 Open Access Article
Open Access ArticleColorimetric detection of fluoride ions by anthraimidazoledione based sensors in the presence of Cu(II) ions†
Amrita
Sarkar
,
Sudipta
Bhattacharyya
and
Arindam
Mukherjee
*
Department of Chemical Sciences, Indian Institute of Science Education and Research Kolkata, Mohanpur 741246, India. E-mail: a.mukherjee@iiserkol.ac.in; Fax: +91-33-25873020
First published on 1st December 2015
Abstract
Anthraquinone based anion receptors have gained importance due to their colorimetric response on sensing a specific anion and the possibility of tuning this property by varying the conjugated moiety (the donor) to the diamine. In this work, we have synthesized and characterized four anthraimidazoledione compounds having 2,5-dihydroxy benzene, 4-(bis(2-chloroethyl)amino)benzene, imidazole and 4-methylthiazole moieties respectively (1–4). All of them were probed for their potential as anion sensors to study the effect of changes in the hydrogen bond donor–acceptor. The p-hydroquinone bound anthraimidazoledione (1) and thioimidazole bound anthraimidazoledione (4) were able to detect both F− and CN− in the presence of other anions Cl−, Br−, I−, H2PO4−, OAc−, NO3− and ClO4−. Both 1 and 4 could not differentiate F− from CN− and provided a similar response to both. The 1H NMR studies of 1 and 4 with F−, showed the formation of [HF2]− at 16.3 ppm and the 19F NMR showed a sharp peak at −145 ppm in both cases. However, although there may be NMR evidence of [HF2]− formation F− may not be detected colorimetrically if the CT band remains almost unchanged, as found for 3. The results emphasize that the change of a hetero atom in the donor moiety of an anthraimidazoledione may render a large difference in sensitivity. In the case of 4 selective detection of F− was possible in the presence of 0.5 equivalent of Cu2+ with the exhibition of a distinct green colour with a Δλ shift of ca. 50 nm in contrast to CN− which showed orange colouration with a Δλ shift of only 15 nm. In the presence of Cu2+ the F− detection limit was 0.038(5) ppm (below the WHO specified level) at a receptor concentration of 25 μM.
Introduction
Anion sensing has become a popular area of research due to its potential role in various biological processes and in environmental chemistry.1–6 The well-known advantages of a highly sensitive and selective anion sensor have led to a surge in research in this area, especially in the past decade.7,8 Among various anionic analytes fluoride and cyanide seem to be of potential interest for their established roles in physiology.9 The benefits of fluoride are well known in the treatments of osteoporosis, orthodontics, enamel demineralisation and as antidepressants.10 However, the excessive intake of fluoride often leads to fluorosis,11–13 urolithiasis9,14 and even cancer.9,15 Cyanide the extremely toxic anion for mammals has extensive industrial applications including gold mining, electroplating, metallurgy, paper, textile and plastic industries.16 The wide range use of cyanide may also be a major cause of contamination of various environmental sources spiking the cyanide amount beyond the safe limit. It is well known that cyanide inhibits cellular respiration by strong interactions with cytochrome a3 of the heme unit.17 A very small amount of cyanide can be lethal to various metabolic functions including cardiac, renal, vascular, respiratory and central nervous systems.18Hence contamination of water sources with cyanide and fluoride is a matter of concern.19 According to World Health Organisation (WHO) and Environment Protection Agency (EPA) the permissible level of fluoride and cyanide in drinking water should be 1.5 and 0.2 ppm respectively.20 Therefore, various methods have been developed for the detection of fluoride and cyanide among which colorimetric sensors have gained much importance due to their straight forward ‘naked-eye’ detection ability allowing them to be an inexpensive detection technique.2,21–34
Three main approaches have been used to develop various anion sensors:
(i) anion binding to the hydrogen bond donor site due to which the electronic properties of the receptor is altered allowing the subsequent detection of anions.5,30,35–46
(ii) Displacement assay, which involves the formation of a complex between an indicator ion and a receptor, followed by the displacement of the indicator by guest anions.47–56 This creates a change in the microenvironment of the receptor resulting in alteration in colours or fluorescence properties.5,56,57
(iii) Generation of new species with different properties upon chemical reaction between anionic species and receptor molecules called chemodosimeters.58–63
The hydrogen bond donating receptors include a wide range of molecules viz. ureas,35,64,65 thioureas,44,66–68 imidazoles/benzimidazoles,21,45,69–75 amides/diamides,76–78 indolocarbazoles,30,79,80 guanidinium derivatives,81 azophenol,31,82 naphthalimides,83–87 pyrrole,88,89 callixpyrrole90–93etc. The benzimidazole derivatives work via the deprotonation of N–H groups which results in either fluorescence quenching,45,94 photoinduced electron transfer (PET)95–97 or intramolecular charge transfer (ICT) processes.21,75,98 The ICT process involves a ‘push–pull’ mechanism between a donor (D) and an acceptor (A) moiety and binding of the negatively charged analyte to the electron deficient acceptor (A) moieties modulates the charge transfer character of the D–A system.21,99,100 In colorimetric anthraquinone-imidazole based receptors the charge transfer band arises mainly from the  . The anthraquinone moieties serve as excellent acceptors due their electron deficient nature and conjugation with different electron rich aromatic donor moieties which help to modulate the charge transfer band that can be exploited for detection.45,101–106
. The anthraquinone moieties serve as excellent acceptors due their electron deficient nature and conjugation with different electron rich aromatic donor moieties which help to modulate the charge transfer band that can be exploited for detection.45,101–106
In this work, we varied the donor system with different aromatic systems including 2,5-dihydroxy benzene, 4-(bis(2-chloroethyl)amino)benzene, imidazole and 4-methylthiazole to prepare four compounds with potential as chemosensors (1–4). Our objective was to study how the variation of the donor type changes the compounds’ behaviour towards the anions of choice viz. fluoride and cyanide. Our endeavour showed that among compounds 1–4, compounds 1 and 4 detect F− and CN− with an almost similar efficiency but could not exhibit any difference in recognition between F− and CN− separately. However, in the presence of Cu2+4 could distinguish F− from CN− and hence compound 4 can act as a receptor for recognition of F− with a detection limit of 0.038(5) ppm at a receptor concentration of 25 μM.
