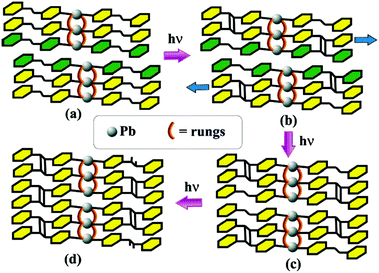 | ||
| Fig. 20 Schematic representation of a two-step photo induced dimerization. Reproduced with permission from ref. 64, Copyright (2010) American Chemical Society. | ||
In a parallel manner, the authors have reported development of CPs using the photoactive monomers. By employing a Zn(II) salt and a mono-dentate N-donor ligand, a monomer [ZnBr2(4spy)2], (spy = trans-2-fluoro-4′-styrylpyridine) was synthesized.65 Upon irradiation, a dimer was obtained which could be reversibly brought back to the monomer by heating. When the dimer was irradiated for a longer duration, a 1D CP was synthesized, the reaction of which was not found to be reversible. The authors further observed a drastic photoluminescence change together with the photopolymerization (Fig. 21). More recently, Medishetty et al. undertook a comprehensive study of the photo-activeness of monomeric systems bearing photo-active olefinic groups.66
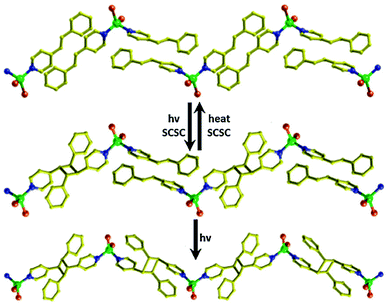 | ||
| Fig. 21 Photo induced cycloaddition reaction in the formation of 1D CP. Reproduced from ref. 65, Copyright (2013) The Royal Society of Chemistry. | ||
A series of Ag(I) centred compounds were synthesized by varying the ligand and anion. All the compounds were found to undergo photo-induced cycloaddition reactions, which provided conclusive evidence for the effect of the shape, nature, and size of the counter-anions on the photo-activity of the compound.
5. Flexibility driven physical and chemical properties
The flexibility endowed by dynamic MOF systems permits investigation of these materials for controlled regulation of certain physical or chemical properties and/or determining their suitability for prospective real-time applications.67,68 A few important results relating to this are discussed next.In an early report, Halder et al. found a flexible Fe(II) centred MOF which exhibited guest-dependent spin-crossover (SCO) properties.69 The authors synthesized a MOF Fe2(azpy)(NCS)4·(EtOH), (azpy = trans-4,4′-azopyridine) where a weak interaction between O–H⋯S was found to prevail. Temperature dependent magnetic susceptibility was recorded followed by desolvation. In the guest-free form, the slightly bent thiocyanate ions were found to be aligned in a linear fashion, which gave rise to structural changes.
Upon measurement of magnetic susceptibility for this phase of the compound, a significant change in the crossover onset temperature was observed. To corroborate with the proposed mechanism, the desolvated phase was dipped separately in EtOH, MeOH and PrOH to obtain resolvated guest included compounds. These phases were found to show similar susceptibility patterns (Fig. 22). In continuation and carrying on from the previous work, the authors synthesized a Fe(II) MOF [Fe(NCS)2(bpbd)2]·{acetone}, (bpbd = 2,3-bis(4′-pyridyl)-2,3-butanediol) comprising free hydroxyl bearing groups in the ligand.70 The O–H⋯S interactions, as previously noted, were found to persist in the interpenetrated packing of the compound, rendering inflexibility to the compound and keeping out any host–guest non-covalent interaction.
Temperature dependent, subtle structural changes were observed for the compound. Temperature dependent magnetic susceptibility measurements were carried out for the as-synthesized phase and the desolvated phase, and these displayed an almost similar tendency, reaffirming the hypothesis of employing a free hydroxyl-based ligand (Fig. 23).
