Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Interaction of alkali acetates with silica supported PdAu†‡§
Elisabeth K.
Hanrieder
,
Andreas
Jentys
* and
Johannes A.
Lercher
*
Department of Chemistry and Catalysis Research Center, Technische Universität München, Lichtenbergstr. 4, 85747 Garching, Germany. E-mail: jentys@tum.de; johannes.lercher@ch.tum.de; Fax: +49 89 289 13544; Tel: +49 89 289 13538 Tel: +49 89 289 13540
First published on 15th July 2016
Abstract
The temperature dependence of the interaction of PdAu alloy particles with alkali acetate promoters (MOAc; M+ = Li+, Na+, K+, Cs+) was studied by X-ray absorption and infrared spectroscopy as well as in situ X-ray diffraction. Alkali acetate promotion on PdAu particles results in irreversible enlargements of the Pd–Pd distances upon heating compared to promoter free PdAu/SiO2. The promoter induces the migration of Pd to the metal particle surface and the formation of a mixed Pd/M+–O layer on the surface of the bimetallic PdAu particles. This structure enhances catalytic reactions by electronically and geometrically modifying the PdAu surface.
1. Introduction
Bimetallic alloy catalysts have attracted significant attention as the presence of a second metal allows to subtly modify activity and selectivity.1–3 Metal combinations exhibiting a continuous range of solubility such as Pd and Au,4 where both elements have similar electronegativity, metal radii and number of valence electrons have been found to be particularly rewarding.5 Formation heats of PdAu alloys are exothermic over the entire composition range, pointing to attractive interactions between the alloy constituents and to a tendency to form long range ordered phases6 such as Au3Pd (stable up to ∼850 °C), Au1Pd1 (stable up to ∼100 °C) and AuPd3 (stable up to ∼870 °C).7,8 Lattice parameters of these bimetallic phases follow Vegard's law and vary from 0.389 nm to 0.406 nm as a function of the composition for PdAu particles with a small negative deviation of 0.0004 nm at about 30 at% Pd.9However, the Pd/Au composition of the bulk and the catalytically active surface can differ markedly4,10 due to differences in surface free energies of Pd (2.043 J m−2)11 and Au (1.626 J m−2).12 Au preferentially decorates the surface in PdAu particles upon heating in vacuum,13 however, we have recently shown that the presence of potassium acetate (KOAc) on PdAu, the trend reverses to surface segregation of Pd under reaction conditions of vinyl acetate (VA) synthesis.14,15 This change is induced by the strong interaction of KOAc with Pd.14,16,17
The activity and selectivity of the structure sensitive VA synthesis reaction are determined by the relative arrangement of active Pd monomers suitably spaced by inert Au atoms on the solid surface.18 Thus, the rate-enhancing effect of KOAc in VA synthesis is attributed to a geometric reordering in PdAu particles. It is unclear, however, whether such local reordering induced by KOAc exists also under “non-reactive” conditions and which impact the nature of the alkali metal has on this process. To address these questions, we systematically studied the impact of the promoter metal nature on the local environment of Pd and Au using a combination of bulk and surface sensitive techniques and to explore the impact on activity and selectivity for the structure sensitive VA synthesis.
2. Experimental
2.1. Synthesis
PdAu/SiO2 with an atomic Pd/Au ratio of 2.0 was prepared via incipient wetness impregnation.19 HAuCl4 and PdCl2 were dissolved in bidistilled water (1 mL g−1 support) and impregnated on SiO2 (HDK®). Subsequent precipitation with sodium carbonate and washing with ammonia solution (pH 8) was carried out to remove chloride ions from the precipitated metal salts. The PdAu/SiO2 precursors were reduced in flowing H2 (100 mL min−1) at 300 °C for 1 h (5 °C min−1). The Pd and Au metal loading was 1.5 wt% each for all catalysts. The monometallic references Pd/SiO2 and Au/SiO2 were synthesized with a metal loading of 3 wt% in the same way. Li+, Na+, K+ and Cs+ were added as acetates to PdAu/SiO2, Pd/SiO2 and Au/SiO2. For the K+ containing catalysts, different counter ions were used including acetate (KOAc), hydroxide (KOH), carbonate (K2CO3) and oxalate (K2C2O4), while maintaining a constant potassium concentration of 1.28 mmol g−1 catalyst. Representative TEM pictures including particle size distributions of PdAu/SiO2 and on PdAu/KOAc/SiO2 are shown in Fig. S1, ESI.§2.2. Elemental analysis
Potassium, palladium and gold contents were determined by atomic absorption spectroscopy (AAS). For this, 50 mg of catalyst was dissolved in a mixture containing 48% hydrofluoric acid and nitro-hydrochloric acid. The spectrometer used was a Solaar M5 Dual Flame graphite furnace AAS (ThermoFisher).2.3. X-ray powder diffraction
X-Ray powder diffraction measurements were conducted on a Philips X'Pert Pro PW 3040/60 system in Bragg–Brentano geometry (θ–2θ-goniometer) using Cu Kα radiation (0.154056 nm) generated at 45 kV and 40 mA and a solid state detector (X'Celerator). In situ experiments were carried out in an Anton Paar HTK 1200 cell under flowing gas atmosphere (N2, H2, synthetic air). The XRD patterns were measured in a 2θ range of 5–70° with a step size of 0.019° s−1. Samples were annealed at 140 °C or 300 °C and cooled to ambient temperature with a heating and cooling rate of 3 °C min−1. The two temperature programs are shown in Fig. S2, ESI.§ The XRD patterns obtained were analyzed with High Score Plus (PANalytical) and compared to Au and Pd references from the Crystallographic Open Database (COD). Alloy compositions were calculated by applying fitted peak positions to Vegard's law.202.4. IR spectroscopy of adsorbed CO
The IR spectra were recorded on a Vertex 70 spectrometer from Bruker Optics at a resolution of 4 cm−1 collecting 100 scans. Samples were pressed into self-supporting wafers (∼10 mg cm−2), activated in vacuum (∼1.0 × 10−7 mbar) and reduced in static H2 (1000 mbar) at 300 °C for 1 h with a heating rate of 5 °C min−1. The samples were outgassed at 300 °C in vacuum for 30 min to remove Pd hydrides before the temperature was decreased either to 100 °C or to −150 °C in 5 mbar He (added to improve the thermal conductivity) to record a spectrum of the activated sample. 1.0 mbar CO was introduced and spectra were recorded until the CO adsorption–desorption equilibrium was established.Analysis of the spectra was carried out using the software Grams AI. The spectra were background corrected and subtracted from the background corrected spectrum of the activated sample to isolate the infrared bands of adsorbed CO. These subtracted spectra were normalized to the integrated area of Si–O overtones between 2107 and 1741 cm−1 of the activated sample. The intensities of the CO absorption bands were evaluated by fitting with a mixed 50/50 Gaussian–Lorentzian function.