Results and discussion
Syntheses
We have synthesized four anthraimidazoledione derivatives (1–4) with four different aldehydes as depicted in Scheme 1. All the compounds were well characterized by 1H NMR, 13C NMR, CHN, IR as well as ESI-MS. The data obtained confirm the purity of the compounds. All compounds presented here are soluble in DMSO-d6. The compounds are not soluble in any chlorinated solvent. Their solubility in polar protic solvents is good enough for the electronic spectral studies and anion recognition. All the compounds are insoluble in water. | ||
| Scheme 1 Representative synthetic scheme for the preparation of 1–4. Reaction conditions applied: ethanol (100 mL), trifluoroacetic acid (catalytic amount), heated to reflux for 16–18 h. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with the addition of various anions (F−, Cl−, Br−, I−, CN−, NO3−, H2PO4−, OAc− and ClO4−) as tetrabutylammonium salts. Water was avoided due to poor solubility of the receptors. All of the four compounds exhibit a charge transfer band around 409–499 nm (Fig. S1, ESI†), which may be assigned to the
1) with the addition of various anions (F−, Cl−, Br−, I−, CN−, NO3−, H2PO4−, OAc− and ClO4−) as tetrabutylammonium salts. Water was avoided due to poor solubility of the receptors. All of the four compounds exhibit a charge transfer band around 409–499 nm (Fig. S1, ESI†), which may be assigned to the  electronic transition.20,21,34,97 Compounds 1 and 4 were found to be sensitive towards F− and CN− as per the results of UV-vis spectroscopic studies. However, 2 and 3 did not show any recognition capability in the presence of the above-mentioned anion using the same receptor concentration as 1 and 4 (i.e. 25 μM) (Fig. S2 and S3, ESI†).
electronic transition.20,21,34,97 Compounds 1 and 4 were found to be sensitive towards F− and CN− as per the results of UV-vis spectroscopic studies. However, 2 and 3 did not show any recognition capability in the presence of the above-mentioned anion using the same receptor concentration as 1 and 4 (i.e. 25 μM) (Fig. S2 and S3, ESI†).
The use of 25 μM of 1 and 4 showed that the ICT bands were red-shifted upon gradual addition of F− and CN− in contrast to other anions. Compound 1 has the 2,5-dihydroxy substituted aryl ring as the donor and anthraquinone as the acceptor. 1 showed a positive but similar colorimetric response in the presence of either F− or CN−. It did not respond to other afore-mentioned common anions probed here (Fig. 1). The colour of the solution turned from yellow to bright orange when F− and CN− solutions were added to the solution of 1 (ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO = 40
DMSO = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Fig. 1). The charge transfer band at 425 nm showed a bathochromic or red shift (Δλ = 59 nm) for both F− and CN−.
1) (Fig. 1). The charge transfer band at 425 nm showed a bathochromic or red shift (Δλ = 59 nm) for both F− and CN−.
When 4 was used as the sensor the red shift of the band at 400 nm was more for both CN− (Δλ = 68 nm) and F− (Δλ = 72 nm) compared to 1. The successive addition of F− and CN− ions to 1 showed a gradual decrease in the absorption band at 425 nm and increase at 484 nm. The saturation limits were achieved with the addition of 1.6 equivalents of F− and 1.4 equivalents of CN− ions. The titration curves for F− and CN− ions are shown in Fig. 2. Successive addition of F− or CN− solution to 4 showed a gradual decrease in absorbance in the range of 401–404 nm along with a concomitant increase in the range of 472–474 nm (Fig. 3). The saturation limit was achieved much earlier for CN− (1.28 equiv., 32 μM) compared to F− (2.48 equiv., 62 μM). The saturation achieved with low excess of the anions suggests that the sensitivity is high towards F− or CN−. The presence of isosbestic points, at 450 nm for 1 (Fig. 2) and 430 nm for 4 (Fig. 3), in the case of either F− or CN−, indicates the presence of two different species at equilibrium. The presence of two different isosbestic points at 350 and 450 nm for 1 and 345 and 430 nm for 4 suggests that the binding stoichiometry may be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Hence we performed a Job's plot for receptors 1 and 4 with F− and CN− and confirmed that the binding ratio is 1
1. Hence we performed a Job's plot for receptors 1 and 4 with F− and CN− and confirmed that the binding ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 4). The data showed that both 1 and 4 are not able to distinguish between F− or CN− and detect both of them. The above results signify that both F− and CN− are almost equally efficient in forming adducts with the –NH proton in 1 and 4 thereby leading to the deprotonation of –NH and decreasing the energy gap between the πimidazole and
1 (Fig. 4). The data showed that both 1 and 4 are not able to distinguish between F− or CN− and detect both of them. The above results signify that both F− and CN− are almost equally efficient in forming adducts with the –NH proton in 1 and 4 thereby leading to the deprotonation of –NH and decreasing the energy gap between the πimidazole and  orbitals rendering a bathochromic shift.
orbitals rendering a bathochromic shift.
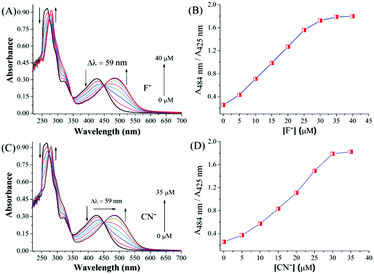 | ||
| Fig. 2 UV-vis spectroscopic titration of 1 (25 μM) with (A) fluoride (0–40 μM), (B) ratiometric plot with fluoride, (C) cyanide (0–35 μM) and (D) ratiometric plot with cyanide. | ||
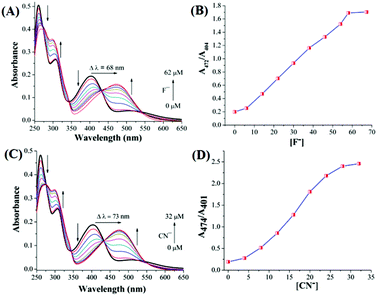 | ||
| Fig. 3 UV-vis spectroscopic titration of 4 (25 μM) with (A) fluoride (0–62 μM), (B) ratiometric plot with fluoride, (C) cyanide (0–32 μM) and (D) ratiometric plot with cyanide. | ||
As mentioned earlier 3, where two imidazole rings are present, did not show anion recognition in the same receptor concentration level as of 1 and 4 (i.e. 25 μM) (Fig. S3 and S4, ESI†). With the addition of up to 6 equivalents of anions (F− and CN−) and 2 equivalents of OH−, 3 hardly showed any red shift of the charge transfer band at 500 nm (Fig. S4, ESI†). Similar to 4 the imidazole protons in 3 are supposed to be susceptible to deprotonation and that should influence the CT band and yet we did not see any change in the spectra at concentrations similar to 1 and 4 so we increased the concentration of 3 to 150 μM and continued adding TBAOH up to 1400 μM. In this case we were able to see a beginning of a ca. 150 nm red shift after the addition of ca. 7 equivalents of OH− which became saturated at ca. 9 equivalents. However, the colour change did not persist long and within 90 min the spectrum reverted to its original position (Fig. S5A, ESI†). The results signify that the deprotonation of the imidazole hydrogen of 3 is difficult compared to 1 and 4 and it also corroborates well since in the presence of a base the colour change of the CT band takes place but after a while reverts to original. We probed the behaviour of 3 with F− and found that it is less sensitive to the addition of F− when compared with 1 and 4 and takes at least 110 equivalents of F− to achieve a similar 50 nm shift, which is saturated at 230 equivalents of F− (Fig. S5B, ESI†). The above phenomenon leads to a poor detection limit of F− by 3 (259 mM; 1054 ppm). In the case of 2 the presence of the weak electron withdrawing bis(2-chloroethylamine) moiety decreases the acidity of the imidazole proton hence the F− or the CN− adduct is weaker and unstable and 2 is not suitable for use as a sensor of the probed anions.
