Gold carbenes, gold-stabilized carbocations, and cationic intermediates relevant to gold-catalysed enyne cycloaddition
Abstract
Cationic gold complexes in which gold is bound to a formally divalent carbon atom, typically formulated as gold carbenes or α-metallocarbenium ions, have been widely invoked in a range of gold-catalyzed transformations, most notably in the gold-catalyzed cycloisomerization of 1,n-enynes. Although the existence of gold carbene complexes as intermediates in gold-catalyzed transformations is supported by a wealth of indirect experimental data and by computation, until recently no examples of cationic gold carbenes/α-metallocarbenium ions had been synthesized nor had any cationic intermediates generated via gold-catalyzed enyne cycloaddition been directly observed. Largely for this reason, there has been considerable debate regarding the electronic structure of these cationic complexes, in particular the relative contributions of the carbene (LAu+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR2) and α-metallocarbenium (LAu–CR2+) forms, which is intimately related to the extent of d → p backbonding from gold to the C1 carbon atom. However, over the past ∼ seven years, a number of cationic gold carbene complexes have been synthesized in solution and generated in the gas phase and cationic intermediates have been directly observed in the gold-catalyzed cycloaddition of enynes. Together, these advances provide insight into the nature and electronic structure of gold carbene/α-metallocarbenium complexes and the cationic intermediates generated via gold-catalyzed enyne cycloaddition. Herein we review recent advances in this area.
CR2) and α-metallocarbenium (LAu–CR2+) forms, which is intimately related to the extent of d → p backbonding from gold to the C1 carbon atom. However, over the past ∼ seven years, a number of cationic gold carbene complexes have been synthesized in solution and generated in the gas phase and cationic intermediates have been directly observed in the gold-catalyzed cycloaddition of enynes. Together, these advances provide insight into the nature and electronic structure of gold carbene/α-metallocarbenium complexes and the cationic intermediates generated via gold-catalyzed enyne cycloaddition. Herein we review recent advances in this area.
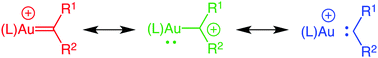
- This article is part of the themed collection: Coinage Metals

 Please wait while we load your content...
Please wait while we load your content...