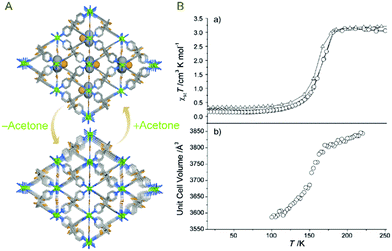 | ||
| Fig. 23 (A) Guest induced reversible structural changes (B) without affecting the magnetic behaviour of host framework. Reproduced with permission from ref. 70, Copyright (2007) Wiley-VCH. | ||
Investigating further, Neville et al. have reported a few studies including SCSC transformations of Fe(II) SCO frameworks,71 a mechanistic understanding of multi-step SCO transitions72 and structural modulations by guest-dependent SCO behaviour of seemingly iso-structural MOFs.73
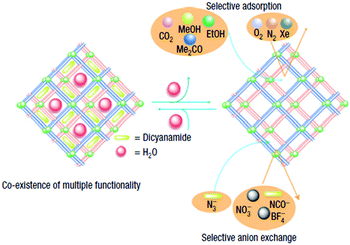
In a distinct domain of investigation, Maji et al. demonstrated the use of the dynamic nature of a two-fold interpenetrated 3D MOF {[Ni(bpe)2(N(CN)2)](N(CN)2)(5H2O)}n, for controlled gas adsorption.32 The compound bears two kinds of porous channels occupied by the solvent (water) and anions separately. Upon desolvation, a noticeable change in colour was observed which was restored upon resolvation. The dehydrated framework bestows a dual function to the compound where N3− anions are exchanged selectively with N(CN)2− ions in competition with NCO−, NO3−, BF4−. The anion-exchanged phase in turn renders an additional feature of significantly enhanced gas adsorption by enlarging the window of the larger pore and squeezed smaller pore, by virtue of the linear shape of the exchanged anion.
In a parallel work, Noro et al. have reported selective CO2 adsorption by a 2D PCP.74 The authors synthesized a Cu(II) centred MOF {[Cu(PF6)(4,4′-bpy)2(MeOH)]·PF6·3MeOH}n, which upon desolvation underwent a change in structure to [Cu(PF6)2(4,4′-bpy)2]n where the uncoordinated PF6− ions replaced the coordinated MeOH molecules in the parent compound. Upon checking the adsorption behaviour towards various gases such as CO2, N2, O2 and Ar, a strong preference and high uptake for CO2 at both low and high temperatures was observed. Theoretical calculations revealed the affinity of the fluorine ions towards CO2 as the primary driving force for the high uptake and low energy regeneration (Fig. 24). CO2 separation based on a similar adsorption mechanism has also been studied by Nugent et al. using a SiF62− bridged N-donor ligand based MOFs.75
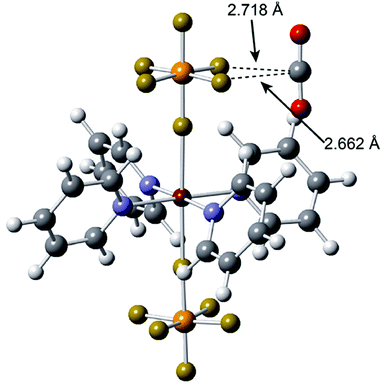 | ||
| Fig. 24 Optimized structure of [Cu(PF6)2(pyridine)4] with CO2 obtained using density functional theory. Reproduced with permission from ref. 74, Copyright (2013) American Chemical Society. | ||
Apart from these properties, such dynamic MOFs have found use as luminescent probes for a few important neutral and ionic analytes. Wang et al. have reported a comprehensive study of structure–property correlation in a flexible MOF [Cu(CN)3L8·(H2O)(CH3CH2OH)]n, (L8 = 2,6-bis((3,5-dimethyl-1H-pyrazol-4-yl)methyl)pyridine) acting as a solvent-vapour sensor for use with the naked eye.76 The compound has an interpenetrated structure which leaves room for pre-organization towards the guest according to its nature and size. Upon desolvation a change in PXRD was found which was restored upon resolvation. Additionally this process was visible under UV irradiation with a notable difference of emitted light frequency.