The obtained differences in the results based on the addition of TBAOH and TBAF/TBACN suggest the use of TBAOH for mechanistic purpose may be useful but the receptors may be much less sensitive to the anions viz. F− and CN− compared to the deprotonation by OH− and hence the predicted mechanism is only an indication of the possible pathway. The binding stoichiometry for the receptors 1 and 4 was evaluated using the Job's plot and it was found that they bind in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio as shown in Fig. 4.
1 ratio as shown in Fig. 4.
We calculated the detectable limit of fluoride and cyanide ions from the ratiometric plot of A484nm/A425nmvs. [anion].107 The apparent association constants for anions were calculated from nonlinear regression analysis and the values are tabulated in Table 1. The apparent binding constants for F− and CN− are in the range of 103 to 104 M−1 showing relatively weak binding with the anions. The association constants in Table 1 show that without the influence of Cu2+, CN− has a higher affinity for receptors 1 and 4 signifying that under such conditions CN− is more efficient in deprotonating the –NH. The UV-vis titrations also show that less CN− is required to achieve saturation compared to the amount of F−. The detection limits were calculated in parts per million (ppm) units. The calculations show that the detection limits are lower than the permitted limit set by WHO or EPA for both fluoride (1.5 ppm) and cyanides (0.2 ppm).20,108 We achieved the lowest detection limit of ca. 0.07(6) ppm for F− in the case of 1 and 0.12(3) ppm for CN− in the case of 4. However, it should be noted that 1 and 4 could not distinguish CN− from F− but we will see later that the presence of Cu2+ helps the selective detection of F−.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Receptors | Anions | Δλ (nm) | K asso (M−1) | Detection limit (ppm) |
|---|---|---|---|---|
| a Experiments were carried out in triplicate. The values in parentheses show standard errors. | ||||
| 1 | F− | 59 | 5.63(8) × 103 | 0.068(6) |
| CN− | 59 | 1.27(3) × 104 | 0.164(4) | |
| 4 | F− | 68 | 1.17(2) × 104 | 0.081(2) |
| CN− | 73 | 4.9(3) × 104 | 0.123(3) | |
1H titration experiment
The mechanism of F− and CN− detection was further explored through NMR using 1H NMR titration experiments for the receptors 1 and 4 in DMSO-d6. With the addition of TBAF solution to 1 we observed that the two singlet peaks at 12.7 and 11.2 ppm for the two phenolic –OH vanished signifying quick chemical exchange or possible deprotonation in the presence of the anion and the singlet NH peak at 9.2 ppm was also broadened (Fig. 5). The broadening of the NH peak suggests that the imidazole hydrogen may be experiencing hydrogen bonding interactions with F−. After the addition of 1.0 equivalent of TBAF the imidazole N–H signal vanished emphasizing that the F− might have deprotonated the –NH group by interactions with the hydrogen trying to form the [HF2]− type of species. Indeed after the addition of 2.5 equivalents of F− one triplet started to appear at ca. 16.3 ppm which is known to be due to the formation of the adduct [HF2]− (Fig. 5). This adduct formation was also confirmed by 19F NMR where we observed a sharp peak around −145 ppm for this same adduct formation (Fig. S6†).109 The deprotonation of imidazole –NH develops a negatively charged ring causing the upfield shift of protons of the anthraquinone moiety. Beside the triplet peak at 16.3 ppm for the [HF2]− adduct, one singlet peak at 14.5 ppm gradually increases at the same time and generation of this singlet peak might be due to the formation of [Nδ−⋯Hδ+⋯Oδ−] as shown in Fig. 5 since if it was due to the formation of [Oδ−⋯Hδ+⋯Fδ−] then it should have appeared as a doublet instead of a singlet, but we only observed a singlet in the spectra (Fig. 5, inset A) which agrees well with similar observations in the literature.110 This peak of [Nδ−⋯Hδ+⋯Oδ−] arises due to the hydrogen bonding of an imidazole nitrogen with one of the –OH groups of the hydroquinone upon deprotonation of the –NH in the imidazole ring.Compound 4 also showed the generation of a triplet peak for [HF2]− at ca. 16.3 ppm with the successive addition of TBAF in the 1H NMR (Fig. S7, inset A, ESI†). The imidazole –NH peak at 7.9 ppm also broadened and ultimately vanished after the addition of 1.25 equivalents of F−. The 19F NMR also confirms the deprotonation since we observed a sharp peak at ca. −145 ppm for the [HF2]− adduct formation (Fig. S8, ESI†). The deprotonation phenomenon was further confirmed from the results of the UV-vis experiment of 1 and 4 with a strong base viz. TBAOH. We observe a similar red shift of ca. 57 nm and 72 nm respectively (Fig. S9, ESI†) comparable to that obtained for F− and CN− using the same receptors.
To study the effect of cyanide addition, 1H NMR titrations were performed with 1 and 4 with TBACN. We could observe the –NH and –OH signals vanishing upon addition of 0.4–1.0 equivalents of TBACN for both 1 and 4 (Fig. S10, S11†) and the same colour change suggests speciation similar to F− for receptors 1 and 4. Hence, the electronic spectroscopy studies could not distinguish between the fluoride and cyanide.
We also probed 1H and 19F titration for 3 using 5.4 × 10−2 M receptor concentration which is close to that taken for the NMR studies of 1 and 4 (4.96 × 10−2 M for 1 and 9.9 × 10−2 M for 4). We observed deprotonation of the imidazole hydrogen and the formation of the [HF2]− adduct both in 1H (triplet at ca. 16.1 ppm) and 19F NMR (doublet at −143 ppm) (Fig. S12–S14†). However, as mentioned earlier there is no F− recognition by 3 at concentrations similar to 1 and 4 and even at higher concentrations (1.4 × 10−3 M) and at least 110 equivalent excess concentration of F− is required for recognition (Fig. S5B, ESI†).
In order to achieve a recognizable difference in the UV-vis spectra due to changes in the interaction we used Cu2+. Since Cu2+ is known to have a stronger interaction with CN− we thought that this will help us based on the numerous literature reports available with Cu2+ enabling cyanide sensing in the presence of a ligand.56,111–114 When we probed, we found that instead of cyanide the F− sensing improved in the presence of Cu2+ as discussed in the next section.
In the presence of Cu2+ ions
The spectral pattern of the CT band in 4 in the presence of Cu2+ showed a distinct effect and we could achieve specific recognition of F−. The successive addition of F− and CN− ions to 4 in the presence of 0.5 equivalent of Cu2+ is displayed in Fig. 6. We have added up to 60 μM (2.4 equivalents) of CN− to obtain only a 15 nm red shift. We did not find the 15 nm shift in the presence of CN− to be sufficient for a good detection so we did not attempt studies on detection of cyanide with 4. The change in Δλ for F− showed a difference of 50 nm compared to that of ca. 15 nm for CN− (Fig. 6 and Fig. S15, ESI†). On addition of approx. half equivalent of Cu2+ with respect to sensor 4, the yellow solution turns orange for all the anions probed except for F−. Addition of up to ca. 5.6 equivalents of Cu2+ alone did not render any considerable change in the CT band (λmax = 400 nm) (Fig. S16A, ESI†). However, in the presence of only 0.5 equivalent Cu2+4 could sense F− specifically as the CT band shifts by 50 nm.In order to probe if deprotonation is one of the causes for recognition even in the presence of Cu2+, we carried out UV-vis titration with up to ca. 1.2 equivalents of TBAOH instead of 1 equivalent of F−. A similar 51 nm red shift was observed. The above data suggest that the deprotonation phenomenon is also acting in the presence of Cu2+ ions (Fig. S16B, ESI†). The Job's plot for F− sensing for 4 in the presence of Cu2+ also indicates a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (Fig. S17, ESI†). Comparatively 1 hardly showed any significant change usable for detection purpose by a shift in the charge transfer band on addition of the other afore-mentioned anions in the presence of Cu2+ (Fig. S18 and S19, ESI†).
