CO2 reforming of methane over highly active La-promoted Ni supported on SBA-15 catalysts: mechanism and kinetic modelling
Abstract
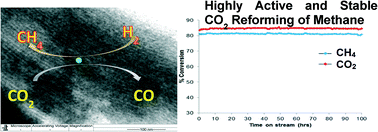
Various Ni catalysts were synthesized by combining a high surface area SBA-15 support, a novel preparation method using an oleic acid precursor to obtain highly dispersed and small Ni particles, and the basic property of La. The activity test shows that the La-promoted Ni/SBA-15 catalyst has much higher catalytic activity and stability than unpromoted Ni/SBA-15 catalysts in the CO2 (dry) reforming of methane (DRM) reaction due to the crucial role of La to simultaneously adsorb CO2 and remove deposited carbon from the Ni metal surface. The optimization of La content shows that only a small amount of La around 1 wt% is necessary to achieve the best catalytic performance. The reaction mechanism was then proposed and kinetic modeling (for pilot scale) was performed to find the rate-determining step (RDS) in the DRM reaction for this 1% La-promoted Ni/SBA-15 catalyst. The obtained fitting result shows that CH4 decomposition is the RDS for this catalyst with an apparent activation energy of around 40.8 kJ mol−1 which is similar to the activation energies reported for common Ni-based supported catalysts.

 Please wait while we load your content...
Please wait while we load your content...