A drastic change in the luminescence of the desolvated compound was observed when it was immersed in several solvents such as acetonitrile, benzene, cyclohexane, cyclohexene, dichloromethane, ethanol, methanol, and tetrahydrofuran. To gain molecular insights into these photophysical observations, the authors synthesized a few compounds which had the guest molecules included. Furthermore, to understand the real-time applicability of the compound, solid-state fluorescence studies towards acetonitrile vapours were investigated. Together with the response towards different concentrations of acetonitrile vapours, the change in fluorescence for solvent mixtures was also examined (Fig. 25).
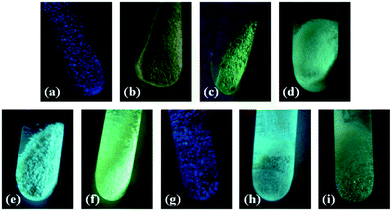 | ||
| Fig. 25 Naked eye fluorescence changes in a guest responsive dynamic MOF upon immersion in several volatile organic solvents (a) parent compound, (b) acetonitrile, (c) methanol, (d) ethanol, (e) tetrahydrofuran, (f) benzene, (g) cyclohexane, (h) cyclohexane (longer time), (i) dichloromethane. Reproduced from ref. 76, Copyright (2013) The Royal Society of Chemistry. | ||
As briefly discussed in the earlier sections,33 a dynamic, luminescent PCP exhibiting guest and anion dependent flexibility has been reported. The ligand used is a π-conjugated molecule, which bestows ligand-based fluorescence to the compound. The parent compound was found to exhibit high solid-state emission primarily because of the intraligand charge transfer. Upon anion-exchange, the fluorescence intensities and positions were perturbed because of plausible changes in the interactions of the anions with the framework (Fig. 26). Such a visible, non-sophisticated tool to monitor anion-exchange/capture process may be useful for important applications in the material/biological fields.
6. Conclusions and future prospects
In this review, a systematic update in the field of flexible MOFs composed of neutral N-donor ligands has been given. A range of exogenous stimuli which trigger structural flexibility/softness to such MOF systems have been discussed. Various chemical stimuli such as framework anions, free guests, coordinated guests and gaseous guests together with physical stimuli (light) have been used in creating flexibility in these kinds of MOF systems. Various flexibility driven functions in these materials (e.g., separation of gases, sensing of small molecules, magnetic properties and so on) which include proper host–guest interactions have been thoroughly examined. For a better understanding of such a flexible MOF system, it is very important to gain structural insights of all the phases. But, to get a single crystal structure after each dynamic process is quite difficult. Thus, using single crystal X-ray diffraction, PXRD, other modern structure determining tools and theoretical methods,77 neutral N-donor ligand based MOFs might show new functions because of their structural flexibility, for the development of new functional materials for various potential applications.Acknowledgements
B. M. is thankful to CSIR for research fellowship, while IISER Pune is acknowledged for the same by B. M., A. V. D.; DST (Project no. GAP/DST/CHE-12-0083) and DST-FIST (SR/FST/CSII-023/2012) are acknowledged for generous financial support.Notes and references
- H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673 CrossRef CAS PubMed.
- J.-R. Li, J. Sculley and H.-C. Zhou, Chem. Rev., 2012, 112, 869 CrossRef CAS PubMed.
- B. Chen, Y. Yang, F. Zapata, G. Lin, G. Qian and E. B. Lobkovsky, Adv. Mater., 2007, 19, 1693 CrossRef CAS.
- B. Chen, L. Wang, F. Zapata, G. Qian and E. B. Lobkovsky, J. Am. Chem. Soc., 2008, 130, 6718 CrossRef CAS PubMed.
- P. Ramaswamy, N. E. Wong and G. K. H. Shimizu, Chem. Soc. Rev., 2014, 43, 5913 RSC.
- D. N. Dybtsev, H. Chun and K. Kim, Angew. Chem., Int. Ed., 2004, 43, 5033 CrossRef CAS PubMed.
- S. Horike, S. Shimomura and S. Kitagawa, Nat. Chem., 2009, 1, 695 CrossRef CAS PubMed.