1 ratio (Fig. S17, ESI†). Comparatively 1 hardly showed any significant change usable for detection purpose by a shift in the charge transfer band on addition of the other afore-mentioned anions in the presence of Cu2+ (Fig. S18 and S19, ESI†).
We found that with the successive addition of F− to 4 in the presence of Cu2+ a gradual decrease in the absorption band at 401 nm was observed with a concomitant increase at 451 nm. The saturation limits were achieved with addition of just 1 equivalent of F−. The apparent association constant of 4 for F− and CN− in the presence of Cu2+ was found to be 1.37(2) × 105 M−1 and the detection limit was achieved to be 0.038(5) ppm in the case of F− (Table 2).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Receptor | Anions | Δλ (nm) | K asso (M−1) | Detection limit (ppm) |
|---|---|---|---|---|
| a Experiments were carried out in triplicate. The values in parentheses show standard errors. | ||||
| 4 | F− | 50 | 1.37(2) × 105 | 0.038(5) |
| CN− | 15 | 1.19(4) × 104 | 0.237(3) | |
Compounds 1–4 have anthraquinone in common but the pendant moieties attached to the imidazole ring fused with anthraquinone are different. We found that 2 and 3 do not exhibit distinct recognition for any of the probed anions but 1 and 4 recognize F− and CN−. In the case of 1 the proton of the imidazole moiety is released in the presence of F− and CN− which renders a spectral change leading to the recognition of the fluoride or cyanide. Since both F− and CN− are equally efficient in interacting with the proton of the imidazole they cannot be distinguished from each other by 1.
The titration experiments of 1–4 with OH− show that 4 is the easiest one to deprotonate as seen from the UV spectral changes (Fig. S20D, ESI†) followed by 1 (Fig. S20A, ESI†) which is also pretty similar in terms of stoichiometry needed to observe the change in the CT band which may be assigned to the deprotonation of the benzimidazole nitrogen. Compounds 2 and 3 however require higher stoichiometries of OH− to observe the bathochromic shift of the CT band as seen in Fig. S20B & C, (ESI†). Receptor 2 requires at least 9 molar equivalents of OH− to initiate the prominent spectral change of the CT band and 3 requires at least 7 molar equivalents of OH− for the same (Fig. S20B and C, ESI†). In contrast for 1 and 4 the saturation for the shift in the charge transfer band is achieved within 1.0 molar equivalent of OH−. The above results signify that the benzimidazole –NH has low acidity in 2 and 3 compared to 1 and 4. Hence, 2 and 3 are not useful as sensors to recognize any of the probed anions including F− and CN−.
The NMR studies showed that HF2− is formed in 3 similar to that reported in the literature and found by us for 1 and 4. However, the NMR studies are performed in greater concentrations and prominent changes are observed with higher equivalents of F− compared to the UV-vis studies. Hence, the formation of HF2− should not be considered as a sufficient indication. Hence the visible colour change to the CT band, rendered by the interaction of the anion to the receptor is necessary for the recognition which is also dependent on the acidity of the –NH proton. The presence of the sulphur atom in 4 is clearly advantageous to render the colour change of the CT band upon interaction of the F− with 4 since it helps the recognition with Cu2+. The presence of Cu2+ should lead to an interaction between the receptor and the Cu2+ although we did not observe any colour change upon addition of Cu2+ to the receptor 1 or 4 (Fig. 6 and S16A, S19A, ESI†) but in the presence of F− and Cu2+ in the case of 4 the change becomes significant which is in contrast to other anions including CN−.
In most cases the probable species formed which renders the colour is not commented on since it is understood that the uncertainties are high. However, based on the evidence obtained we propose a plausible speciation for the F− detection by 4 (Scheme 2). We strongly feel that prediction of the possible interactions would lead to better understanding of the possible species responsible for recognition in future. The above speciation is based on the following: (i) we have seen that in the presence of TBAOH and in the presence of F− the colour change is the same using similar concentrations of the anions. Hence, deprotonation may be occurring in both cases leading to the formation of HF2− which supports that species II may be forming in solution rendering the colouration. (ii) The position of HF2− is almost the same in both the cases of 1 and 4 suggesting that they may not be influenced by other atoms of 1 and 4i.e. the formed HF2− may be independent from the negatively charged receptor molecule and helps recognition supporting the formation of II (Scheme 2). (iii) In the presence of Cu2+ added to the solution of 1 or 4 we do not see much shift in the native CT-band but when F− is added to 4 there is a similar shift to that observed in the presence of TBAOH and Cu2+. Hence here V may be the species forming in solution rendering the colouration. The small shift of 15 nm for the CT band in the case of addition of CN− in the presence of Cu2+ shows that the interaction of CN− in the presence of Cu2+ in 4 is weaker than F− which is supported by the obtained association constant (Table 2). Based on the results, 4 is not suitable for CN− recognition.
The structural difference of receptor 1 renders a different speciation in the presence of Cu2+, which does not increase the acidity of the –NH proton of the benzimidazole sufficient enough such that it may be deprotonated by F− or CN−. However, due to the stronger deprotonation ability of TBAOH the CT band of 1 shows a shift (Fig. S16 and S19, ESI†) similar to that in the case of 4 (in the presence of F− and Cu2+). The data also show that a higher concentration of OH− is required in 1 to observe changes in the CT band similar to 4 suggesting that the F− may not be able to form the anion of 1 diminishing its recognition capability.
Our preliminary computational studies by the DFT level of theory with the B3LYP function and 6-31G(d) basis set showed that the charge transfer bands are indeed from  (Fig. S21 and Table S1, ESI†) as assigned earlier in the literature,20,21,34,97 and the bathochromic shift in the CT band upon recognition of F− and CN− is due to deprotonation of the benzimidazole –NH, as found from the TDDFT calculations of the deprotonated optimized structure of 1 and 4 (Fig. S22, ESI†).
(Fig. S21 and Table S1, ESI†) as assigned earlier in the literature,20,21,34,97 and the bathochromic shift in the CT band upon recognition of F− and CN− is due to deprotonation of the benzimidazole –NH, as found from the TDDFT calculations of the deprotonated optimized structure of 1 and 4 (Fig. S22, ESI†).
