Open Access Article
Open Access ArticleSelective hydrogenation of arenes to cyclohexanes in water catalyzed by chitin-supported ruthenium nanoparticles†
Yuna
Morioka
a,
Aki
Matsuoka
a,
Kellie
Binder
b,
Benjamin R.
Knappett
b,
Andrew E. H.
Wheatley
*b and
Hiroshi
Naka
*a
aDepartment of Chemistry and Research Center for Materials Science, Nagoya University, Chikusa, Nagoya, 464-8602, Japan. E-mail: h_naka@nagoya-u.jp
bDepartment of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW, UK. E-mail: aehw2@cam.ac.uk
First published on 24th June 2016
Abstract
The selective hydrogenation of aromatic compounds to cyclohexanes was found to be promoted by chitin-supported ruthenium nanoparticles (Ru/chitin) under near-neutral, aqueous conditions without the loss of C–O/C–N linkages at benzylic positions.
The catalytic hydrogenation of arenes is a straightforward and hugely important method by which cyclohexanes are produced.1,2 However, a major issue with this transformation is the need to suppress the competitive hydrogenolysis of reactive carbon–heteroatom linkages (e.g. C–O and C–N bonds) at benzylic positions.3,4 Attempts to address this problem typically focus on using acetic acid as a (co-)solvent in the presence of late transition metal catalysts such as PtO2,5a,b Rh–PtO2,5c RuO2,6a Ru/Al2O3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6b and Rh/Al2O3.7 The most frequently used catalysts are Ru/Al2O3 or, substantially more expensive, Rh/Al2O3. However, irrespective of the choice of catalyst, the use of acidic reaction media is incompatible with substrates bearing acid-sensitive functionalities such as epoxides and tertiary benzylic alcohols. Recently studied catalysts that have allowed the selective arene hydrogenation of benzyl alcohols or ethers in the absence of acidic additives include Rh/AlO(OH),8a Ru/MCM-41,8b Ru/CNF-P,9a,b Rh/CNF-T9c and Ru/HPS-NR3Cl.9d In particular, Motoyama and Nagashima elegantly demonstrated the simultaneous tolerance of epoxide and benzylic C–O functionalities using Rh/CNF-T or Ru/HPS-NR3Cl.9c,d However, with the exception of the Ru/HPS-NR3Cl system, which utilized H2O as a solvent,9d these reactions were typically run in hydrocarbons or polyethylene glycol.10,11 The development of catalytic systems that operate under aqueous conditions remains strongly in demand, since it promises a route to functionalized, water-soluble cyclohexanes for applications in materials and biological sciences.4g,12 We here disclose that chitin-supported ruthenium nanoparticles (Ru/chitin) efficiently catalyze arene hydrogenation under aqueous conditions without hydrogenolyzing C–O/C–N bonds at the benzylic positions.
6b and Rh/Al2O3.7 The most frequently used catalysts are Ru/Al2O3 or, substantially more expensive, Rh/Al2O3. However, irrespective of the choice of catalyst, the use of acidic reaction media is incompatible with substrates bearing acid-sensitive functionalities such as epoxides and tertiary benzylic alcohols. Recently studied catalysts that have allowed the selective arene hydrogenation of benzyl alcohols or ethers in the absence of acidic additives include Rh/AlO(OH),8a Ru/MCM-41,8b Ru/CNF-P,9a,b Rh/CNF-T9c and Ru/HPS-NR3Cl.9d In particular, Motoyama and Nagashima elegantly demonstrated the simultaneous tolerance of epoxide and benzylic C–O functionalities using Rh/CNF-T or Ru/HPS-NR3Cl.9c,d However, with the exception of the Ru/HPS-NR3Cl system, which utilized H2O as a solvent,9d these reactions were typically run in hydrocarbons or polyethylene glycol.10,11 The development of catalytic systems that operate under aqueous conditions remains strongly in demand, since it promises a route to functionalized, water-soluble cyclohexanes for applications in materials and biological sciences.4g,12 We here disclose that chitin-supported ruthenium nanoparticles (Ru/chitin) efficiently catalyze arene hydrogenation under aqueous conditions without hydrogenolyzing C–O/C–N bonds at the benzylic positions.