2.5. X-ray absorption spectroscopy at the Pd-K and Au-L3-edge
X-Ray absorption spectra were measured at HASYLAB (DESY, Hamburg/Germany) on the beamlines X1 and C (electron energy of 4.5 GeV, average current of 100 mA). Spectra were recorded in transmission mode at the Pd-K edge (E0 = 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 eV) using Si(311) crystals and Au-L3 edge (E0 = 11
350 eV) using Si(311) crystals and Au-L3 edge (E0 = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 919 eV) using Si(111) crystals. The samples were pressed into self-supporting wafers with weights to obtain a total absorbance of 2.0 to optimize the signal to noise ratio. The samples were activated at 300 °C with 5 °C min−1 in He (100 mL min−1), reduced at 300 °C for 1 h in H2 and flushed with 150 mL min−1 He before cooling to liquid nitrogen temperature to record 2–3 spectra per sample.
919 eV) using Si(111) crystals. The samples were pressed into self-supporting wafers with weights to obtain a total absorbance of 2.0 to optimize the signal to noise ratio. The samples were activated at 300 °C with 5 °C min−1 in He (100 mL min−1), reduced at 300 °C for 1 h in H2 and flushed with 150 mL min−1 He before cooling to liquid nitrogen temperature to record 2–3 spectra per sample.
EXAFS data were processed with IFEFFIT, Athena and Artemis.21 The scattering contributions of the pre and post-edge were removed by a third-order polynomial function. The oscillations were weighted by k2 and Fourier transformed within the limits k = 2.1–12 Å−1 for the Pd-K edge and k = 2.8–12 Å−1 for the Au-L3 edge. The EXAFS functions from PdAu/LiOAc/SiO2 are compared to PdAu/SiO2 in Fig. S3, ESI.§
The amplitude reduction factor So2 was determined for bulk references and set to 0.9 for Au and 1.0 for Pd during analysis. Multiple-edge fitting was carried out with following constraints.
| NAuPd = NPdAuxPd/xAu | (1) |
| rAuPd = rPdAu | (2) |
| σAuPd = σPdAu | (3) |
3. Results and discussion
3.1. Pd surface enrichment on PdAu particles induced by MOAc (M+ = Li+, Na+, K+, Cs+)
Electronic and geometric effects of MOAc promotor (M+ = Li+, Na+, K+ and Cs+) on surface properties of the PdAu particles were studied by IR spectra of CO adsorbed at −150 °C on PdAu/MOAc/SiO2. Bands for CO adsorbed on OH groups of SiO2 at 2157 cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22,23 and with trace concentrations of cations at 2184 cm−1
22,23 and with trace concentrations of cations at 2184 cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24,25 were observed. The stretching frequency v(C
24,25 were observed. The stretching frequency v(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of physisorbed CO appeared at 2135 cm−1.26,27 CO adsorbed on Au atoms in a linear mode at 2104 cm−1,2,28–30 while on Pd atoms, both linear and bridged adsorption modes were observed at 2109 cm−1 and 1991 cm−1, respectively.29,31–33
O) of physisorbed CO appeared at 2135 cm−1.26,27 CO adsorbed on Au atoms in a linear mode at 2104 cm−1,2,28–30 while on Pd atoms, both linear and bridged adsorption modes were observed at 2109 cm−1 and 1991 cm−1, respectively.29,31–33
On bimetallic PdAu surfaces the broad band between 2135–2000 cm−1 consists of two contributions for CO linearly bound to Au (2128/2106 cm−1) and of two bands for CO linearly bound to Pd (2084/2063 cm−1). The band at 2128 cm−1 is attributed to CO on Au atoms surrounded by Pd (linear CO on “Au next to Pd”) and the band at 2106 cm−1 is assigned to CO linearly bound on Au atoms in proximity to other Au atoms (linear CO on “Au next to Au”). The band at 2084 cm−1 is attributed to CO chemisorbed on larger Pd islands (linear CO on “Pd next to Pd”) and the band at 2063 cm−1 to linearly adsorbed CO on Pd atoms surrounded by Au (linear CO on “Pd next to Au”).34
Alloying of Pd with Au shifts electron density towards the element with the larger fraction of empty valence states.35 Lee et al. suggested that Au gains sp-type electrons and loses d-electrons, whereas Pd loses sp-electrons and gains d-electrons.36 The increase in the electron density in the d-states of Pd leads to a stronger electron (back-)donation into the antibonding π* orbitals of CO, which shifts the CO stretching vibration to lower wavenumbers.29 In contrast, the band of linearly adsorbed CO on Au in proximity to Pd appeared at higher wavenumbers, as the electron density in the d-bands of Au decreases by the interaction with Pd.14
Fig. 1 presents the IR spectra of CO adsorbed on PdAu/MOAc/SiO2 (M+ = Li+, Na+, K+, Cs+) at −150 °C.