Experimental
Materials and methods
All chemicals and solvents were purchased from commercial sources. 1,2-Diaminoanthraquinone and other reagents were purchased from Sigma-Aldrich and Spectrochem. Solvents are of spectroscopic and GC grade and purchased from Merck. Melting points for the compounds were measured in a SECOR India melting point apparatus and the uncorrected values are reported. UV-visible measurements were performed using a Perkin Elmer lambda 35 spectrophotometer. FT-IR spectra were recorded using a Perkin-Elmer SPECTRUM RX I spectrometer in KBr pellets. 1H & proton decoupled 13C NMR spectra were recorded using either a JEOL ECS 400 MHz or a Bruker Avance III 500 MHz spectrometer at room temperature and the chemical shifts are reported in parts per million (ppm). Elemental analyses were performed on a Perkin-Elmer 2400 series II CHNS/O series. ESI-MS spectra were recorded using a micromass Q-Tof micro™ (Waters) in ESI +ve mode electrospray ionization. The isolated yields were reported for analytically pure compounds.Syntheses and characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 266 (44068), 319 (13530), 424 (12692). IR (KBr, cm−1) 3391 (s), 2358 (s), 1668 (s), 1495 (s), 1330 (s), 1294 (s), 1261 (s), 1218 (m), 717 (s); ESI-MS (CH3OH), m/z (calc.): 379.21(379.07) [M + Na]+.
1) 266 (44068), 319 (13530), 424 (12692). IR (KBr, cm−1) 3391 (s), 2358 (s), 1668 (s), 1495 (s), 1330 (s), 1294 (s), 1261 (s), 1218 (m), 717 (s); ESI-MS (CH3OH), m/z (calc.): 379.21(379.07) [M + Na]+.
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2C
2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2Cl) (Fig. S39, ESI†); 13C-NMR (125 MHz, DMSO-d6) δ 183.2 (CO), 182.1 (CO), 158.2 (ArC), 149.9 (ArC), 148.7 (ArC), 134.3 (ArC), 134.1 (ArC), 133.2 (ArC), 133.1 (ArC), 132.9 (ArC), 129.8 (ArC), 129.7 (ArC), 127.0 (ArC), 126.7 (ArC), 126.1 (ArC), 123.8 (ArC), 123.3 (ArC), 120.9 (ArC), 117.8 (ArC), 116.8 (ArC), 111.5 (ArC), 51.8 (CH2Cl), 41.1 (
2Cl) (Fig. S39, ESI†); 13C-NMR (125 MHz, DMSO-d6) δ 183.2 (CO), 182.1 (CO), 158.2 (ArC), 149.9 (ArC), 148.7 (ArC), 134.3 (ArC), 134.1 (ArC), 133.2 (ArC), 133.1 (ArC), 132.9 (ArC), 129.8 (ArC), 129.7 (ArC), 127.0 (ArC), 126.7 (ArC), 126.1 (ArC), 123.8 (ArC), 123.3 (ArC), 120.9 (ArC), 117.8 (ArC), 116.8 (ArC), 111.5 (ArC), 51.8 (CH2Cl), 41.1 (![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2CH2Cl) (Fig. S40, ESI†); UV-vis λmax/nm (ε/dm3 mol−1 cm−1) in CH3CN–DMSO (50
H2CH2Cl) (Fig. S40, ESI†); UV-vis λmax/nm (ε/dm3 mol−1 cm−1) in CH3CN–DMSO (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 266 (34
1) 266 (34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550), 315 (29
550), 315 (29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520), 462 (20
520), 462 (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 190); IR (KBr, cm−1) 3444 (s), 2922 (w), 1660 (s), 1607 (s), 1489 (s), 1326 (s), 1294 (s), 1184 (m), 1008 (w), 719 (s); ESI-MS (CH3OH), m/z (calc.): 464.33 (464.09) [M + H]+.
190); IR (KBr, cm−1) 3444 (s), 2922 (w), 1660 (s), 1607 (s), 1489 (s), 1326 (s), 1294 (s), 1184 (m), 1008 (w), 719 (s); ESI-MS (CH3OH), m/z (calc.): 464.33 (464.09) [M + H]+.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 260 (36
1) 260 (36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 575), 499 (7574). IR (KBr, cm−1) 3416 (s), 3368 (s), 1667 (s), 1624 (s), 1588 (s), 1531 (s), 1441 (s), 1329 (s), 1301 (s), 1165 (w), 840 (m), 715 (s); ESI-MS (CH3OH), m/z (calc.): 315.56 (315.09) [M + H]+.
575), 499 (7574). IR (KBr, cm−1) 3416 (s), 3368 (s), 1667 (s), 1624 (s), 1588 (s), 1531 (s), 1441 (s), 1329 (s), 1301 (s), 1165 (w), 840 (m), 715 (s); ESI-MS (CH3OH), m/z (calc.): 315.56 (315.09) [M + H]+.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 260 (22
1) 260 (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400), 406 (5600), 504 (3100). IR (KBr, cm−1) 3433 (s), 2373 (s), 1665 (s), 1583 (s), 1328 (s), 1293 (s), 1216 (s), 1048 (w), 1005 (m), 714 (s); ESI-MS (CH3OH), m/z (calc.): 346.17(346.06) [M + H]+.
400), 406 (5600), 504 (3100). IR (KBr, cm−1) 3433 (s), 2373 (s), 1665 (s), 1583 (s), 1328 (s), 1293 (s), 1216 (s), 1048 (w), 1005 (m), 714 (s); ESI-MS (CH3OH), m/z (calc.): 346.17(346.06) [M + H]+.
UV-vis titration of 1 and 4 with F− and CN−
20 μL of DMSO solution of 1 and 4 (10−2 M) were added in 800 μL acetonitrile solution to make the final concentration 25 μM. Acetonitrile solutions of tetrabutylammonium fluoride (TBAF) and tetrabutylammonium cyanide (TBACN) (10−3 μM) were added to the solution of 1 and 4 successively. After 2 minutes the UV-vis spectral data were recorded at room temperature.Determination of binding ratios (Jobs’ plot)
1 and 4 were prepared in a DMSO mixture (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to achieve 10−2 μM concentration. Likewise TBAF and TBACN solutions were prepared in the same concentration (10−2 μM) in acetonitrile. Nine sets of sample solutions containing receptors 1 and 4 with F− and CN− were prepared in vials varying the mole fraction of 1 or 4 from 0.1 to 0.9. Thus different volumes of the receptor (1 or 4) and analyte solution (TBAF or TBACN) were added to vary the mole fraction from 0.1 to 0.9 keeping the total volume same in each case. After shaking the vials for a few minutes, the UV-vis spectra were recorded. The Job's plots were obtained by plotting ΔA vs. mole fraction of 1 or 4.
1) to achieve 10−2 μM concentration. Likewise TBAF and TBACN solutions were prepared in the same concentration (10−2 μM) in acetonitrile. Nine sets of sample solutions containing receptors 1 and 4 with F− and CN− were prepared in vials varying the mole fraction of 1 or 4 from 0.1 to 0.9. Thus different volumes of the receptor (1 or 4) and analyte solution (TBAF or TBACN) were added to vary the mole fraction from 0.1 to 0.9 keeping the total volume same in each case. After shaking the vials for a few minutes, the UV-vis spectra were recorded. The Job's plots were obtained by plotting ΔA vs. mole fraction of 1 or 4.