- C. Serre, C. Mellot-Draznieks, S. Surble, N. Audebrand, Y. Filinchuk and G. Ferey, Science, 2007, 315, 1828 CrossRef CAS PubMed.
- G. Kumar Kole and J. J. Vittal, Chem. Soc. Rev., 2013, 42, 1755 RSC.
- A. Schneemann, V. Bon, I. Schwedler, I. Senkovska, S. Kaskel and R. A. Fischer, Chem. Soc. Rev., 2014, 43, 6062 RSC.
- Z. Chang, D.-H. Yang, J. Xu, T.-L. Hu and X.-H. Bu, Adv. Mater., 2015, 27, 5432–5441 CrossRef CAS PubMed.
- M. C. Das and P. K. Bharadwaj, J. Am. Chem. Soc., 2009, 131, 10942 CrossRef CAS PubMed.
- Y.-Q. Chen, G.-R. Li, Z. Chang, Y.-K. Qu, Y.-H. Zhang and X.-H. Bu, Chem. Sci., 2013, 4, 3678 RSC.
- J.-P. Ma, Y. Yu and Y.-B. Dong, Chem. Commun., 2012, 48, 2946 RSC.
- H. Fei, M. R. Bresler and S. R. J. Oliver, J. Am. Chem. Soc., 2011, 133, 11110 CrossRef CAS PubMed.
- H. Fei, C. S. Han, J. C. Robins and S. R. J. Oliver, Chem. Mater., 2013, 25, 647 CrossRef CAS.
- X. Li, H. Xu, F. Kong and R. Wang, Angew. Chem., Int. Ed., 2013, 52, 13769 CrossRef CAS PubMed.
- B. Manna, B. Joarder, A. V. Desai, A. Karmakar and S. K. Ghosh, Chem. – Eur. J., 2014, 20, 12399 CrossRef CAS PubMed.
- K. Biradha, K. V. Domasevitch, B. Moulton, C. Seward and M. J. Zaworotko, Chem. Commun., 1999, 1327 RSC.
- M. C. Das, S. K. Ghosh, E. C. Sañudo and P. K. Bharadwaj, Dalton Trans., 2009, 1644 RSC.
- W. L. Leong and J. J. Vittal, Chem. Rev., 2011, 111, 688 CrossRef CAS PubMed.
- A. Karmakar, A. V. Desai and S. K. Ghosh, Coord. Chem. Rev., 2015 DOI:10.1016/j.ccr.2015.08.007.
- C.-P. Li, J. Chen, C.-S. Liub and M. Du, Chem. Commun., 2015, 51, 2768 RSC.
- J.-P. Zhang, P.-Q. Liao, H.-L. Zhou, R.-B. Lin and X.-M. Chen, Chem. Soc. Rev., 2014, 43, 5789 RSC.
- O.-S. Jung, Y. J. Kim, Y.-A. Lee, H. K. Chae, H. G. Jang and J. Hong, Inorg. Chem., 2001, 40, 2105 CrossRef CAS PubMed.
- S. Muthu, J. H. K. Yip and J. J. Vittal, J. Chem. Soc., Dalton Trans., 2002, 4561 RSC.
- S. Kitagawa, R. Kitaura and S.-i. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS PubMed.
- O. M. Yaghi and H. Li, J. Am. Chem. Soc., 1996, 118, 295 CrossRef CAS.
- K. S. Min and M. P. Suh, J. Am. Chem. Soc., 2000, 122, 6834 CrossRef CAS.
- O.-S. Jung, Y. J. Kim, Y.-A. Lee, J. K. Park and H. K. Chae, J. Am. Chem. Soc., 2000, 122, 9921 CrossRef CAS.
- S.-I. Noro, R. Kitaura, M. Kondo, S. Kitagawa, T. Ishii, H. Matsuzaka and M. Yamashita, J. Am. Chem. Soc., 2002, 124, 2568 CrossRef CAS PubMed.
- T. K. Maji, R. Matsuda and S. Kitagawa, Nat. Mater., 2007, 6, 142 CrossRef CAS PubMed.
- B. Manna, A. K. Chaudhari, B. Joarder, A. Karmakar and S. K. Ghosh, Angew. Chem., Int. Ed., 2013, 52, 998 CrossRef CAS PubMed.