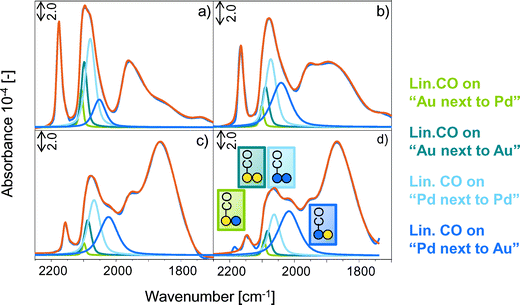 | ||
| Fig. 1 IR spectra of 1.0 mbar CO adsorbed on PdAu/SiO2 promoted with (a) LiOAc, (b) NaOAc, (c) KOAc and (d) CsOAc at −150 °C. Green lines represent linearly adsorbed CO on Au, blue lines CO on Pd. | ||
The presence of MOAc on PdAu led (i) to a shift of the stretching frequencies assigned to linear and bridged CO to lower wavenumbers, (ii) to a broadening of linear and bridged bands of adsorbed CO, (iii) to a preferential adsorption of CO on bridged rather than on linear sites and (iv) to a change in the relative band areas of linearly adsorbed CO on Au and Pd. These changes increased with increasing alkali metal radius from Li+, Na+, K+ to Cs+.
The gradual red-shift (Fig. 2) of the linear and bridged CO bands from Li+ to Cs+ promoted PdAu is attributed to the electron donating effect of the promoter towards the metal d-orbitals. This leads to an enhanced back donation from Pd and Au into the 2π* antibonding orbitals of CO, which strengthens the metal–carbon bond, but weakens the carbon–oxygen bond.37–42
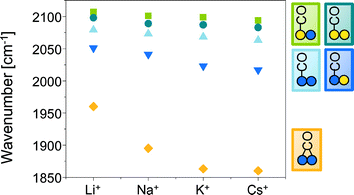 | ||
| Fig. 2 IR band positions [cm−1] of linearly and bridged adsorbed CO in dependence of the promoter M+ in PdAu/MOAc/SiO2 (M+ = Li+, Na+, K+, Cs+). | ||
The impact of the metal cation increased from Li+ (hard Lewis acid site) to Cs+ (soft Lewis acid site). The cations are considered to produce a long-range electronic effect mediated by the substrate, which is effective over several interatomic distances.40 These electronic effects of MOAc on PdAu as well as the physical presence of cation are able to reduce the intermolecular dipole–dipole coupling of CO, which is manifested by an increase in the wavenumber of the v(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) vibration with increasing surface coverage.43
O) vibration with increasing surface coverage.43
Finally the decrease in the frequency of the CO stretching vibration could be related to the reduction of the concentration of continuous Pd adsorption sites for CO by the presence of K+ and thus the extent of dipole–dipole coupling.44 The electron-donating alkali metals M+ increase the binding energy of reactive molecules to the metal by enhancing back donation, leading, e.g., to a higher heat of adsorption of CO.37,38,41 Therefore, we attribute the shift of the CO stretching vibration to electronic effects resulting from the interaction with MOAc.
The increased widths at half height of the CO band hints to an enhanced surface heterogeneity induced by the presence of MOAc, which resulted in an enhanced disorder in the adsorbed CO overlayer. The continuous shift of CO bands results from the varying distances between CO to M+. The shorter the distance between CO and M+, the larger the CO band shift.39
The preferential adsorption of CO in bridged rather than linear form and the change in integral intensity of linearly adsorbed CO on Au and Pd indicates a MOAc induced enrichment of Pd on the bimetallic PdAu surface. The Pd enrichment increasing from Li+ to Cs+ was monitored by the decreasing concentration of CO adsorbed on Au than on Pd as well as the decreasing ratio between linear/bridged adsorbed CO and the increasing overall Pd/Au ratio derived from IR spectra. Table 1 compiles the surface concentrations [10−6 mol g−1] and fractions [%] for CO adsorbed on PdAu/MOAc/SiO2 calculated from absorption coefficients of CO on pure Au and Pd.14
| PdAu/MOAc/SiO2 | Au next to Pd | Au next to Au | Pd next to Pd | Pd next to Au | Linear/bridged CO | Pd/Au |
|---|---|---|---|---|---|---|
| M+ = | [10−6 mol g−1] ([%]) | [mol g−1/mol g−1] | ||||
| — | 2.9(11) | 8.7(33) | 12(45) | 3.0(11) | 29.0 | 1.3 |
| Li+ | 3.0(6) | 8.3(18) | 25(54) | 9.8(21) | 20.3 | 3.1 |
| Na+ | 1.9(4) | 5.9(14) | 20(47) | 15(35) | 14.5 | 4.5 |
| K+ | 0.9(2) | 4.9(12) | 18(43) | 18(44) | 11.2 | 6.3 |
| Cs+ | 1.1(3) | 3.2(8) | 13(30) | 24(59) | 11.8 | 8.5 |
The fractions of Au on PdAu, i.e., “Au next to Pd” and “Au next to Au” decreased from 11% to 3% and from 33% to 8%, respectively, from the parent PdAu/SiO2 to increasing main quantum number of the promoter. At the same time, the fraction of “Pd next to Pd” decreased from 45% to 30%, while that of “Pd next to Au” strongly increased from 11% to 59%. The significant increase of the band for CO on “Pd next to Au” is accompanied by a decrease of “Au next to Pd” from Li+ to Cs+. However, these bands are complementary and, thus, they should both either increase or decrease. The large shift in band position of “Pd next to Pd” and minor “Pd next to Au” to lower wavenumber (Fig. 2) and the behavior of “Pd next to Pd” (maximum fraction at Li+) indicated that these Pd domains were in contact with MOAc. Due to these significant band shifts and broadenings, the bands assigned to “Pd next to Pd” and “Pd next to Au” are overlapping. As consequence (Table 1) the fraction of “Pd next to Pd” is underestimated and “Pd next to Au” is overestimated when going from Li+ to Cs+.