1H and 19F NMR titrations
Receptor 1 (10.6 mg, 0.01 mmol) was dissolved in DMSO-d6 (600 μL) and TBAF (1 M) in DMSO-d6 was added into the solution of receptor 1. After shaking the solution for a minute, 1H NMR spectra were obtained at room temperature. For compounds 3 and 4 the same methods were followed.Conclusions
The results emphasize that in the detection of F− and CN− using anthraimidazolediones the deprotonation of the benzimidazole –NH in the presence of the anion renders the recognition due to the shift of the CT band. We have seen that variation of the donor arm regulates the deprotonation and hence the recognition ability. When the donor arm has thioimidazole or p-hydroquinone it was possible to detect F− and CN−. However, selectivity for F− increased in the presence of Cu2+ due to its coordinating ability to the receptor and the influence on the deprotonation of the benzimidazole –NH. The detection limit of 0.038 ppm and selectivity for F− in the case of 4 is encouraging. The results signify that although the NMR studies reveal the formation of HF2− in the case of 1, 3 and 4, 3 cannot recognize F− at concentrations similar to 1 and 4. The UV-vis spectral studies with OH− to probe the acidity of the benzimidazole –NH show that benzimidazole –NH is most acidic in 4 followed by 1 and relatively much less acidic in 2 and 3. It should be noted that the formation of HF2− may be good evidence to predict the mechanism but does not necessarily comment on the recognition sensitivity. The presence of sulphur in the heterocyclic ring influences the anion recognition properties in a positive fashion at lower concentrations. In fact when we compare the activity of 4 with the rest in the series we also see that the presence of S in the heterocycle helps distinct recognition of F− from CN− in the presence of Cu2+.Acknowledgements
We earnestly acknowledge DST for financial support via project no. SB/S1/IC-02/2014. We also thank IISER Kolkata for infra-structural support. A.S. thanks IISER Kolkata for providing the doctoral fellowship. S.B. is thankful to CSIR, India for the research fellowship.Notes and references
- P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486–516 CrossRef CAS.
- R. Martinez-Manez and F. Sancenon, Chem. Rev., 2003, 103, 4419–4476 CrossRef CAS PubMed.
- P. A. Gale and R. Quesada, Coord. Chem. Rev., 2006, 250, 3219–3244 CrossRef CAS.
- M. Wenzel, J. R. Hiscock and P. A. Gale, Chem. Soc. Rev., 2012, 41, 480–520 RSC.
- P. A. Gale and C. Caltagirone, Chem. Soc. Rev., 2015, 44, 4212–4227 RSC.
- S. Kubik, Chem. Soc. Rev., 2010, 39, 3648–3663 RSC.
- T. S. Snowden and E. V. Anslyn, Curr. Opin. Chem. Biol., 1999, 3, 740–746 CrossRef CAS PubMed.
- P. A. Gale, Coord. Chem. Rev., 2003, 240, 1 CrossRef CAS.
- D. L. Ozsvath, Rev. Environ. Sci. Bio/Technol., 2009, 8, 59–79 CrossRef CAS.
- M. Mousny, S. Omelon, L. Wise, E. T. Everett, M. Dumitriu, D. P. Holmyard, X. Banse, J. P. Devogelaer and M. D. Grynpas, Bone, 2008, 43, 1067–1074 CrossRef CAS PubMed.
- Y. Yu, W. Yang, Z. Dong, C. Wan, J. Zhang, J. Liu, K. Xiao, Y. Huang and B. Lu, Fluoride, 2008, 41, 134–138 CAS.
- O. Fejerskov, F. Manji and V. Baelum, J. Dent. Res., 1990, 69, 692–700 CrossRef PubMed , discussion 721.
- S. Erdal and S. N. Buchanan, Environ. Health Perspect., 2005, 113, 111–117 CrossRef CAS PubMed.
- P. P. Singh, M. K. Barjatiya, S. Dhing, R. Bhatnagar, S. Kothari and V. Dhar, Urol. Res., 2001, 29, 238–244 CrossRef CAS PubMed.
- M. M. Grice, B. H. Alexander, R. Hoffbeck and D. M. Kampa, J. Occup. Environ. Med., 2007, 49, 722–729 CrossRef CAS PubMed.
- R. R. Dash, A. Gaur and C. Balomajumder, J. Hazard. Mater., 2009, 163, 1–11 CrossRef CAS PubMed.
- H. B. Leavesley, L. Li, K. Prabhakaran, J. L. Borowitz and G. E. Isom, Toxicol. Sci., 2008, 101, 101–111 CrossRef CAS PubMed.
- B. Ballantyne, Fundam. Appl. Toxicol., 1983, 3, 400–408 CrossRef CAS PubMed.
- S. Ayoob and A. K. Gupta, Crit. Rev. Environ. Sci. Technol., 2006, 36, 433–487 CrossRef CAS.
- S. Saha, A. Ghosh, P. Mahato, S. Mishra, S. K. Mishra, E. Suresh, S. Das and A. Das, Org. Lett., 2010, 12, 3406–3409 CrossRef CAS PubMed.
- N. Kumari, S. Jha and S. Bhattacharya, J. Org. Chem., 2011, 76, 8215–8222 CrossRef CAS PubMed.
- T. Gunnlaugsson, M. Glynn, G. M. Tocci, P. E. Kruger and F. M. Pfeffer, Coord. Chem. Rev., 2006, 250, 3094–3117 CrossRef CAS.
- C. Suksai and T. Tuntulani, Chem. Soc. Rev., 2003, 32, 192–202 RSC.
- V. Kumar, M. P. Kaushik, A. K. Srivastava, A. Pratap, V. Thiruvenkatam and T. N. G. Row, Anal. Chim. Acta, 2010, 663, 77–84 CrossRef CAS PubMed.
- L. M. Zimmermann-Dimer and V. G. Machado, Dyes Pigm., 2009, 82, 187–195 CrossRef CAS.
- G.-Y. Qing, Y.-B. He, Y. Zhao, C.-G. Hu, S.-Y. Liu and X. Yang, Eur. J. Org. Chem., 2006, 1574–1580 CrossRef CAS.
- D. Aldakov and P. Anzenbacher Jr., J. Am. Chem. Soc., 2004, 126, 4752–4753 CrossRef CAS PubMed.
- R. Nishiyabu and P. Anzenbacher Jr., J. Am. Chem. Soc., 2005, 127, 8270–8271 CrossRef CAS PubMed.
- J. Yoo, M.-S. Kim, S.-J. Hong, J. L. Sessler and C.-H. Lee, J. Org. Chem., 2009, 74, 1065–1069 CrossRef CAS PubMed.
- X. He, S. Hu, K. Liu, Y. Guo, J. Xu and S. Shao, Org. Lett., 2006, 8, 333–336 CrossRef CAS PubMed.
- D. H. Lee, K. H. Lee and J.-I. Hong, Org. Lett., 2001, 3, 5–8 CrossRef CAS PubMed.
- R. Nishiyabu and P. Anzenbacher Jr., Org. Lett., 2006, 8, 359–362 CrossRef CAS PubMed.
- C. Suksai and T. Tuntulani, Top. Curr. Chem., 2005, 255, 163–198 CAS.
