Hydroalumination of alkynyl-aminophosphines as a promising tool for the synthesis of unusual phosphines: P–N bond activation, a transient phosphaallene, a zwitterionic AlP2C2 heterocycle and a masked Al/P-based frustrated Lewis pair†
Abstract
Treatment of the new alkynyl-chlorophosphine, Mes-P(Cl)–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CMe3, with LiNR2 afforded various unprecedented aminophosphines, Mes-P(NR2)–C
C–CMe3, with LiNR2 afforded various unprecedented aminophosphines, Mes-P(NR2)–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CMe3, which showed a fascinating diversity in their reactivity towards H-AltBu2. NMe2 and NEt2 derivatives yielded the hydroalumination products Mes-P(NR2)–C(AltBu2)
C–CMe3, which showed a fascinating diversity in their reactivity towards H-AltBu2. NMe2 and NEt2 derivatives yielded the hydroalumination products Mes-P(NR2)–C(AltBu2)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)–CMe3 which have an Al–N and an activated P–N bond. Elimination of aluminium amide yielded the transient 3H-phosphaallene, Mes-P
C(H)–CMe3 which have an Al–N and an activated P–N bond. Elimination of aluminium amide yielded the transient 3H-phosphaallene, Mes-P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
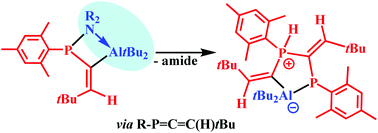
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)-CMe3, which finally afforded a five-membered AlP2C2 heterocycle with an Al–P bond and two exocyclic C
C(H)-CMe3, which finally afforded a five-membered AlP2C2 heterocycle with an Al–P bond and two exocyclic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. This heterocycle is directly formed with the sterically shielded iPr2N- and dimethylpiperidinophosphines. A unique R2AlH adduct resulted from the NPh2 and N(SiMe3)2 substituted phosphines. It may be viewed as an Al/P-based frustrated Lewis pair (FLP) which coordinates an R2AlH moiety. The heterocyclic AlP2C2 compound is formed in the final step of this reaction. Hydroalumination with Et2AlH yielded [Mes-P(H)–C(AlEt2)
C bonds. This heterocycle is directly formed with the sterically shielded iPr2N- and dimethylpiperidinophosphines. A unique R2AlH adduct resulted from the NPh2 and N(SiMe3)2 substituted phosphines. It may be viewed as an Al/P-based frustrated Lewis pair (FLP) which coordinates an R2AlH moiety. The heterocyclic AlP2C2 compound is formed in the final step of this reaction. Hydroalumination with Et2AlH yielded [Mes-P(H)–C(AlEt2)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)–CMe3]2 which features an Al2P2C2 heterocycle and two Al–P bonds. This dimer resembles the class of hidden or masked FLPs which show a reactivity similar to uncoordinated FLPs. Its unique structural motif is a P–H bond which may result in a new type of FLP chemistry.
C(H)–CMe3]2 which features an Al2P2C2 heterocycle and two Al–P bonds. This dimer resembles the class of hidden or masked FLPs which show a reactivity similar to uncoordinated FLPs. Its unique structural motif is a P–H bond which may result in a new type of FLP chemistry.
- This article is part of the themed collection: Phosphorus Chemistry: Discoveries and Advances

 Please wait while we load your content...
Please wait while we load your content...