Therefore, the overall linear/bridged CO as well as the Pd/Au surface ratio was calculated using the absorption coefficients14 of adsorbed CO on monometallic Au/SiO2 and Pd/SiO2. It decreased from 20.3 to 11.8, while the overall surface Pd/Au ratio increased from 3.1 to 8.5 when going from Li- to CsOAc. Acetates covalently bound to Li+ show a lower strength of interaction with Pd in PdAu than acetates from CsOAc. The effect of acetate ions on PdAu and thus, the Pd surface enrichment increased towards CsOAc.
The effect of Pd surface enrichment upon promotion with MOAc depends on the overall as well as on the surface Pd/Au and the Pd/K+ ratio. Previous studies15 reveal the equilibrium between the Pd/MOAc layer and more or less soluble KPd-acetate species during vinyl acetate synthesis.14 Independent from the synthesis procedure, PdAu particles undergo interdiffusion already at room temperature45 – during reaction, the thermodynamic stable Pd1Au1 phase is formed.
Having shown that MOAc induced PdAu particle reordering leads to an enrichment of Pd on the surface (increasing from Li to Cs acetate) under “non-reactive” heating, we investigate in the next step if the promoter has also an influence on the metal–metal distances.
3.2. Interaction of MOAc (M+ = Li+, Na+, K+, Cs+) with Pd surface enriched PdAu
The influence of LiOAc on the structure of the bimetallic PdAu particles was further studied by EXAFS. Table 2 shows the metal–metal distances r and the coordination numbers N in PdAu/SiO2 compared to PdAu/LiOAc/SiO2 determined by multiple edge fitting with first shell analysis of Pd/Li+ and Au/Li+ contributions.| Sample | Au–Pd | Au–Au | Pd–Au | Pd–Pd | Pd–M+ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| r/Å | N | r/Å | N | r/Å | N | r/Å | N | r/Å | N | |
| PdAu/SiO2 | 2.77 | 4.5 | 2.80 | 7.3 | 2.77 | 2.2 | 2.75 | 7.9 | — | — |
| PdAu/LiOAc/SiO2 | 2.78 | 4.8 | 2.79 | 7.2 | 2.78 | 2.4 | 2.80 | 7.1 | 2.14 | 0.8 |
The addition of LiOAc to PdAu/SiO2 enlarged the Pd–Pd distances compared to PdAu/SiO2. The coordination numbers for Pd (NPdM = NPdPd + NPdAu) and for Au (NAuM = NAuPd + NAuAu) allow to distinguish a core–shell particle from one with a random alloy structure. In presence of a Pd enriched surface, NPdM will be smaller than NAuM because surface atoms have fewer neighbors than atoms in the core. On the other hand in a random alloy structure NPdM is close to NAuM.33 The presence of Pd1Au1 phases can be deduced from a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio between NPd–Pd and NPd–Au (the same applies for the coordination numbers of Au).16 For the samples studied, the total coordination numbers for Pd (∼10) were lower than that of Au (∼12), which indicates that Au occupied with preference sites in the core of the particles, while Pd atoms were located with preference on the surface. With the addition of LiOAc NPdPd decreased from 7.9 to 7.1 indicating an enhanced surface segregation of Pd in presence of the promotor.
1 ratio between NPd–Pd and NPd–Au (the same applies for the coordination numbers of Au).16 For the samples studied, the total coordination numbers for Pd (∼10) were lower than that of Au (∼12), which indicates that Au occupied with preference sites in the core of the particles, while Pd atoms were located with preference on the surface. With the addition of LiOAc NPdPd decreased from 7.9 to 7.1 indicating an enhanced surface segregation of Pd in presence of the promotor.
For (PdAu)corePdshell structures, the Pd atoms in the shell adopt the bond length of the Au atoms in the core up to a thickness of the shell approximating 1 nm.33,46 On PdAu/LiOAc/SiO2 the Pd–Pd distance was significantly longer by ∼0.05 Å (2.75–2.80 Å) compared to the distances in PdAu/SiO2, whereas mixed interatomic Pd–Au and Au–Pd distances increased only slightly from 2.77 to 2.78 Å. The Pd lattice expansion after LiOAc impregnation suggests the formation of a Pd/Li+ mixed adlayer on the PdAu alloy particle.16 First shell analysis of the EXAFS of the promoted sample indicates a Pd/Li+ distance of 2.14 Å, which agrees well with the Pd and Li+ radii (rPd = 1.39 Å, rLi+ = 0.75 Å). The small coordination number NPd/Li+ of 0.8, which averages over all Pd atoms present in the sample, additionally underlines that the Pd/Li+ adlayer is formed as a thin shell on the alloy particle core.
The fit of the EXAFS oscillations improved significantly by including Pd/Li+ contributions, while Au/Li+ interactions were excluded as first shell analysis yielded nonrealistic parameters (i.e., unacceptable distances and partly negative coordination numbers).
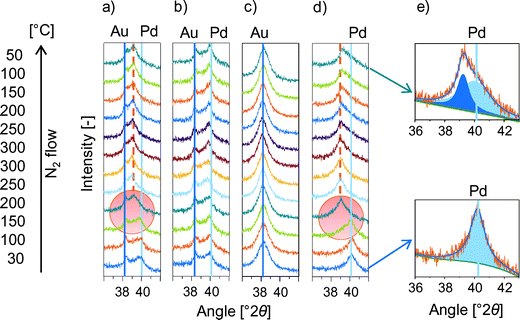
The changes in the interatomic Pd distances upon promoter impregnation and activation were first studied by XRD using the KOAc as model and then expanded to the other alkali acetates. The in situ XRD measured in N2 flow in presence and absence of KOAc on mono- and on bimetallic catalysts is shown Fig. 3. The Pd(111) and PdxAuy(111) diffraction peaks were observed between 36 and 42° 2θ for (a) PdAu/KOAc/SiO2, (b) PdAu/SiO2, (c) Au/KOAc/SiO2 and (d) Pd/KOAc/SiO2. On fresh PdAu/KOAc/SiO2 catalysts, Au- and Pd-rich (Pd0Au100, Pd89Au11) as well as bimetallic particles composed of Pd54Au46 were observed (Fig. 3a).14 While the Pd(111) reflex shifted from 40.0° to 39.3° 2θ after heating to 200 °C and further to 39.1° 2θ at 250 °C. The total shift of Pd(111) by Δθ = −0.9° to lower angles was irreversible upon cooling to room temperature. This KOAc induced shift in the Pd(111) reflex is attributed to the elongation of the Pd–Pd bond distance of ∼0.055 Å, which is in good agreement with the increase in the Pd–Pd bond distance observed by EXAFS (∼0.05 Å).