- H. Miyaji, W. Sato and J. L. Sessler, Angew. Chem., Int. Ed., 2000, 39, 1777–1780 CrossRef CAS.
- V. Amendola, D. Esteban-Gomez, L. Fabbrizzi and M. Licchelli, Acc. Chem. Res., 2006, 39, 343–353 CrossRef CAS PubMed.
- F. Han, Y. Bao, Z. Yang, T. M. Fyles, J. Zhao, X. Peng, J. Fan, Y. Wu and S. Sun, Chem. – Eur. J., 2007, 13, 2880–2892 CrossRef CAS PubMed.
- F. D'Souza and O. Ito, Chem. Commun., 2009, 4913–4928 RSC.
- S. E. Garcia-Garrido, C. Caltagirone, M. E. Light and P. A. Gale, Chem. Commun., 2007, 1450–1452 RSC.
- X. Mei and C. Wolf, Chem. Commun., 2004, 2078–2079 RSC.
- J. Cai, B. P. Hay, N. J. Young, X. Yang and J. L. Sessler, Chem. Sci., 2013, 4, 1560–1567 RSC.
- R. Zadmard and T. Schrader, J. Am. Chem. Soc., 2005, 127, 904–915 CrossRef CAS PubMed.
- M. Jablonski and A. J. Sadlej, J. Phys. Chem. A, 2007, 111, 3423–3431 CrossRef CAS PubMed.
- R. D. Falcone, N. M. Correa, M. A. Biasutti and J. J. Silber, Langmuir, 2000, 16, 3070–3076 CrossRef CAS.
- W.-X. Liu and Y.-B. Jiang, Org. Biomol. Chem., 2007, 5, 1771–1775 CAS.
- X. Peng, Y. Wu, J. Fan, M. Tian and K. Han, J. Org. Chem., 2005, 70, 10524–10531 CrossRef CAS PubMed.
- G. Cooke and V. M. Rotello, Chem. Soc. Rev., 2002, 31, 275–286 RSC.
- S. L. Wiskur, H. Ait-Haddou, J. J. Lavigne and E. V. Anslyn, Acc. Chem. Res., 2001, 34, 963–972 CrossRef CAS PubMed.
- M. Comes, G. Rodriguez-Lopez, M. D. Marcos, R. Martinez-Manez, F. Sancenon, J. Soto, L. A. Villaescusa, P. Amoros and D. Beltran, Angew. Chem., Int. Ed., 2005, 44, 2918–2922 CrossRef CAS PubMed.
- M. Comes, E. Aznar, M. Moragues, M. D. Marcos, R. Martinez-Manez, F. Sancenon, J. Soto, L. A. Villaescusa, L. Gil and P. Amoros, Chem. – Eur. J., 2009, 15, 9024–9033 CrossRef CAS PubMed.
- M. Comes, M. D. Marcos, R. Martinez-Manez, F. Sancenon, J. Soto, L. A. Villaescusa and P. Amoros, Chem. Commun., 2008, 3639–3641 RSC.
- E. J. O'Neil and B. D. Smith, Coord. Chem. Rev., 2006, 250, 3068–3080 CrossRef.
- S. Khatua, S. H. Choi, J. Lee, K. Kim, Y. Do and D. G. Churchill, Inorg. Chem., 2009, 48, 2993–2999 CrossRef CAS PubMed.
- K. Ghosh, A. R. Sarkar, A. Samadder and A. R. Khuda-Bukhsh, Org. Lett., 2012, 14, 4314–4317 CrossRef CAS PubMed.
- T. Zhang and E. V. Anslyn, Org. Lett., 2006, 8, 1649–1652 CrossRef CAS PubMed.
- M. M. Linn, D. C. Poncio and V. G. Machado, Tetrahedron Lett., 2007, 48, 4547–4551 CrossRef CAS.
- X. Lou, D. Ou, Q. Li and Z. Li, Chem. Commun., 2012, 48, 8462–8477 RSC.
- B. T. Nguyen and E. V. Anslyn, Coord. Chem. Rev., 2006, 250, 3118–3127 CrossRef CAS.
- S.-J. Hong, J. Yoo, S.-H. Kim, J. S. Kim, J. Yoon and C.-H. Lee, Chem. Commun., 2009, 189–191 RSC.
- H. J. Kim, K. C. Ko, J. H. Lee, J. Y. Lee and J. S. Kim, Chem. Commun., 2011, 47, 2886–2888 RSC.
- J. Du, M. Hu, J. Fan and X. Peng, Chem. Soc. Rev., 2012, 41, 4511–4535 RSC.
- R. Martinez-Manez and F. Sancenon, Coord. Chem. Rev., 2006, 250, 3081–3093 CrossRef CAS.
- H. Lu, Q. Wang, Z. Li, G. Lai, J. Jiang and Z. Shen, Org. Biomol. Chem., 2011, 9, 4558–4562 CAS.
- J. Isaad and F. Salauen, Sens. Actuators, B, 2011, 157, 26–33 CrossRef CAS.
- V. Amendola, L. Fabbrizzi and L. Mosca, Chem. Soc. Rev., 2010, 39, 3889–3915 RSC.
- Y. Wu, X. Peng, J. Fan, S. Gao, M. Tian, J. Zhao and S. Sun, J. Org. Chem., 2007, 72, 62–70 CrossRef CAS PubMed.
- R. M. Duke, J. E. O'Brien, T. McCabe and T. Gunnlaugsson, Org. Biomol. Chem., 2008, 6, 4089–4092 CAS.
- K. Okamoto, T. Yamamoto, M. Akita, A. Wada and T. Kanbara, Organometallics, 2009, 28, 3307–3310 CrossRef CAS.
- S. Nishizawa, R. Kato, T. Hayashita and N. Teramae, Anal. Sci., 1998, 14, 595–597 CrossRef CAS.
- Z. Xu, N. J. Singh, S. K. Kim, D. R. Spring, K. S. Kim and J. Yoon, Chem. – Eur. J., 2011, 17, 1163–1170 CrossRef CAS PubMed.
- Y. Cui, H.-J. Mo, J.-C. Chen, Y.-L. Niu, Y.-R. Zhong, K.-C. Zheng and B.-H. Ye, Inorg. Chem., 2007, 46, 6427–6436 CrossRef CAS PubMed.
- Z. Xu, S. K. Kim, S. J. Han, C. Lee, G. Kociok-Kohn, T. D. James and J. Yoon, Eur. J. Org. Chem., 2009, 3058–3065 CrossRef CAS.
- T. Ghosh, B. G. Maiya and M. W. Wong, J. Phys. Chem. A, 2004, 108, 11249–11259 CrossRef CAS.
- H.-T. Niu, D. Su, X. Jiang, W. Yang, Z. Yin, J. He and J.-P. Cheng, Org. Biomol. Chem., 2008, 6, 3038–3040 CAS.
- Z. Xu, K. Kim Sook and J. Yoon, Chem. Soc. Rev., 2010, 39, 1457–1466 RSC.
- C. Marin-Hernandez, L. E. Santos-Figueroa, M. E. Moragues, M. M. M. Raposo, R. M. F. Batista, S. P. G. Costa, T. Pardo, R. Martinez-Manez and F. Sancenon, J. Org. Chem., 2014, 79, 10752–10761 CrossRef CAS PubMed.
- P. Piatek and J. Jurczak, Chem. Commun., 2002, 2450–2451 RSC.
- M. Querol, J. W. Chen, R. Weissleder and A. Bogdanov Jr., Org. Lett., 2005, 7, 1719–1722 CrossRef CAS PubMed.
- Y. Zhang and S. Jiang, Org. Biomol. Chem., 2012, 10, 6973–6979 CAS.
- P. A. Gale, Chem. Commun., 2008, 4525–4540 RSC.
- A. K. Mahapatra, G. Hazra and P. Sahoo, Beilstein J. Org. Chem., 2010, 6(12), 1–8 Search PubMed.
- R. J. T. Houk, S. L. Tobey and E. V. Anslyn, Top. Curr. Chem., 2005, 255, 199–229 CAS.
- D. H. Lee, J. H. Im, S. U. Son, Y. K. Chung and J.-I. Hong, J. Am. Chem. Soc., 2003, 125, 7752–7753 CrossRef CAS PubMed.
- R. M. Duke, E. B. Veale, F. M. Pfeffer, P. E. Kruger and T. Gunnlaugsson, Chem. Soc. Rev., 2010, 39, 3936–3953 RSC.
- D. Cui, X. Qian, F. Liu and R. Zhang, Org. Lett., 2004, 6, 2757–2760 CrossRef CAS PubMed.
- P. Mukhopadhyay, Y. Iwashita, M. Shirakawa, S.-i. Kawano, N. Fujita and S. Shinkai, Angew. Chem., Int. Ed., 2006, 45, 1592–1595 CrossRef CAS PubMed.
- X. Zhang, L. Guo, F.-Y. Wu and Y.-B. Jiang, Org. Lett., 2003, 5, 2667–2670 CrossRef CAS PubMed.
- N. Sakai, J. Mareda, E. Vauthey and S. Matile, Chem. Commun., 2010, 46, 4225–4237 RSC.
- P. Anzenbacher Jr., Top. Heterocycl. Chem., 2010, 24, 205–235 Search PubMed.
- M. V. R. Raju and H.-C. Lin, Org. Lett., 2013, 15, 1274–1277 CrossRef CAS PubMed.
- A. J. Evans, S. E. Matthews, A. R. Cowley and P. D. Beer, Dalton Trans., 2003, 4644–4650 RSC.
- P. A. Gale, M. B. Hursthouse, M. E. Light, J. L. Sessler, C. N. Warriner and R. S. Zimmerman, Tetrahedron Lett., 2001, 42, 6759–6762 CrossRef CAS.
- X. H. Sun, W. Li, P. F. Xia, H.-B. Luo, Y. Wei, M. S. Wong, Y.-K. Cheng and S. Shuang, J. Org. Chem., 2007, 72, 2419–2426 CrossRef CAS PubMed.
- P. Anzenbacher Jr., K. Jursikova and J. L. Sessler, J. Am. Chem. Soc., 2000, 122, 9350–9351 CrossRef.
- R. M. F. Batista, S. P. G. Costa and M. M. M. Raposo, J. Photochem. Photobiol., A, 2013, 259, 33–40 CrossRef CAS.
- K. Yoshida, T. Mori, S. Watanabe, H. Kawai and T. Nagamura, J. Chem. Soc., Perkin Trans. 2, 1999, 393–398 RSC.
- J. Shao, Y. Qiao, H. Lin and H. Lin, J. Fluoresc., 2009, 19, 183–188 CrossRef CAS PubMed.
- R. M. F. Batista, E. Oliveira, S. P. G. Costa, C. Lodeiro and M. M. M. Raposo, Org. Lett., 2007, 9, 3201–3204 CrossRef CAS PubMed.
- D. Mondal, M. Bar, D. Maity and S. Baitalik, J. Phys. Chem. C, 2015, 119, 25429–25441 CAS.
- A. Kovalchuk, J. L. Bricks, G. Reck, K. Rurack, B. Schulz, A. Szumna and H. Weisshoff, Chem. Commun., 2004, 1946–1947 RSC.
- C.-Y. Chen, J.-H. Ho, S.-L. Wang and T.-I. Ho, Photochem. Photobiol. Sci., 2003, 2, 1232–1236 CAS.
- D. Jimenez, R. Martinez-Manez, F. Sancenon and J. Soto, Tetrahedron Lett., 2002, 43, 2823–2825 CrossRef CAS.
- D. A. Jose, D. K. Kumar, B. Ganguly and A. Das, Org. Lett., 2004, 6, 3445–3448 CrossRef CAS PubMed.
- F.-y. Wu, M.-h. Hu, Y.-m. Wu, X.-f. Tan, Y.-q. Zhao and Z.-j. Ji, Spectrochim. Acta, Part A, 2006, 65A, 633–637 CrossRef CAS PubMed.
- H. Miyaji and J. L. Sessler, Angew. Chem., Int. Ed., 2001, 40, 154–157 CrossRef CAS.
- P. Anzenbacher Jr., M. A. Palacios, K. Jursikova and M. Marquez, Org. Lett., 2005, 7, 5027–5030 CrossRef PubMed.
- K. Wannajuk, M. Jamkatoke, T. Tuntulani and B. Tomapatanaget, Tetrahedron, 2012, 68, 8899–8904 CrossRef CAS.
- S. Anbu, S. Shanmugaraju, R. Ravishankaran, A. A. Karande and P. S. Mukherjee, Dalton Trans., 2012, 41, 13330–13337 RSC.
- P. B. Maithani, R. Gurjar, R. Banerjee, B. K. Balaji, S. Ramachandran and R. Singh, Curr. Sci., 1998, 74, 773–777 CAS.
- C. B. Rosen, D. J. Hansen and K. V. Gothelf, Org. Biomol. Chem., 2013, 11, 7916–7922 CAS.
- P. M. Tolstoy, S. N. Smirnov, I. G. Shenderovich, N. S. Golubev, G. S. Denisov and H.-H. Limbach, J. Mol. Struct., 2004, 700, 19–27 CrossRef CAS.
- S.-Y. Chung, S.-W. Nam, J. Lim, S. Park and J. Yoon, Chem. Commun., 2009, 2866–2868 RSC.
- X. Chen, S.-W. Nam, G.-H. Kim, N. Song, Y. Jeong, I. Shin, S. K. Kim, J. Kim, S. Park and J. Yoon, Chem. Commun., 2010, 46, 8953–8955 RSC.
- X. Lou, L. Zhang, J. Qin and Z. Li, Chem. Commun., 2008, 5848–5850 RSC.
- H. S. Jung, J. H. Han, Z. H. Kim, C. Kang and J. S. Kim, Org. Lett., 2011, 13, 5056–5059 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed UV-vis spectral data, fitting plots, 1H, 19F NMR titration data, 1H and 13C NMR characterization data. See DOI: 10.1039/c5dt03209a |
| This journal is © The Royal Society of Chemistry 2016 |