- C. P. Li, J. Guo and M. Du, Inorg. Chem. Commun., 2013, 38, 70 CrossRef CAS.
- S. Hou, Q.-K. Liu, J.-P. Ma and Y.-B. Dong, Inorg. Chem., 2013, 52, 3225 CrossRef CAS PubMed.
- B. Manna, S. Singh, A. Karmakar, A. V. Desai and S. K. Ghosh, Inorg. Chem., 2015, 54, 110 CrossRef CAS PubMed.
- A. Karmakar, A. V. Desai, B. Manna, B. Joarder and S. K. Ghosh, Chem. – Eur. J., 2015, 21, 7071 CrossRef CAS PubMed.
- J. J. Vittal, Coord. Chem. Rev., 2007, 251, 1781 CrossRef CAS.
- L. Carlucci, G. Ciani, M. Moret, D. M. Proserpio and S. Rizzato, Angew. Chem., Int. Ed., 2000, 39, 1506 CrossRef CAS.
- K. Biradha, Y. Hongo and M. Fujita, Angew. Chem., Int. Ed., 2002, 41, 3395 CrossRef CAS.
- K. Biradha and M. Fujita, Angew. Chem., Int. Ed., 2002, 41, 3392 CrossRef CAS.
- K. Uemura, S. Kitagawa, K. Fukui and K. Saito, J. Am. Chem. Soc., 2004, 126, 3817 CrossRef CAS PubMed.
- Y. Inokuma, T. Arai and M. Fujita, Nat. Chem., 2010, 2, 780 CrossRef CAS PubMed.
- W. M. Bloch and C. J. Sumby, Chem. Commun., 2012, 48, 2534 RSC.
- W. M. Bloch and C. J. Sumby, Eur. J. Inorg. Chem., 2015, 2015, 3723 CrossRef CAS.
- A. Karmakar, B. Manna, A. V. Desai, B. Joarder and S. K. Ghosh, Inorg. Chem., 2014, 53, 12225 CrossRef CAS PubMed.
- B. Manna, A. V. Desai, N. Kumar, A. Karmakar and S. K. Ghosh, CrystEngComm, 2015 10.1039/C5CE00139K.
- A. Malik, P. Peedikakkal and J. J. Vittal, Cryst. Growth Des., 2011, 11, 4697 Search PubMed.
- M. H. Zeng, S. Hu, Q. Chen, G. Xie, Q. Shuai, S. L. Gao and L. Y. Tang, Inorg. Chem., 2009, 48, 7070 CrossRef CAS PubMed.
- D. Bradshaw, J. E. Warren and M. J. Rosseinsky, Science, 2007, 315, 977 CrossRef CAS PubMed.
- A. Aslani and A. Morsali, Chem. Commun., 2008, 3402 RSC.
- C.-F. Zhuang, J. Zhang, Q. Wang, Z.-H. Chu, D. Fenske and C.-Y. Su, Chem. – Eur. J., 2009, 15, 7578 CrossRef CAS PubMed.
- X.-M. Liu, B.-Y. Wang, W. Xue, L.-H. Xie, W.-X. Zhang, X.-N. Cheng and X.-M. Chen, Dalton Trans., 2012, 41, 13741 RSC.
- J. L. C. Rowsell, E. C. Spencer, J. Eckert, J. A. K. Howard and O. M. Yaghi, Science, 2005, 309, 1350 CrossRef CAS PubMed.
- H. Sato, W. Kosaka, R. Matsuda, A. Hori, Y. Hijikata, R. V. Belosludov, S. Sakaki, M. Takata and S. Kitagawa, Science, 2014, 343, 167 CrossRef CAS PubMed.
- D. Li and K. Kaneko, Chem. Phys. Lett., 2001, 334, 50 CrossRef.
- A. Kondo, H. Noguchi, S. Ohnishi, H. Kajiro, A. Tohdoh, Y. Hattori, W.-C. Xu, H. Tanaka, H. Kanoh and K. Kaneko, Nano Lett., 2006, 6, 2581 CrossRef CAS PubMed.