The temperature induced miscibility of bimetallic PdAu particles is independent of the presence of KOAc (Fig. 3b). The absence of a shift of the Au(111) peak in PdAu/KOAc/SiO2 as well as in monometallic Au/KOAc (Fig. 3c) confirmed that the KOAc adsorption did not influence the Au–Au distance, while the Pd(111) diffraction peak shifted to lower angles by −1° after KOAc adsorption in monometallic Pd/KOAc/SiO2 (Fig. 3d). The asymmetric shape of the Pd(111) reflection after cooling to 50 °C indicated the presence of two overlapping contributions at 39.26° and 40.05° 2θ (see Fig. 3e for the fit) corresponding to Pd–Pd distance enlargements of 0.055 Å and 0.002 Å. Consequently, in situ XRD results confirm the presence of a Pd/K+ shell on Pd or PdAu implying that Pd migrates from the particle bulk to the alloy surface upon KOAc loading.
The next part addresses the question whether the Pd–Pd distance depends on the size of the promoter ions by comparing Li-, Na-, K- and Cs-acetate promoted PdAu/SiO2 (Fig. S4, ESI§). In order to determine the starting temperature of the Pd–Pd bond expansion, the temperature was increased from 100 °C to 140 °C with steps of 10 °C allowing the system to equilibrate for 2 h at each temperature. The position of the Pd(111) peak was independent on the type of promoter. However, the temperature of the Pd–Pd bond expansion slightly decreased from 120 °C for PdAu/LiOAc/SiO2 to 110 °C for Na-, K- and Cs-OAc impregnated PdAu/SiO2. This indicates that the formation of the Pd/M+ adlayer is accompanied by acetate decomposition to COx and H2 catalyzed by Pd.47 To test this hypothesis, the K+ counter anion was varied from acetate to (non-decomposable) OH−, CO32−, C2O42−. The Pd–Pd bond enlargement increased in the order OH− < CO32− < C2O42− < OAc− (Fig. S5b, ESI§) underlining that Pd/M+ formation is induced by counter ion transformation. As observed by in situ XRD (Fig. S5§), acetate as bidentate ligand favors the formation of the Pd/K+–O layer of K+ on the Pd surface layer of PdAu particles thereby favoring the dynamic reordering of PdAu by the formation of homogeneous K2Pd2(OAc)6 species.15
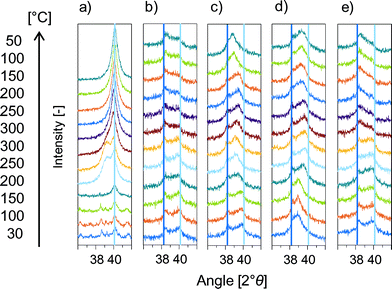
Summarizing, IR, XAS and in situ XRD showed the temperature and counter ion dependent, but alkali cation radius independent formation of a Pd/M+ adlayer on PdAu alloy particles. The formation of PdCx, Pd hydride or Pd oxide species, which result in a similar Pd(111) peak shift,48–51 was excluded by additional in situ XRD experiments in Fig. 4.
Adsorbed carbon from decomposed acetates47,52 migrates to the Pd bulk to form PdCx leading to a downshift of the Pd reflections corresponding to a Pd–Pd bond enlargement of 0.075 Å.50,53 The presence of PdCx instead of a Pd/K+ mixed adlayer was not only excluded because of the difference in Pd bond enlargement (0.055 Å compared to 0.075 Å) but also by several independent experiments. Pd(OAc)2 impregnated Pd/SiO2 should show the Pd(111) shift in the absence of KOAc due to Pd(OAc)2 decomposition that creates PdCx. (Fig. 4a) Decomposition of thermally instable Pd(OAc)2 above 140 °C led to the expected, however reversible in contrast to the irreversible Pd(111) shift for PdAu/KOAc/SiO2. The absence of PdCx from decomposed acetate was confirmed by applying NH4OAc impregnated PdAu/SiO2 (Fig. 4b). NH4OAc decomposed, but generated tetragonal PdO at 34.0, 54.8 and 60.8° 2θ54 and no shift of Pd(111) due to PdCx. Additionally, possible PdCx species in PdAu51 can be removed by H2 treatment at 180 °C.55 Thus, PdAu/KOAc/SiO2 was heated in N2 to observe the downshift of Pd(111) and then in H2 to remove possible PdCx species (Fig. 4c). As expected, the Pd(111) peak shift occurred above 150 °C in N2 but it was irreversible in H2 strongly suggesting the absence of PdCx.
Pd-hydride formation in H2/N2 as cause for the irreversible downshift of Pd(111) in PdAu/KOAc/SiO2 is also excluded since hydrides decomposed above 150 °C leading to reversible Pd(111) shifts (Fig. 4d).
Formation of PdO on Pd(111)56–59 was reported to form above 470 K and 2 × 10−2 Pa.60 In order to check for PdO, in situ XRD was performed with PdAu/KOAc/SiO2 in synthetic air (Fig. 4e). The Pd(111) reflex did not shift but became lower in intensity as soon as additional peaks for tetragonal PdO54 formed. Consequently, the formation of bulk PdO can be excluded.