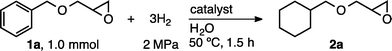
In recent work we established that Ru/chitin serves as an efficient catalyst for the hydration of nitriles to amides under aqueous conditions.13,14 Based on this result, we reasoned that Ru/chitin should also be suited to the chemoselective hydrogenation of functionalized arenes because the nitrile hydration operated under near-neutral conditions13 and supported ruthenium nanoparticles are known to be good catalysts for arene hydrogenation.6,8b,9a,b,10a–d,15 As shown in Table 1, the activity of Ru was tested in the hydrogenation of benzyl glycidyl ether (1a) to cyclohexylmethyl glycidyl ether (2a). This reaction allowed the monitoring of both reactivity and selectivity for arene hydrogenation over hydrogenolysis at the benzylic position or acid-/base-mediated opening of the oxirane ring.9c,16 Currently known catalysts effective in this transformation are limited to just two tailor-made systems: Rh/CNF-T (rt, 12 h)9c and Ru/HPS-NR3Cl (30 °C, 24 h).9d,17 Ru/chitin can be prepared by simple impregnation–reduction using inexpensive RuCl3·3H2O, NaBH4 and commercially available chitin under aqueous conditions and in the absence of capping agents.13 Results demonstrate that the hydrogenation of 1a was effectively catalyzed by Ru/chitin. When a mixture of 1a (1.0 mmol), H2O (5 mL) and Ru/chitin (0.8 wt%, 0.008 mmol of Ru, 0.8 mol% Ru) was stirred at 50 °C under a H2 atmosphere (2 MPa), the hydrogenation was completed within 1.5 h and cyclohexane 2a was obtained in 98% yield (Table 1, entry 1). ICP-AES on the Ru/chitin catalyst before and after the hydrogenation cycle established that only negligible leaching of Ru (4.2 ppm) took place during the catalytic test. The hydrogenation proceeded in water with no detectable loss of the C–O linkages in the substrates, there being no appreciable formation of side products 3a–6a. This result was reproducible (1H NMR yields of 2a in separate runs: 97, 95 and 97%). Product 2a could be isolated in 84% yield after removal of the catalyst and SiO2 column chromatography, with Ru contamination proving lower than the detection limit of ICP-AES (<1 ppb). Results demonstrate that both ruthenium and chitin were necessary for selective arene hydrogenation (entry 1 vs. entries 2–5). Moreover, heterogeneous catalysts prepared analogously to Ru/chitin but using cellulose, chitosan, γ-Al2O3 or carbon promoted arene hydrogenation but with lower selectivity (entries 6–9). Among these, hydrogenation with Ru/cellulose15c was also found to be distinctly effective, although appreciable amounts of side-products were formed through hydrogenolysis (Table 1, entry 6). Results obtained using commercially available catalysts are summarized in entries 10–14. Rh/Al2O3 (Sigma-Aldrich) proved efficient (2a: 92% yield) but induced partial hydrogenolysis to 3a or 4a (entry 11). Lastly, Ru/C (TCI)18 was also a moderately good catalyst (2a: 87% yield) but caused competing epoxide ring-opening (entry 12).19
| Entry | Catalyst | Conv. of 1ab (%) | Yield of 2ab (%) | Combined yield of 3a and 4ac (%) | Combined yield of 5a and 6ab (%) |
|---|---|---|---|---|---|
| a Conditions: 1a (1.0 mmol), catalyst (0.8 mol% Ru) and H2O (5 mL) at 50 °C for 1.5 h under H2 (2 MPa). b Determined by 1H NMR using mesitylene as an internal standard. c GC-MS yield using n-octane as an internal standard. d Purchased from commercial suppliers. e 5 wt% Ru or Rh. f 10 wt% Pd. | |||||
| 1 | Ru/chitin | >99 | 98 | <1 | <1 |
| 2 | None | <1 | <1 | <1 | <1 |
| 3 | Chitin | 4 | <1 | <1 | <1 |
| 4 | RuCl3·3H2O | >99 | 75 | 6 | 12 |
| 5 | RuO2d | 1 | <1 | 1 | <1 |
| 6 | Ru/cellulose | >99 | 97 | 2 | <1 |
| 7 | Ru/chitosan | 47 | 41 | <1 | <1 |
| 8 | Ru/γ-Al2O3 | 40 | 36 | 3 | <1 |
| 9 | Ru/C | 51 | 43 | 4 | 1 |
| 10 | Ru/Al2O3d,e | 19 | 15 | 2 | <1 |
| 11 | Rh/Al2O3d,e | >99 | 92 | 5 | <1 |
| 12 | Ru/Cd,e | >99 | 87 | 4 | 7 |
| 13 | Rh/Cd,e | >99 | 24 | 25 | 38 |
| 14 | Pd/Cd,f | 96 | <1 | 25 | 69 |
|
|||||
Results in Table 2 demonstrate that Ru/chitin-promoted selective arene hydrogenation was compatible with benzylic C–O or C–N linkages in alcohol, ether, amide and amino functionalities in a wide range of substrates (1b–kNa). The corresponding cyclohexanes 2b–kNa were obtained in good-to-excellent isolated yields, with the products typically being isolated by distillation or column chromatography after simply removing the catalyst by filtration or centrifugation. Hydrogenation of significantly acid-sensitive benzyl alcohols 1d and 1e proceeded without loss of the C–O bonds (Table 2, entries 3 and 4).20 Marginal amounts of side-products were detected when using 1d–fNa (entries 3–5). The absolute configurations of (S)-1c, (R)-1fNa and (S)-1j·HCl were retained under our reaction conditions (entries 2, 5 and 9).21 Unfortunately, double hydrogenation of dibenzyl ether or dibenzylamine·HCl was sluggish due to low reactivity and competitive hydrogenolysis at the benzylic positions.22 However, arene 1h could be doubly hydrogenated to give the dicyclohexyl analogue 2h in excellent yield (entry 7). By virtue of the aqueous conditions used, hydrophilic sodium carboxylates 1fNa and 1kNa as well as ammonium salts 1i·HCl and 1j·HCl could be effectively converted to give the corresponding salts of substituted cyclohexanes (entries 5, 8–10). In particular, the hydrogenation of 1kNa to 2kNa represents an important route for preparing non-standard amino acid-bearing hydrophobic cyclohexyl rings from more accessible aromatic analogues.4g,12 In a similar vein, the hydrogenation of sodium phenylalanate (1lNa) with Ru/chitin also gave the corresponding cyclohexyl-bearing amino acid 2lNa in high yield (entry 11). The substrate scope of other typical aromatic rings is summarized in Table S3.†
| Entry | Substrate (1) (conditions) | Conv.b (%) | Product (2) | Yieldb,c (%) |
|---|---|---|---|---|
| a Conditions: 1 (0.50 or 1.0 mmol), Ru/chitin (0.8 mol% Ru), H2O (5 mL) and H2 (2 MPa). b Determined by 1H NMR using mesitylene as an internal standard. c Isolated yield in parentheses. d Low yield due to the volatile nature of the product. e Absolute configuration and optical purity were determined by a polarimeter and chiral GC analyses. f Side-products were detected: entry 3, 4d (3%); entry 4, 6e (7%); entry 5, 4fNa (6%). g As indicated by the supplier. h Yield of carboxylic acid (R)-2f after the addition of HCl aq. i Optical purity was not determined. j Yield of 2·HCl after the addition of HCl aq. | ||||
| 1 |
|
>99 |
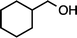
|
90 [66d] |
| 1b (100 °C, 6 h) | 2b | |||
| 2 |
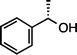
|
>99 |
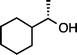
|
95 [92] |
(S)-1c (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R = 97 R = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)e (50 °C, 3 h) 3)e (50 °C, 3 h) |
(S)-2c (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R = 94 R = 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6)e 6)e |
|||
| 3 |
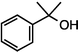
|
>99f |
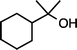
|
90 [88] |
| 1d (50 °C, 3 h) | 2d | |||
| 4 |
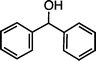
|
>99f |
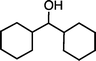
|
