Highly efficient synthesis of flavones via Pd/C-catalyzed cyclocarbonylation of 2-iodophenol with terminal acetylenes†
Abstract
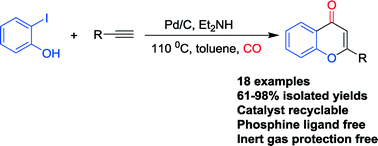
A highly efficient and selective Pd/C-catalyzed ligand-free cyclocarbonylation reaction for the synthesis of flavones has been developed. Various flavone derivatives were isolated in excellent yields with excellent functional group tolerances. Additionally, catalyst reuse experiments were performed successfully as well.

 Please wait while we load your content...
Please wait while we load your content...