Summarily, our findings on the nature of interstitial PdCx species, on Pd oxide and hydride species and the general finding that Au suppressed carbide formation in PdAu particles61,62 led us to the conclusion that exclusively M+ from MOAc (M+ = Li+, Na+, K+, Cs+) induced the Pd–Pd bond enlargement. The fact that the Pd–Pd distance does not change as a function of the cation suggests that the cations form a surface layer on top of Pd inducing the elongation of the Pd–Pd distance in analogy to the widening of M–M distances increase upon hydrogen adsorption on metal nanoparticles.
3.3. Chemical composition and nature of the Pd/promoter+ adlayer
Having shown that the migration of Pd to the PdAu particle surface and the formation of a mixed Pd/M+ (M+ = Li+, Na+, K+, Cs+) shell on the bimetallic PdAu core is induced by the presence of alkali acetates, we discuss the chemical nature of this adlayer.Four locations of M+ on PdAu/MOAc/SiO2 can be considered, i.e., (i) physisorbed MOAc species, (ii) interactions of M+ with the silica support,63 (iii) interaction of M+ on the Pd/M+ adlayer (Fig. 5) and (iv) binding of M+ within the Pd/M+ adlayer.
The samples were washed with water in order to differentiate the strength of interaction. It is expected that physisorbed MOAc species are easily removed by washing, while more strongly interacting M+ ions on SiO2 and on/in Pd/M+ have higher stability. Thus, these components would be still detected after washing by chemical analysis of the washed KOAc/SiO2 (concentration of K+ in SiO2) and PdAu/KOAc/SiO2 samples (concentration of K+ in SiO2 plus Pd/M+) (Table 3). All samples initially contained identical K+ concentrations (∼4.6 wt%); 0.208 wt% K+ remained on washed KOAc/SiO2 and a significant higher K+ concentration (0.330 wt%) was found on washed PdAu/KOAc/SiO2.
| Sample | Potassium concentration [wt%] | |
|---|---|---|
| Unwashed sample | Washed sample | |
| KOAc/SiO2 | 4.64 | 0.208 |
| PdAu/KOAc/SiO2 | 4.61 | 0.330 |
The difference in K+ concentrations on both washed samples (0.122 wt%) allows to determine the K+ concentration at or within the Pd/K+ adlayer. With a dispersion of 24% (average PdAu particle size of 3.6 nm), a Pd/Au molar surface ratio of 6.3 (from the IR spectrum of adsorbed CO14) and a total Pd loading of 1.4 wt%, about 2.7 × 10−5 mol Pd g−1 is located on the surface of the bimetallic particles. The agreement with the concentration of K+ in Pd/K+ (3.1 × 10−5 mol K+ g−1) indicates the formation of a Pd/K+ monolayer with a ratio between K+ and Pd surface atoms of ∼1.
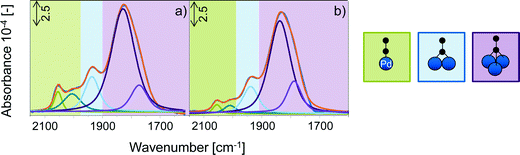
Having established the composition of Pd/K+ as monolayer, its chemical nature was studied in more detail. To preserve the electroneutrality M+ is expected to be in contact to oxygen species either in form of acetate or its oxidic decomposition products. In order to check for oxygen counter ions of K+ in Pd/K+, IR spectra of CO adsorbed on activated PdAu/KOAc/SiO2 and PdAu/KOH/SiO2 at 100 °C were compared (Fig. 6a and b). KOH, which partly forms K2CO3 in air, is stable in oxidic form on the catalyst surface during activation at 300 °C and, thus, the electronic influence of K+ and O species on Pd can be observed by comparing the IR spectra of CO adsorbed on PdAu/KOH/SiO2 and on PdAu/KOAc/SiO2 at 100 °C. At this temperature, CO adsorbs only on Pd surface atoms in the bimetallic PdAu particles (Fig. S6, ESI§). Both spectra in Fig. 6 exhibited five CO bands between 2150 and 1600 cm−1 (Table 4). Linearly adsorbed CO on Pd sites has stretching frequencies of 2055 and ∼2007 cm−1. Bridged adsorbed CO on two neighboring Pd species appeared at 1937 cm−1 and the two CO bands at 1832 and 1780 cm−1 are attributed to threefold bridged adsorbed CO. Similar positions of the CO bands on both samples point to the formation of K+-oxide species from KOAc during temperature treatment. The shift of the band for threefold bridged CO species to higher wavenumbers on PdAu/KOH/SiO2 (1837 and 1788 cm−1) compared to PdAu/KOAc/SiO2 (1829 and 1773 cm−1) can be attributed to local CO–K+ interactions with KOH. This indicates that K+ from KOH remains accessible for CO adsorption and is not “incorporated” into the Pd enriched PdAu surface. In contrast, K+ from KOAc formed a mixed Pd/K+ layer on PdAu. The lower ratio between linear and multifold adsorbed CO and the lower total Pd surface concentration (Table 4) on PdAu/KOH/SiO2 compared with PdAu/KOAc/SiO2 indicates that KOH covered and blocks the PdAu surface, whereas K+ from KOAc was incorporated in the outer Pd layer of PdAu and generated the Pd/K+–O adlayer.
| PdAu/x/SiO2 | Band positions [cm−1] | Linear/multifold | Concentration of Pd [10−5 mol g−1] | ||
|---|---|---|---|---|---|
| Linear | Bridged | Threefold bridged | |||
| KOAc | 2055, 2006 | 1937 | 1829, 1773 | 3.5 | 2.3 |
| KOH | 2055, 2009 | 1938 | 1837, 1788 | 1.5 | 1.0 |
Fresh PdAu/MOAc/SiO2 indicates partial oxidation of Pd either in form of Pd+ or Pd2+ within the Pd/M+–O adlayer which is supposed to be the precursor for homogeneous Pd2+/M+ acetate species in the reaction to vinyl acetate.
4. Conclusions
The promoters MOAc (M+ = Li+, Na+, K+, Cs+) on PdAu/SiO2 are located on the bimetallic particles as well as on the support (see Fig. 7). On SiO2, MOAc forms M+-silicates, while on the metal particles it induces the segregation of Pd to the surface of PdAu, generating a Pd/M+-oxide adlayer during annealing. The formation of the Pd/K+-oxide monolayer shell on PdAu particles exerts electronic and geometric changes in the catalysts accompanied by decomposition of acetates and leads to an increase in Pd–Pd bond length. These differences are characterized by two effects on Pd at the outermost layer. The Pd atoms, which are pulled out beyond the K+ layer and form a “mixed” layer are located at irregular intervals (and do not contribute to the larger regular Pd–Pd distances). The Pd atoms enriched at the surface of the PdAu particle have an enhanced distance independent of the nature of the alkali cation, pointing to an unspecific interaction with the outer surface layer. Both Pd will contribute to catalysis, but with a rate significantly lower than the PdAu alloy. For VA synthesis, however, the Pd surface lattice mismatch modified by KOAc confines the reactants in small regions and limits in this manner side reactions. Additionally, the weakening of the interaction of the reactants with the metal sites enhances reaction selectivities and activities in the structure sensitive VA synthesis reaction.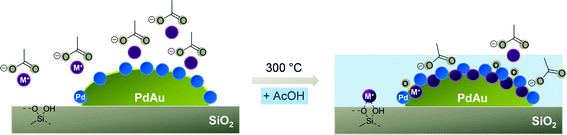 | ||
| Fig. 7 Formation of the Pd/M+-oxide adlayer on PdAu/MOAc/SiO2 (M+ = Li+, Na+, K+, Cs+) during activation and AcOH adsorption. | ||
Acknowledgements
Financial support by Wacker Chemie AG and fruitful discussions within the Wacker-Institut für Siliciumchemie is gratefully acknowledged. Xaver Hecht and Martin Neukamm are acknowledged for their experimental support.References
- M. Chen and D. W. Goodman, Chin. J. Catal., 2008, 29, 1178–1186 CrossRef CAS.
- M. S. Chen, K. Luo, T. Wei, Z. Yan, D. Kumar, C. W. Yi and D. W. Goodman, Catal. Today, 2006, 117, 37–45 CrossRef CAS.
- A. E. Baber, H. L. Tierney and E. C. H. Sykes, ACS Nano, 2010, 4, 1637–1645 CrossRef CAS PubMed.
- E. G. Allison and G. C. Bond, Catal. Rev., 1972, 7, 233–289 CAS.
- H. P. Myers, L. Wallden and B. Karlsson, Philos. Mag., 1968, 18, 725–744 CrossRef CAS.
- D. Kumar, M. S. Chen and D. W. Goodman, Catal. Today, 2007, 123, 77–85 CrossRef CAS.
- H. Okamoto and T. Massalski, J. Phase Equilib., 1985, 6, 229–235 CAS.
- R. Elliott and F. Shunk, J. Phase Equilib., 1982, 2, 482–484 Search PubMed.
- A. Maeland and T. B. Flanagan, Can. J. Phys., 1964, 42, 2364–2366 CrossRef CAS.
- W. M. H. Sachtler and P. Van Der Plank, Surf. Sci., 1969, 18, 62–79 CrossRef CAS.
- L. Z. Mezey and J. Giber, Jpn. J. Appl. Phys., 1982, 21, 1569–1571 CrossRef CAS.
- R. Anton, H. Eggers and J. Veletas, Thin Solid Films, 1993, 226, 39–47 CrossRef CAS.
- C. W. Yi, K. Luo, T. Wei and D. W. Goodman, J. Phys. Chem. B, 2005, 109, 18535–18540 CrossRef CAS PubMed.
- E. K. Hanrieder, A. Jentys and J. A. Lercher, ACS Catal., 2015, 5776–5786, DOI:10.1021/acscatal.5b01140.
- E. K. Hanrieder, A. Jentys and J. A. Lercher, J. Catal., 2016, 333, 71–77 CrossRef CAS.
- S. Simson, A. Jentys and J. A. Lercher, J. Phys. Chem. C, 2013, 117, 8161–8169 CAS.
- S. Simson, A. Jentys and J. A. Lercher, J. Phys. Chem. C, 2015, 119, 2471–2482 CAS.
- M. Chen, D. Kumar, C.-W. Yi and D. W. Goodman, Science, 2005, 310, 291–293 CrossRef CAS PubMed.
- H.-J. Eberle, R. Heidenreich and J. Weis, Ger. DE 102006058800 A1, 2008.06.19, 2008 Search PubMed.
- L. Vegard, Z. Phys., 1921, 5, 17–26 CrossRef CAS.
- B. Ravel and M. Newville, J. Synchrotron Radiat., 2005, 12, 537–541 CrossRef CAS PubMed.
- W. K. Kuhn, J. Szanyi and D. W. Goodman, Surf. Sci., 1992, 274, L611–L618 CrossRef CAS.
- M. Mihaylov, K. Hadjiivanov and H. Knözinger, Catal. Lett., 2001, 76, 59–63 CrossRef CAS.
- T. Montanari, L. Castoldi, L. Lietti and G. Busca, Appl. Catal., A, 2011, 400, 61–69 CrossRef CAS.
- T. Montanari, R. Matarrese, N. Artioli and G. Busca, Appl. Catal., B, 2011, 105, 15–23 CrossRef CAS.
- T. P. Beebe, P. Gelin and J. T. Yates Jr, Surf. Sci., 1984, 148, 526–550 CrossRef CAS.
- M. Mihaylov, H. Knözinger, K. Hadjiivanov and B. C. Gates, Chem. Ing. Tech., 2007, 79, 795–806 CrossRef CAS.
- J. Shen, J. Hill, R. Watwe, S. G. Podkolzin and J. A. Dumesic, Catal. Lett., 1999, 60, 1–9 CrossRef CAS.
- E. L. Kugler and M. Boudart, J. Catal., 1979, 59, 201–210 CrossRef CAS.
- D. C. Meier and D. W. Goodman, J. Am. Chem. Soc., 2004, 126, 1892–1899 CrossRef CAS PubMed.
- E. Ozensoy and D. Wayne Goodman, Phys. Chem. Chem. Phys., 2004, 6, 3765–3778 RSC.
- T. Wei, J. Wang and D. W. Goodman, J. Phys. Chem. C, 2007, 111, 8781–8788 CAS.
- F. Gao and D. W. Goodman, Chem. Soc. Rev., 2012, 41, 8009–8020 RSC.
- D. Rainer, J. Vac. Sci. Technol., A, 1997, 15, 1653–1662 CAS.
- J. A. Rodriguez and D. W. Goodman, Science, 1992, 257, 897–903 CAS.
- Y.-S. Lee, Y. Jeon, Y.-M. Chung, K.-Y. Lim, C.-N. Whang and S.-J. Oh, J. Korean Phys. Soc., 2000, 37, 451–455 CAS.
- C. T. Campbell and D. W. Goodman, Surf. Sci., 1982, 123, 413–426 CrossRef CAS.
- J. E. Crowell, E. L. Garfunkel and G. A. Somorjai, Surf. Sci., 1982, 121, 303–320 CrossRef CAS.
- J. E. Crowell and G. A. Somorjai, Appl. Surf. Sci., 1984, 19, 73–91 CrossRef CAS.
- E. L. Garfunkel, M. H. Farias and G. A. Somorjai, J. Am. Chem. Soc., 1985, 107, 349–353 CrossRef CAS.
- E. L. Garfunkel, J. E. Crowell and G. A. Somorjai, J. Phys. Chem., 1982, 86, 310–313 CrossRef CAS.
- E. L. Garfunkel and G. A. Somorjai, Surf. Sci., 1982, 115, 441–454 CrossRef CAS.
- A. F. Gusovius, T. C. Watling and R. Prins, Appl. Catal., A, 1999, 188, 187–199 CrossRef CAS.
- F. Stoop, F. J. C. M. Toolenaar and V. Ponec, J. Catal., 1982, 73, 50–56 CrossRef CAS.
- A. Sellidj and B. E. Koel, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 8367–8376 CrossRef CAS.
- A. Sárkány, O. Geszti and G. Sáfrán, Appl. Catal., A, 2008, 350, 157–163 CrossRef.
- M. Bowker, C. Morgan and V. P. Zhdanov, Phys. Chem. Chem. Phys., 2007, 9, 5700–5703 RSC.
- S. Nakamura and T. Yasui, J. Catal., 1970, 17, 366–374 CrossRef CAS.
- S. A. H. Zaidi, J. Catal., 1981, 68, 255–263 CrossRef CAS.
- S. B. Ziemecki, G. A. Jones, D. G. Swartzfager, R. L. Harlow and J. Faber, J. Am. Chem. Soc., 1985, 107, 4547–4548 CrossRef CAS.
- M. Bonarowska, A. Malinowski, W. Juszczyk and Z. Karpiński, Appl. Catal., B, 2001, 30, 187–193 CrossRef CAS.
- N. Aas and M. Bowker, J. Chem. Soc., Faraday Trans., 1993, 89, 1249–1255 RSC.
- M. Bowker, C. Morgan and J. Couves, Surf. Sci., 2004, 555, 145–156 CrossRef CAS.
- J. Waser, H. A. Levy and S. W. Peterson, Acta Crystallogr., 1953, 6, 661–663 CrossRef CAS.
- M. Bonarowska, J. Pielaszek, V. A. Semikolenov and Z. Karpiński, J. Catal., 2002, 209, 528–538 CrossRef CAS.
- H. Conrad, G. Ertl, J. Küppers and E. E. Latta, Surf. Sci., 1977, 65, 245–260 CrossRef CAS.
- B. A. Banse and B. E. Koel, Surf. Sci., 1990, 232, 275–285 CrossRef CAS.
- S. Yang, A. Maroto-Valiente, M. Benito-Gonzalez, I. Rodriguez-Ramos and A. Guerrero-Ruiz, Appl. Catal., B, 2000, 28, 223–233 CrossRef CAS.
- A. M. Venezia, L. F. Liotta, G. Pantaleo, V. La Parola, G. Deganello, A. Beck, Z. Koppány, K. Frey, D. Horváth and L. Guczi, Appl. Catal., A, 2003, 251, 359–368 CrossRef CAS.
- E. H. Voogt, A. J. M. Mens, O. L. J. Gijzeman and J. W. Geus, Surf. Sci., 1997, 373, 210–220 CrossRef CAS.
- Y. F. Han, D. Kumar, C. Sivadinarayana, A. Clearfield and D. W. Goodman, Catal. Lett., 2004, 94, 131 CrossRef CAS.
- M. Bowker and C. Morgan, Catal. Lett., 2004, 98, 67 CrossRef CAS.
- M.-M. Pohl, J. Radnik, M. Schneider, U. Bentrup, D. Linke, A. Brückner and E. Ferguson, J. Catal., 2009, 262, 314–323 CrossRef CAS.
Footnotes |
| † The authors declare no competing financial interest. |
| ‡ All authors have given approval to the final version of the manuscript. |
| § Electronic supplementary information (ESI) available: Additional information about EXAFS fit results, IR of adsorbed CO, XRD and in situ IR spectra are listed. See DOI: 10.1039/c6cy01228k |
| This journal is © The Royal Society of Chemistry 2016 |