79 [75] |
| 1e (50 °C, 6 h) | 2e | |||
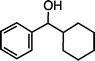
|
13 [17] | |||
| 2ea | ||||
| 5 |
|
91f |
|
85 [82h] |
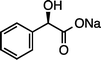
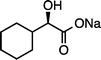
(R)-1fNa (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R = 1 R = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99)g (100 °C, 3 h) 99)g (100 °C, 3 h) |
(R)-2fNa (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R = 1 R = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99)e 99)e |
|||
| 6 |
|
>99 |
|
99 [93] |
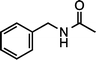
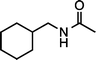
| 1g (100 °C, 3 h) | 2g | |||
| 7 |
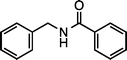
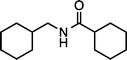
|
>99 |
|
97 [95] |
| 1h (100 °C, 3 h) | 2h | |||
| 8 |
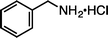
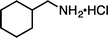
|
>99 |
|
93 [92] |
| 1i·HCl (100 °C, 3 h) | 2i·HCl | |||
| 9 |
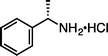
|
>99 |
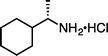
|
96 [91] |
(S)-1j·HCl (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R = 99 R = 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)e (100 °C, 3 h) 1)e (100 °C, 3 h) |
(S)-2j·HCl (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R = 96 R = 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4)e 4)e |
|||
| 10 |
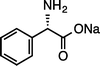
|
>99 |
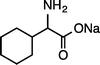
|
96 [90j] |
| (S)-1kNa (100 °C, 3 h) | 2kNa | |||
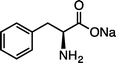
| 11 |
|
>99 |
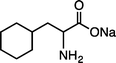
|
95 [91j] |
| (S)-1lNa (100 °C, 6 h) | 2lNa | |||
|
||||
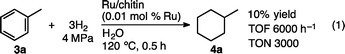
The hydrogenation of toluene (3a) by Ru/chitin in water showed a turnover frequency (TOF) of 6000 h−1 and a turnover number (TON) of 3000 based on the amount of consumed H2 (eqn (1)). These values are higher than or comparable to those in previously reported hydrogenations of 3a in water with other Ru or Rh catalysts (Ru: TOF and TON, 10–2700 h−1 and 240–2700; Rh: 100–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 and 300).4e,9d,10
000 h−1 and 300).4e,9d,10
The reusability of the Ru/chitin catalyst was tested over seven consecutive reductions of 1a, with the catalyst being recovered by centrifugation each time. Results indicated only a modest loss of catalytic activity and selectivity (hydrogenation of 1a: 1st run, 98% yield; 2nd run, 96% yield; 3rd run, 94%; 4th run, 90%; 5th run, 89%; 6th run, 87%; 7th run, 87%, Table S4†). This behavior was investigated by HRTEM analysis (Fig. 1). Analysis after either 1 or 6 hydrogenation cycles suggested that the nanoparticles continued to incorporate pristine metal, with an observed d-spacing of 2.14–2.17 Å attributed to the Ru(002) plane of Ru0. However, nanoparticle sintering was clearly observed after repeated testing, with the mean particle size growing from 2.3 ± 0.3 nm in the fresh catalyst (Fig. 1a) to 3.5 ± 0.8 nm after 6 hydrogenation cycles (Fig. 1c). TEM, EDX and XRD analyses of the as-prepared Ru catalysts (Fig. S1–S9†) suggest that 2–3 nm nanoparticles represent both the most efficient and selective of the catalysts tested. Interestingly, results point to the inexpensive polysaccharide supports chitin and cellulose accommodating such particles (Table S5 and Fig. S10†) more readily than other commercially available supports do when using the same preparative route. Though one commercially sourced Ru/C catalyst contains comparably-sized nanoparticles, the selectivity is lower than Ru/chitin or Ru/cellulose (Fig. S8 and Table S5†).
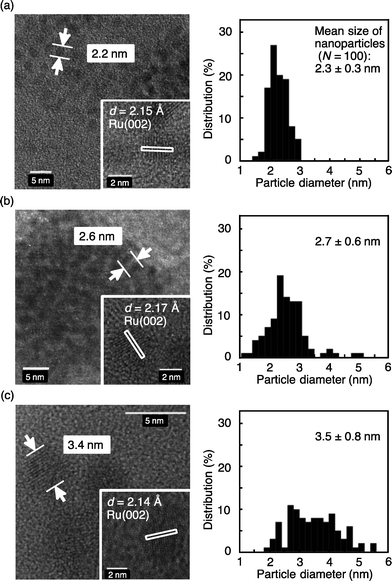 | ||
| Fig. 1 TEM images and particle size distributions of Ru/chitin: (a) as prepared, (b) after 1 cycle and (c) after 6 cycles of hydrogenation of 1a. | ||
In summary, we have prepared 2–3 nm chitin-supported ruthenium nanoparticles in the absence of additional capping agents. They have promoted efficient hydrogenation of arenes to cyclohexanes under near-neutral, aqueous conditions, with hydrogenation taking place to the exclusion of hydrogenolysis of normally reactive C–O and C–N linkages. Of importance, preliminary data point to the use of this readily available, environmentally benign support material being synonymous with the generation of nanoparticles whose dimensions provide both excellent conversion and selectivity. Ongoing work is seeking to more precisely investigate morphological changes exhibited by Ru/chitin in order to counteract the modest loss of activity after multiple hydrogenation cycles and to assess the possibility of developing these systems in a microfluidic context.
This work was financially supported by the Ichihara International Scholarship Foundation (to H. N.), the Institute for Quantum Chemical Exploration (to H. N.), MEXT (Japan) through its program “Integrated Research on Chemical Synthesis” (to H. N.) and the Royal Society through its International Exchange Scheme (to A. E. H. W. and H. N.). K. B. and B. R. K. thank the UK EPSRC (EP/J500380/1). Y. M. and A. M. acknowledge the IGER program at NU. We thank Professors R. Noyori (NU), S. Saito (NU) and K. Shimizu (Hokkaido U) for their helpful comments. Unprocessed data for this paper are available at the University of Cambridge data repository (see http://dx.doi.org/10.17863/CAM.432).
Notes and references
- M. J. Palframan and A. F. Parsons, Product class 4: Five Membered and Larger-Ring Cycloalkanes, in Science of Synthesis, ed. H. Hiemstra, E. Schaumann and M. S. Baird, Thieme, Stuttgart, 2009, vol. 48, section 48.4, pp. 647–693 Search PubMed.
- (a) S. Nishimura, Hydrogenation of Aromatic Compounds, in Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis, John Wiley & Sons, Inc., New York, 2001, ch. 11, pp. 414–496 Search PubMed; (b) F. Foubelo and M. Yus, Reduction/Hydrogenation of Aromatic Rings, in Arene Chemistry, ed. J. Mortier, John Wiley & Sons, Inc., New Jersey, 2015, pp. 339–364 Search PubMed.
- (a) H. Adkins and L. W. Covert, J. Phys. Chem., 1931, 35, 1684–1691 CrossRef CAS; (b) Y. Takagi, T. Naito and S. Nishimura, Bull. Chem. Soc. Jpn., 1964, 37, 585–587 CrossRef CAS.
- Selective arene hydrogenation remains a major issue in current synthetic chemistry: (a) I. S. Park, M. S. Kwon, K. Y. Kang, J. S. Lee and J. Park, Adv. Synth. Catal., 2007, 349, 2039–2047 CrossRef CAS; (b) R. Baumgartner, G. K. Stieger and K. McNeil, Environ. Sci. Technol., 2013, 47, 6545–6553 CAS; (c) C. Moreno-Marrodan, F. Liguori, E. Mercadé, C. Godard, C. Claver and P. Barbaro, Catal. Sci. Technol., 2015, 5, 3762–3772 RSC; (d) W. Gu, M. M. Stalzer, C. P. Nicholas, A. Bhattacharyya, A. Motta, J. R. Gallagher, G. Zhang, J. T. Miller, T. Kobayashi, M. Pruski, M. Delferro and T. J. Marks, J. Am. Chem. Soc., 2015, 137, 6770–6780 CrossRef CAS PubMed; (e) C.-H. Pélisson, A. Denicourt-Nowicki and A. Roucoux, ACS Sustainable Chem. Eng., 2016, 4, 1834–1839 CrossRef; (f) M. M. Stalzer, C. P. Nichloas, A. Bhattacharyya, A. Motta, M. Deferro and T. J. Marks, Angew. Chem., Int. Ed., 2016, 55, 5263–5267 CrossRef CAS PubMed; (g) B. Sun and G. Süss-Fink, J. Organomet. Chem., 2016, 812, 81–86 CrossRef CAS.
- (a) S. Nishimura, Bull. Chem. Soc. Jpn., 1959, 32, 1155–1157 CrossRef CAS; (b) P.-E. Tong, P. Li and A. S. C. Chan, Tetrahedron: Asymmetry, 2001, 12, 2301–2304 CrossRef CAS; (c) S. Nishimura and H. Taguchi, Bull. Chem. Soc. Jpn., 1963, 36, 353–355 CrossRef CAS.
- (a) Y. Takagi, T. Naito and S. Nishimura, Bull. Chem. Soc. Jpn., 1964, 37, 585–587 CrossRef CAS; (b) Z. Tang, Z. Yang, X. Chen, L. Cun, A. Mi, Y. Jiang and L. Gong, J. Am. Chem. Soc., 2005, 127, 9285–9289 CrossRef CAS PubMed.
- (a) J. H. Stocker, J. Org. Chem., 1962, 27, 2288–2289 CrossRef CAS; (b) S. Siegel, Rhodium on Alumina, in Encyclopedia of Reagents for Organic Synthesis, ed. L. A. Paquette, D. Crich, P. L. Fuchs and G. A. Molander, Wiley, Chichester, 2009, vol. 11, pp. 8559–8563 Search PubMed; (c) F. Jing, M. R. Smith III and G. L. Baker, Macromolecules, 2007, 40, 9304–9312 CrossRef CAS.
- (a) I. S. Park, M. S. Kwon, K. Y. Kang, J. S. Lee and J. Park, Adv. Synth. Catal., 2007, 349, 2039–2047 CrossRef CAS; (b) H.-W. Lin, C. H. Yen and C.-S. Tan, Green Chem., 2012, 14, 682–687 RSC.
- (a) Y. Motoyama, M. Takasaki, K. Higashi, S.-H. Yoon, I. Mochida and H. Nagashima, Chem. Lett., 2006, 35, 876–877 CrossRef CAS (CNF-P = platelet carbon nanofibers); (b) M. Takasaki, Y. Motoyama, K. Higashi, S.-H. Yoon, I. Mochida and H. Nagashima, Chem. – Asian J., 2007, 2, 1524–1533 CrossRef CAS PubMed; (c) Y. Motoyama, M. Takasaki, S.-H. Yoon, I. Mochida and H. Nagashima, Org. Lett., 2009, 11, 5042–5045 CrossRef CAS PubMed (CNF-T = tubular carbon nanofibers); (d) L. Gao, K. Kojima and H. Nagashima, Tetrahedron, 2015, 71, 6414–6423 CrossRef CAS (HPS-NR3Cl = ammonium salts of hyperbranched polystyrene).
- For examples of Ru or Rh nanoparticle catalysts for arene hydrogenation in water, see: Ru: (a) A. Nowicki, Y. Zhang, B. Léger, J.-P. Rolland, H. Bricout, E. Monflier and A. Roucoux, Chem. Commun., 2006, 296–298 RSC; (b) A. Nowicki, V. Le Boulaire and A. Roucoux, Adv. Synth. Catal., 2007, 349, 2326–2330 CrossRef CAS; (c) L. Song, X. Li, H. Wang, H. Wu and P. Wu, Catal. Lett., 2009, 133, 63–69 CrossRef CAS; (d) C. Hubert, A. Denicourt-Nowicki, A. Roucoux, D. Landy, B. Leger, G. Crowyn and E. Monflier, Chem. Commun., 2009, 1228–1230 RSC; Rh: (e) C. Hubert, A. Denicourt-Nowicki, J.-P. Guégan and A. Roucoux, Dalton Trans., 2009, 7356–7358 RSC; (f) C. Hubert, A. Denicourt-Nowicki, P. Beaunier and A. Roucoux, Green Chem., 2010, 12, 1167–1170 RSC; (g) E. G. Bilé, R. Sassine, A. Denicourt-Nowicki, F. Launay and A. Roucoux, Dalton Trans., 2011, 40, 6524–6531 RSC.
- During the review process for this manuscript, arene hydrogenation of benzyl ethers by the Ru/carbon-nitrogen matrix was reported: X. Cui, A.-E. Surkus, K. Junge, C. Topf, J. Radnik, C. Kreyenschulte and M. Beller, Nat. Commun., 2016, 7, 11326 CrossRef PubMed.
- (a) P. J. Corringer, J. H. Weng, B. Ducos, C. Durieux, P. Boudeau, A. Bohme and B. P. Roques, J. Med. Chem., 1993, 36, 166–172 CrossRef CAS PubMed; (b) R. Hartung, F. Hitzel-Zerrahn, T. Müller and J. Pietsch, US Pat., 8148430B2, 2012 Search PubMed; (c) D. J. Ager and I. Prakash, Org. Process Res. Dev., 2003, 7, 164–167 CrossRef CAS.
- A. Matsuoka, T. Isogawa, Y. Morioka, B. R. Knappett, A. E. H. Wheatley, S. Saito and H. Naka, RSC Adv., 2015, 5, 12152–12160 RSC.
- Chitin is the second most abundant polysaccharide in nature: Chitin: Formation and Diagenesis, ed. N. S. Gupta, Springer, New York, 2011 Search PubMed.
- (a) L. M. Rossi and G. Machado, J. Mol. Catal. A: Chem., 2009, 298, 69–73 CrossRef CAS; (b) F. Schwab, M. Lucas and P. Claus, Angew. Chem., Int. Ed., 2011, 50, 10453–10456 CrossRef CAS PubMed; (c) M. Kaushik, H. M. Friedman, M. Bateman and A. Moores, RSC Adv., 2015, 5, 53207–53210 RSC; (d) A. Gual, C. Godard, S. Castillon and C. Claver, Dalton Trans., 2010, 39, 11499–11512 RSC; (e) P. Lara, K. Philippot and B. Chaudret, ChemCatChem, 2013, 5, 28–45 CrossRef CAS.
- 2a can also be a (co-)monomer for preparing epoxide resins.
- (a) Ref 9c: 1a (0.5 mmol), Rh/CNF-T (10 mg, 0.2 wt% Rh, 0.04 mol% Rh), H2 (1 MPa), n-hexane (1 mL), rt, 12 h; 2a, >99% yield; (b) Ref 9d: 1a (1 mmol), Ru/HPS-NR3Cl (0.3 mol% Ru), H2 (3 MPa), H2O (1 mL), 30 °C, 24 h; 2a, 96% yield.
- Ru/C and Rh/C are excellent, general catalysts for arene hydrogenation: T. Maegawa, A. Akashi, K. Yaguchi, Y. Iwasaki, M. Shigetsura, Y. Monguchi and H. Sajiki, Chem. – Eur. J., 2009, 15, 6953–6963 CrossRef CAS PubMed.
- The origin of high selectivity in the hydrogenation of 1a with Ru/chitin remains unclear. Coordination of hydroxyl groups in chitin to the Ru metal does not seem to suppress catalytic activity for side reactions; the selectivity in the hydrogenation with Rh/Al2O3, Ru/C or Rh/C was not significantly altered by adding ethylene glycol (Table S2,† entries 1–3). Meanwhile, the coordination of acetamide groups to the Ru metal may partly account for the high selectivity of Ru/chitin; addition of N-acetylethanolamine increased the selectivity for arene hydrogenation with Rh/C (Table S2,† entry 5).
- T. L. Amyes, J. P. Richard and M. Novak, J. Am. Chem. Soc., 1992, 114, 8032–8041 CrossRef CAS.
- The reason for a small amount of racemization in Table 2, entries 2 and 9 is not entirely clear, but we assume it to result from dehydrogenation of alcohol or amine·HCl followed by hydrogenation of the resulting ketones and iminium salts. This view is based on the following observations: (1) in Table 2, entry 4, ketone 6e formed in 7% yield; (2) hydrogenation of acetophenone with Ru/chitin under the standard conditions yielded cyclohexyl methyl ketone (20%), 1c (27%) and 2c (52%).
- Hydrogenation of dibenzyl ether (100 °C, 6 h, 91% conv.) gave a mixture of bis(cyclohexylmethyl) ether (27%), benzyl cyclohexylmethyl ether (38%), 2b (7%) and other unidentified products. Hydrogenation of dibenzylamine·HCl (100 °C, 5 h, >99% conv.) gave a mixture of bis(cyclohexylmethyl)amine·HCl (21%) and 2i·HCl (74%).
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cy00899b |
| This journal is © The Royal Society of Chemistry 2016 |