- A. Kondo, H. Noguchi, L. Carlucci, D. M. Proserpio, G. Ciani, H. Kajiro, T. Ohba, H. Kanoh and K. Kaneko, J. Am. Chem. Soc., 2007, 129, 12362 CrossRef CAS PubMed.
- A. Kondo, H. Kajiro, H. Noguchi, L. Carlucci, D. M. Proserpio, G. Ciani, K. Kato, M. Takata, H. Seki, M. Sakamoto, Y. Hattori, F. Okino, K. Maeda, T. Ohba, K. Kaneko and H. Kanoh, J. Am. Chem. Soc., 2011, 133, 10512 CrossRef CAS PubMed.
- R. Kotani, A. Kondo and K. Maeda, Chem. Commun., 2012, 48, 11316 RSC.
- I.-H. Park, A. Chanthapally, Z. Zhang, S. S. Lee, M. J. Zaworotko and J. J. Vittal, Angew. Chem., Int. Ed., 2014, 53, 414 CrossRef CAS PubMed.
- M. Nagarathinam and J. J. Vittal, Angew. Chem., Int. Ed., 2006, 45, 4337 CrossRef CAS PubMed.
- M. Nagarathinam and J. J. Vittal, Chem. Commun., 2008, 438 RSC.
- A. M. P. Peedikakkal and J. J. Vittal, Inorg. Chem., 2010, 49, 10 CrossRef CAS PubMed.
- R. Medishetty, T. T. S. Yap, L. L. Koh and J. J. Vittal, Chem. Commun., 2013, 49, 9567 RSC.
- R. Medishetty, S. C. Sahoo, C. E. Mulijanto, P. Naumov and J. J. Vittal, Chem. Mater., 2015, 27, 1821 CrossRef CAS.
- G. Ferrey and C. Serre, Chem. Soc. Rev., 2009, 38, 1380 RSC.
- S. Tominaka, H. Hamoudi, T. Suga, T. D. Bennett, A. B. Cairns and A. K. Cheetham, Chem. Sci., 2015, 6, 1465 RSC.
- G. J. Halder, C. J. Kepert, B. Moubaraki, K. S. Murray and J. D. Cashion, Science, 2002, 298, 1762 CrossRef CAS PubMed.
- S. M. Neville, B. Moubaraki, K. S. Murray and C. J. Kepert, Angew. Chem., Int. Ed., 2007, 46, 2059 CrossRef CAS PubMed.
- S. M. Neville, G. J. Halder, K. W. Chapman, M. B. Duriska, P. D. Southon, J. D. Cashion, J.-F. Letard, B. Moubaraki, K. S. Murray and C. J. Kepert, J. Am. Chem. Soc., 2008, 130, 2869 CrossRef CAS PubMed.
- G. J. Halder, K. W. Chapman, S. M. Neville, B. Moubaraki, K. S. Murray, J.-F. Letard and C. J. Kepert, J. Am. Chem. Soc., 2008, 130, 17552 CrossRef CAS PubMed.
- S. M. Neville, G. J. Halder, K. W. Chapman, M. B. Duriska, B. Moubaraki, K. S. Murray and C. J. Kepert, J. Am. Chem. Soc., 2009, 131, 12106 CrossRef CAS PubMed.
- S.-I. Noro, Y. Hijikata, M. Inukai, T. Fukushima, S. Horike, M. Higuchi, S. Kitagawa, T. Akutagawa and T. Nakamura, Inorg. Chem., 2013, 52, 280 CrossRef CAS PubMed.
- P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Ma, B. Space, L. Wojtas, M. Eddaoudi and M. J. Zawarotko, Nature, 2013, 495, 80 CrossRef CAS PubMed.
- J.-H. Wang, M. Li and D. Li, Chem. Sci., 2013, 4, 1793 RSC.
- A. U. Ortiz, A. Boutin, A. H. Fuchs and F.-X. Coudert, Phys. Rev. Lett., 2012, 109, 195502 CrossRef PubMed.
Footnote |
| † These authors have contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |